Abstract
This review evaluates oilseed crop soybean endophytic bacteria, their prospects, and challenges for sustainable agriculture. Soybean is one of the most important oilseed crops with about 20–25% protein content and 20% edible oil production. The ability of soybean root-associated microbes to restore soil nutrients enhances crop yield. Naturally, the soybean root endosphere harbors root nodule bacteria, and endophytic bacteria, which help increase the nitrogen pool and reclamation of another nutrient loss in the soil for plant nutrition. Endophytic bacteria can sustain plant growth and health by exhibiting antibiosis against phytopathogens, production of enzymes, phytohormone biosynthesis, organic acids, and secondary metabolite secretions. Considerable effort in the agricultural industry is focused on multifunctional concepts and bioprospecting on the use of bioinput from endophytic microbes to ensure a stable ecosystem. Bioprospecting in the case of this review is a systemic overview of the biorational approach to harness beneficial plant-associated microbes to ensure food security in the future. Progress in this endeavor is limited by available techniques. The use of molecular techniques in unraveling the functions of soybean endophytic bacteria can explore their use in integrated organic farming. Our review brings to light the endophytic microbial dynamics of soybeans and current status of plant microbiome research for sustainable agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, diverse oilseed crops are cultivated for edible oil production to safeguard humans from malnutrition and related illnesses [1]. Their production rate differs from one country to another due to adaptation and growth under different weather conditions by region (e.g., temperate, tropical, and subtropical) [2]. The major type of oilseed crops are canola, groundnut, palm oil, sunflower, soybean, peanut, rapeseed, and cottonseed [3]. In 2020/2021, statistics of USDA showed an account of 362.05 soybeans, 68.87 rapeseed, 49.46 sunflower seed, 47.79 peanuts, and 41.80 of cottonseed, 19.96 palm kernel, and 5.75 copra world oilseed production (million metric tons) with soybean estimated of about a 90% production in the USA [4]. Also, in Sub-Saharan Africa, Nigeria produces and exports a larger percentage of soybean annually.
Soybeans are leguminous plants in the family Fabaceae. Interest in soybean cultivation relies on their economic value, the edible oil-producing potential of about 20%, and protein content of 20–25% [5]. Notably, soybeans serve as an inexpensive and excellent source of high-quality edible oil and protein for humans as compared to other leguminous crops and animal protein [6], and can be a supplement food source for livestock. Yet, soybean’s market value and maximum utilization are less explored in many countries [7]. Soybean can be processed into composite food products, substituting animal proteins, i.e., eggs, meat, and milk.
The uncertainties and challenges facing soybean cultivation may include poor and inefficient farming systems, drought, disease invasion, pest attack, lack of disease-resistant cultivars, etc. [8,9,10]. Diseases such as stem and root blight, bacterial leaf blight, downy mildew, bacterial pustule, rust, purple seed stain, frog-eye leaf spot, brown spot, charcoal rot, and soybean mosaic virus are the most common peculiar to soybean [11]. The control of disease in plants and crops under storage can be achieved by either biological, chemical, or physical means. Therefore, adopting proper control measures against phytopathogens in soybean can sustain plant health and crop productivity.
From antiquity, farmers adopted diverse cropping systems (crop rotation, mixed farming, organic farming, etc.) and agricultural practices (e.g., agrochemicals, irrigation, and harrowing) to mitigate bottlenecks limiting the cultivation of soybean and other food crops. Over time, agrochemical use has been a major concern to environmentalists, ecologists, and microbiologists due to the negative impact on the ecosystem [12, 13]. The peculiarity of these challenges is not limited to soybean cultivation alone, but other economical food crops.
In recent times, research efforts are on the increase to devise a sustainable means of improving soybean, and other food crop production in order to help solve food scarcity, hunger, and malnutrition [14]. Because of the environmental threats posed by the synthetic fertilizer application and the incessant population increase, the need to employ biorational approaches and sustainable measures to enhance soybean production has become imperative. Naturally, soybean houses endophytic microbes capable of increasing the nitrogen pool in the soil to enhance plant nutrition for higher productivity [15]. The natural occurrence of these nitrogen-fixing bacteria is a promising way to reclaim lost soil nutrients for food production to meet the demand of the ever-growing population and relieve farmers of the cost and over-dependence on chemical fertilizers by farmers. Thus, harnessing endophytic bacteria as bioinoculants to oppose chemical fertilizers is critical as the best alternative.
The plant root endosphere represents discreet regions occupied by diverse, endophytic microorganisms [16], where these microbes exhibit mutualistic, neutral, or antagonistic relationships with the host plants. The emphasis on the root-associated bacteria will be most considered in this review, as soybean root nodules naturally contain diverse nitrogen-fixing bacteria (NFB) [17]. The complementary effects of root-associated bacteria and root nodule NFB can positively influence plant growth and survival under nitrogen-limiting soils [18]. Here we emphasize that the nitrogen-fixing potential of endophytic bacteria in leaves, stems, seeds, flowers, ovules, etc. may be of greater importance in plant growth when compared to root nodules NFB only. Nevertheless, comparative studies of these bacteria from various plant organs upon inoculation under greenhouse and field experiments are required to ascertain this claim, requiring further studies.
The molecular insights into plant–microbe interactions have unveiled important functions of some endophytic microbes, which suggests their maximum exploration as bioinoculants in sustaining plant growth and health [19]. For instance, a few beneficial nodule endophytic microbes associated with soybeans have been assessed under greenhouse and field trials to enhance soybean yield and in vitro screening for their antimicrobial properties against phytopathogens [20].
The interdependence of endophytic bacteria with the host plants confers beneficial effects in soybeans and other food crops, such that it stimulates plant growth promoters, antibiosis activity against phytopathogens for plant health, defense against oxidative stress, and yield enhancement without any pathogenic effects [21, 22]. Limited information is available in the literature on the plant growth stimulation and biocontrol potential of endophytic bacteria inhabiting soybean, thus limiting their ecological services. Nevertheless, exploring endophytic bacteria as bioinoculants can provide several opportunities in mitigating diverse agricultural problems, such as biotic and abiotic stress, and climate change. Furthermore, addressing the challenges and uncertainties limiting plant microbiome biotechnologically will ultimately reveal the amazing realities of incorporating endophytic resources from soybean and other food crops into agricultural management. Our review brings to light the endophytic microbial dynamics of soybeans and current status of plant microbiome research for sustainable agriculture.
The Soybean Microbiome
It is essential to evaluate the diversity and population of endophytes in soybean plants in different environments, as a knowledge of this would serve as a background to promote their usage as biofertilizers, soil amendments, plant growth enhancers, and biocontrol agents with the overall aim of increasing different plant yield. Despite the success recorded in soybean’s endophytic microbiome research with promises to achieve future agricultural productivity [10, 15, 23], there is still a need for further studies. Sustaining plant health is paramount, as it is relatively mirrored in crop yield. The antimicrobial compounds and metabolites naturally found in economical plants, coupled with the biocontrol potential of some endophytic microbes, can contribute to plant health by reducing plant pathogenicity [24]. Inefficient control of plant pathogens results in yield loss to crops [25]. To help ameliorate these threats, in vitro screening of novel endophytic bacteria from economic plants for antimicrobial activity became important in identifying targeted biocontrol agents to specific pathogens in the host plants [26].
Taking account of key certain environmental factors that influence microbial community structure by monitoring different ecological niches is vital to ascertain specific environmental factors influencing microbial diversity in plants. For instance, in the phyllosphere, a limited supply of nutrients, ultraviolet light, humidity, temperature, oxygen concentration, pH, etc. influence the microbiome in this niche [27]. In the root endosphere, pathogens, nutrient deposition, and versatility might be key factors influencing the diversity of the microbes in different plants. The effect of ultraviolet “B” has been reported to influence the bacterial community structure in the soybean phyllosphere [28]. The factors (geographical location, carbohydrates, amino acids, and other soil nutrients) influence the microbial diversity in the root endosphere [29]. The soil-inhabiting microbes and some phyllosphere endophytic microbes can withstand high ultraviolet radiation due to the presence of pigments, i.e., melanin, xanthomonadine, and carotenoids [30]. The microorganisms found in the same ecological niche can be differentiated based on their characterization, genetic composition, and metabolic activities [30].
Plant endosphere ecology comprises microbial domains found in the below (root, sometimes seeds) and above (stem, leaf, seed, flower, and ovule) plant parts [31]. The microbial population and diversity in the plant root may be dissimilar compared to the other plant parts. The root endophytes are influenced by the exudate-secondary metabolites released into the soil-root environment [32]. Mina, Pereira, Lino-Neto, and Baptista [33] stated that the endophyte diversity in the different organs of a particular plant is mediated by the physical and chemical properties. This claim was relative to soybean as de Almeida Lopes, Carpentieri‐Pipolo, Oro, Stefani Pagliosa, and Degrassi [34] observed a similarity in the diversity of microorganisms in soybean.
Endophytic Bacteria Associated with Soybeans
Studies on the functional traits exhibited by endophytic bacteria associated with soybean and Arabidopsis aim to reveal their significance in agriculture, industry, and medicine [15, 35]. The effects of some endophytic bacteria from legumes and other food crops on plant growth are presented in Table 1. Hence, the advantages of beneficial endophytic bacteria (e.g., plant production, growth, secondary metabolites) found in different food crops from various plant habitats remains crucial in plant growth promotion, inducing plant tolerance to harsh environmental conditions and disease control. These researchers reported Citrobacter freundii and Enterobacter asburiae from the root and stem; Kosakonia cowanii, Pantoea agglomerans, and Variovorax paradoxus from the root and leaf; Staphylococcus aureus from the stem and leaf; and Enterobacter ludwigii from the root, stem, and leaf of soybean. Likewise, Dubey, Saiyam, Kumar, Hashem, Abd_Allah and Khan [15] and Brunda, Jahagirdar, and Kambrekar [36] also isolated Bacillus pumilus from the stem and leaf of the soybean plant, which aligns closely with the claims of de Almeida Lopes, Carpentieri‐Pipolo, Oro, Stefani Pagliosa, and Degrassi [34], who observed similar bacterium in different organs of soybean. Hence, it is crucial to carry out more research to have a deeper understanding of the inherent factors affecting the diversity of endophytes in different plant parts for maximum exploration in solving agricultural problems.
The selection of endophytic bacteria based on taxonomy and functions can help understand diverse bacteria communities in different plants [37]. Plants of the same species may have different bacteria compositions and associations, depending on the location, genotype, cropping system, climatic conditions, and growth stage [38].
The genomic data available on the microbes from soybean with unique metabolic features reveal their genetic variation. The notable genes involved in flagella biosynthesis (flg, fil, flh), chemotaxis (cheABRVWZ, mpc), IAA synthesis (trpABCDE), nitrogen fixation (iscU), and phosphate solubilization (pstABCS) identified in the genome of Pseudomonas fluorescens BRZ63 isolated from rapeseed may be responsible for the bacterium functions in enhancing plant growth and disease control [39]. A study by Adeleke, Ayangbenro, and Babalola [40] reported genes involved in nitrogen fixation, phosphate transport and solubilization, siderophore production, secretion systems, iron transport, flagella, flagella biosynthesis, and phytohormones in the genome of endophytic Bacillus cereus T4S isolated from sunflower, which enhanced sunflower yield. Furthermore, studies should also be intensified on soybean to unravel the genes in their different endophytes, enhancing plant growth.
Plant–microbe cooperation can modulate the transfer of certain genetic traits in the host plant by genome modulation, which may assist plants in acquiring novel traits and in boosting their adaptation mode of actions in diverse environments. The level of genetic communication in the root-soil interface facilitates microbial infiltration into the plants [41]. However, the similar genetic complexity between rhizosphere microbes and endophytic microbes provide new insights into their colonization pattern into the root endosphere and become endophytes [42]. Therefore, the mechanisms employed by soybean endophytic microbes in plant growth promotion need to be understood to ascertain their roles in the plant endosphere.
Endophytic Fungi Associated with Soybean
Providing information on endophytic fungi (EF) inhabiting the root of soybean can help unravel the prospects of soybean in sustainable crop production. The plant growth–promoting attributes of bacteria and fungi inhabiting the root of plants may share significant similarities depending on the sample type, isolation source, and growth conditions [43, 44]. EF employs multifunctional strategies for plant growth and protection against biotic and abiotic stressors [45]. Unraveling the community structure and complex plant–microbe synergies in the host plants has made the science of endophyte interesting as a way of maximizing their bio-products (bioinoculants) to ensure food security [46, 47].
The EF which forms part of plant lifestyle with a strong affinity in the root endosphere due to the presence of cell organelle (mycelia) can be explored in agriculture [48,49,50]. Despite the ecological services of plant-associated EF, there is still a need to further investigate EF associated with soybean. For instance, the biocontrol potential of EF isolated from rapeseed against Botrytis cinerea and Sclerotinia sclerotiorum, which causes gray mold and Sclerotinia stem root, has necessitated further their exploration [51]. A study by Sallam, Ali, Seleim, and Bagy [10] reported antagonistic activity of the endophytic fungus Trichoderma spp. isolated from the soybean against Rhizoctonia solani, which reduces their effect on soybean yield under greenhouse experiments. Other research findings (to mention but a few) on the plant growth promotion and antifungal attributes of EF against plant pathogens were evident in literature due to phytohormone and metabolite secretions [25, 51,52,53].
The biotechnological potential of diverse EF in the production of therapeutic agents and antibiotics revealed their beneficial effect on plant immunity and growth enhancement [54]. The mechanism of action and factors influencing the diversity of root-associated endophytic bacteria and root-associated EF may be similar, possibly based on the same source of identification. Some identifiable EF isolated from the root, stem, and leaves of soybean with detailed biological activities for sustainable plant health includes Trichoderma asperellum, T. longibrachiatum, and T. atroviride [10], Colletotrichum spp., Pestalotiopsis spp., Botryosphaeria spp., Diaporthe spp. [55], and Fusarium, Alternata [56]. Despite their multifaceted attributes in plant growth promotion, disease suppressiveness, stress alleviation, metal reduction, and nutrient mineralization [57,58,59], there is still a need for more studies into the EF colonizing the root of soybean.
Methodologies and Bottlenecks Limiting the Endophytic Study
The identification of endophytes in their host plants is somewhat difficult because some endophytic microbes might not be easy to culture in the laboratory [60], while some are viable but non-culturable. Hence, the use of culture-dependent and culture-independent methods remain important as the case may be. In the use of culture-dependent methods, the population of microbes are easily evaluated, while in contrast, the culture-independent methods are more useful in assessing the entire microbiome in the samples [61]. Culture-dependent methods, which involve microbial isolation on nutrient-rich microbiological media under specific revolutionized growth conditions, are important to determine microbial physiology and genes and screening for plant growth-promoting traits [62].
Conversely, this technique is laborious, revealing detailed microbial diversity and networking in econiches. Also, the proliferation of undesirable microorganisms on the cultured plates, which compete for nutrients needed by the desirable microorganisms, has been identified as a major challenge when isolating endophytic microbes by culturing methods [63]. Hence, the application of culture-independent methods is profound in characterizing yet-to-be cultured microorganisms. Authors Alain and Querellou [64], Torsvik and Ovresas [65], and Afzal, Shinwari, Sikandar, and Shahzad [63] stated that culturable bacteria represent about 0.0001–1% of the total endophytes in plants. Hence, the interest of researchers on purposeful research design should be considered before selecting a method for isolating endophytic microbes.
Endophytes can be cultured on agar plates, and then microbial DNA can be extracted before carrying out polymerase chain reaction (PCR). Garcias-Bonet, Arrieta, de Santana, Duarte, and Marbà [66] employed a commercial DNA extraction kit specific for plant DNA extraction to extract microbial endophytic DNA and used primer meant for the bacteria domain to carry out the PCR procedure. However, it should be noted that when amplifying a specific region of bacteria DNA, the mitochondria and chloroplast DNA found in plants may have a close resemblance to that of endophytes; hence, this method might not be too appropriate. In this light, next-generation sequencing is recommended without denaturing gradient gel electrophoresis (DGGE) analysis. Piccolo, Ferraro, Alfonzo, Settanni, Ercolini, Burruano, and Moschetti [67] demonstrated the use of fluorescence in situ hybridization (FISH) technique in studying endophytic microbes. However, this can only be done in the natural habitat, thus making the laboratory isolation complicated.
On the other hand, Ikeda, Kaneko, Okubo, Rallos, Eda, Mitsui, Sato, Nakamura, Tabata, and Minamisawa [68] developed a procedure to enrich bacterial cells when isolating unculturable endophytes from the stem of a soybean by fractionalizing the homogenated soybean stem. This method was achieved by differential centrifugation and Nycodenz density gradient centrifugation. This method proved effective compared to when DNA was isolated from the soybean stem due to the higher intensity and number of amplicons of the bacteria when the efficiency of the bacteria cell was fortified using ribosomal intergenic spacer analysis. Equally, Lundberg, Yourstone, Mieczkowski, Jones, and Dangl [69] also worked on an improved technique for 16S ribosomal rRNA sequencing, where unique template molecules were tagged before PCR by mapping amplicon sequences (to their original templates), which help to prevent error and bias arising from the amplification process. This method uses a base pair sequence with a higher temperature (melting) than the primer set, which is designed to attach to the host’s DNA.
The culture-independent methods are more advanced due to attention drawn to them which facilitated more research to improve them. For instance, a modern analytical approach has been documented to advance the science of the plant microbiome [70]. The use of combined stable isotope probing (SIP) and nanoscale secondary ion mass spectrometry techniques (NanoSIMS) coupled with advanced Raman spectroscopy-based single cell–based methods have been envisaged in studying plant microbiome in situ and to determine their biological functions in the bioremediation of complex pollutants from metal-polluted soil [71]. More importantly, the specific metabolic functions of endophytic microbes can be better understood by combining SIP with other molecular methods, such as qPCR, finger printing, and cloning.
Dos Santos and Olivares [72] reported the use of microcosm combined with bacteria stocks as a reference to determine bacteria assemblage in the root of plants and their plant growth-promoting potential. Also, Hartman, van der Heijden, Roussely-Provent, Walser, and Schlaeppi [73] reported a microcosm approach in elucidating the bacteria diversity and function in the root of red clover. Furthermore, Hartman, van der Heijden, Roussely-Provent, Walser, and Schlaeppi [73] revealed a significant reduction in the growth of red clover upon mono-inoculation with Flavobacterium compared to the co-inoculation of red clover with root microbiome, which enhanced plant growth by reducing the negative effect of mono-inoculation of red clover with Flavobacterium. Finally, a microcosm study performed by Eldridge, Travers, Val, Ding, Wang, Singh, and Delgado‐Baquerizo [74] reported diverse microbiome and their functions on 15 plant species growing in terrestrial habitats to reveal the preference of plant-associated microbes and their importance in plant germination. It will be interesting to fashion out how these modern approaches can be employed in the science of endophytes to better understand endosphere biology.
Different molecular approaches exist for the identification of endophytic bacteria and the combination of recent molecular approaches, such as genome sequencing and metagenome. The use of DNA extracted from the root of plants can be employed in unraveling microbial community structure and functions in soybean. The extracted DNA from plant tissues after surface sterilization with water, hypochlorite, or ethanol for endophytic studies might contain a certain proportion of plant DNA, which is needed to be depleted using appropriate sequencing techniques and plantforms (e.g., Illumina, PacBio, and DNA fingerprinting). Many techniques exist for DNA fingerprinting. These include restriction fragment length polymorphism (RFLP), simple sequence repeat (SSR), terminal-RFLP, rapid amplified polymorphic DNA, amplified fragment length polymorphism, inter-SSR, single-stranded conformation polymorphism, and DGGE [75]. The analysis of diverse plant microbiomes based on genetic composition can be achieved by the real-time polymerase chain reaction, FISH, automated version of ribosomal intergenic spacer analysis, terminal restriction fragment length polymorphisms, and DGGE, and phospholipid and fatty acid have also been documented [72, 76,77,78].
It is noteworthy to understand the use of molecular methods in identifying yet-to-be microbial endophytes by using appropriate methods to maximally recover endophytic DNA after the extraction process. The advent of PCR-based approaches in the Plant-Microbial Genome Project has provided vast advantages and opportunities for the detection, multiplication, quantification, and synthesis of copies of DNA in large amounts, differentiated from one another [79]. PCR techniques have been widely employed for the detection of diverse genes responsible for microbial functions [80]. The PCR and DNA sequencing aims at measuring the presence, taxonomy, and functions of plant microbiome from various samples, although, despite the importance of these techniques, there are limitations surrounding the PCR amplification process and DNA sequencing, mostly when extracting DNA from plant samples. The limitations include (i) contamination during the DNA extraction for PCR reaction and library preparation which may affect the DNA integrity, resulting in result errors and false outcome; (ii) primers’ design which require some previous sequence information; and (iii) the specific PCR product obtained during amplification process may be altered from one microbe to another based on non-specific binding of primers to other identical targeted sequences [81]. Piccolo, Ferraro, Alfonzo, Settanni, Ercolini, Burruano, and Moschetti [67] demonstrated the use of FISH technique in studying endophytic microbes; however, this can only be done in the natural habitat, thus making the laboratory isolation complicated. Furthermore, addressing these limitations specific to sequences may help devise approaches for the normalization of sequenced data to reveal microbial composition in its entirety. The use of PCR coupled with other sequence-based approaches is promising with more insights into plant microbiome gene combinations [71].
The advent of advanced molecular techniques for endophytes’ identification has succeeded in DNA fingerprinting, for instance, the use of omics approaches where DNA is retrieved from bacteria to evaluate the diversity, functions, genes, metabolites, transcripts, and proteins with the aid of next-generation sequencing. The DNA fingerprinting methods have been overtaken by more technical procedures, such as metagenomics which involves DNA extraction from the total bacteria population using next-generation sequencing [82]. This method has proven to better unravel the total endophytes from plant tissues compared to the fingerprinting techniques. Aside from omics approaches, the use of microscopy techniques, epifluorescence light microscopy, bright-field light microscopy, interferential and differential contrast light microscopy, scanning electron, and transmission electron microscopy in determining visual evidence of microbial colonization patterns in plants, has been documented [72, 76, 83].
The use of culture-independent techniques, which involved DNA/RNA extraction from environmental samples coupled with omics approaches, has revolutionized the science of endophyte microbiology in generating large sequence datasets. This next-generation sequencing approach involving no DNA cloning has been employed to unveil the community structure, diversity, taxonomic and functional profiling, metabolites, and metabolic pathways of the plant microbiome [72]. So far, the few research efforts utilizing next-generation sequencing from soybeans and other food crops revealed their taxonomic and functional attributes of endophytes in different plant species (Table 2).
Furthermore, addressing these limitations specific to sequences may help devise approaches for the normalization of sequenced data to reveal microbial composition in its entirety. The use of PCR coupled with other sequence-based approaches is promising with more insights into plant microbiome gene combinations [71]. The combination of recent molecular approaches, such as genome sequencing and metagenome using DNA extracted from the root of plants, can be employed in unraveling microbial community structure and functions in soybean. More importantly, the specific metabolic functions of endophytic microbes can be better understood by combining SIP with other molecular methods, such as qPCR, finger printing, and cloning.
The advancement in plant microbiome studies has revealed certain traits, which mediate their functions, such as secondary metabolites, genetic information, proteins, and transcripts using culture-dependent and culture-independent techniques [84,85,86]. Modern approaches to studying diverse endophytic microbes and functions are being employed to understand the colonization pattern for plant–microbe interactions based on host specificity and signaling networking for microbial communications linked to root exudation [87]. The genes involved in flagellation, chemotaxis, motility, and biofilm formation has been reported in many bacteria strains, which facilitate their attachment/adherence, penetration, and colonization in the host plants [88, 89]. The host plants’ specific signal-networking and plant–microbe communications can reveal how microbes exhibit mutual relationships and antagonistic toward the phytopathogens by triggering host immune responses [12]. Aside from genes involved in beneficial bacterial colonization, other genes have also been documented to partake in microbial biological processes. For instance, the genes involved in carbohydrate metabolism, phytohormone synthesis, secretion systems, biocontrol activity, and oxidative stress identified in the genome of endophytic bacteria from sunflower, apricot, and poplar that are important in agriculture, biotechnology, and industry have been documented [90,91,92].
In line with the aforementioned approaches and conventional techniques, studying the plant microbiome can be easier. Hence, it is recommended to compare the different recovering or identifying endophytes. This would assist in selecting the best method to use to identify endophytic microbe from plant samples. On the other hand, both the culture-dependent and culture-independent methods of endophyte analysis can help have a broader view of the diversity and population of plant endophytes and their functional attributes in the ecosystem. Briefly, the advantages and disadvantages of the techniques and approaches employed in the study of plant-associated microbes are highlighted in Table 3.
Complexity of Plant Microbiome in Plant Ecosystem
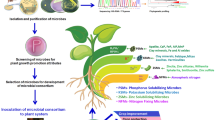
The microbes recruited into the plant endosphere and those inhabiting the external root environment contribute to plant growth in diverse ways, as shown in Fig. 1. Reports by Ku et al. [93] showed root surface and hair colonization by an endophytic bacterium, B. cereus, in Chinese cabbage, soybean, and wheat, with evidence in understanding the mode of actions of plant microbes and how they influence plant growth. Aside from endosphere and rhizosphere research findings, fewer studies have documented the microbiome inhabiting the antosphere, caulosphere, carposhere, and spermosphere. Research into microbiome in the plant environments, such as rhizosphere, root, seed, and stem, have been documented, and their possible use in agricultural biotechnology is profound.
For instance, Kumawat et al. [94] reported an increase in the growth, symbiotic efficacy, nutrient acquisition, and yield of soybean co-inoculated with endophytic Pseudomonas oryzihabitans and Bradyrhizobium spp. Also, an increase in the crop yield, oil content, antioxidant content, seed quality, carbohydrates, and chemical composition (protein and lipid) of soybean inoculated with endophytic Bacillus amyloliquefaciens has been reported by Sheteiwy et al. [23], which suggests their future exploration as bioinoculants in growing soybean under drought stress. Because plants harbor diverse number of microorganisms, the better understanding of their complexity and functional traits will help unraveled their biological activities.
Rhizosphere and Bulk Soil Microbiome
The rhizosphere (plant microhabitats) represents soil regions closer to the plant root environment [95]. The rhizosphere is often referred to as a “hotspot” for microbial activities due to the excess release of root exudates, which supply the required energy for microbial metabolic activities [96]. The response of soil microbes to the diverse chemical compounds and varied soil parameters, which favors soil microflora, can be an indicator for selecting them over others. The shaping of the rhizosphere microbiome can be a function of the quantity of exudate released from one plant to another. Some examples of secondary metabolite organic compounds include amino acids, phenols, organic acids, sugars, siderophores, polysaccharides, etc. When released from plant roots, it influences a higher microbial population in the rhizosphere than in bulk soil [97].
Bulk soil is the soil that is equidistance away from the rhizosphere region without root penetration [98]. The microbial inhabitant in the bulk soil can be less in diversity due to the fewer organic compounds than the rhizosphere soil inhabitants with identical species. Some examples of bacteria occupying the rhizosphere root environment include Bradyrhizobium diazoefficiens, Bacillus subtilis, B. velezensis, etc. [99,100,101]. High microbial colonization, diversity, and activities are easily mediated by rhizodeposition, reducing compared to the adjacent or bulk soil [102].
Usually, the variations in the rhizosphere microbial communities in soybean and other food crops can be linked to the geographical locations, growing seasons, crop rotation, plant growth stages, cultivars, farm practices, etc. The identification of diverse bacterial phyla, such as Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Gemmatimonadetes, and Proteobacteria from rhizosphere soils under the different growing conditions and soil types, has been reported with greater influence on the bacterial diversity [103, 104]. Hence, there is a need for further research to ascertain if the microbes present in soybean across multiple locations are different, since there is less information of the soybean rhizosphere microbial communities.
Due to the nodule formation in the root of soybean, the endosymbiotic relationship with nitrogen-fixing bacteria can be an advantage in releasing excess quality, and quantity of root exudate different from non-nodulating plants to help establish discreet microbial biomass in the rhizosphere [18]. High-throughput sequencing in determining the rhizosphere bacterial and fungal communities of rapeseed have revealed varied operational taxonomic units at seedling, flowering, and maturity stages [105]. The assessment of a bacterial community in rapeseed using a molecular ecology network with random matrix theory showed bacteria genera, such as Rhizobium, Flavobacterium, and Pseudomonas, at the network level [106].
Interestingly, research on rhizosphere microbiome with the view of mapping out strategies for their incorporation into agriculture has been emphasized in recent times [107,108,109]. Nevertheless, the presence of pathogens may influence rhizosphere microbes in many ecological processes. Furthermore, the source of rhizosphere microbes is important as most of them may be introduced into the soil through seed planting [110].
Seed Microbiome
Aside from the rhizosphere microbiome [111], research advancements have shown microbial composition on the surface and internal tissue of seeds [76, 112] can be beneficial or pathogenic. The beneficial microbes influence seed growth at pre-germination, germination, flowering, and maturation stages [113]. The recruitment of microbiome in seeds can be achieved by vertical (from the mother plant) or horizontal (environment) transmission [114]. Vertical transmission of seed endophytes is believed to originate from the leaves and flowering parts. Upon planting into the soil, the seed undergoes imbibition, which enables them to absorb soil nutrients and then germinate. During the imbibition process, the release of metabolic compounds in the spermosphere region, i.e., soil-seed environment, creates an attractive environment for the soil microbes to compete with the natural soil pathogens [115]. At this stage, the seed microbiome infiltrate or release to the soil environment via horizontal transfer.
Seed endophytic bacteria and their mode of transmission enabled them to occupy diverse niches, such as the pericarp, seed embryo or cotyledon, and endosperm [116]. The transmission of seed endophytes may differ depending on the organ location. For instance, endophytic in the pericarp are horizontally transmitted, while those colonizing the endosperm and embryo are easily transmitted by vertical processes [117]. More research should be done on soybean to understand how endophytes are transferred in their root region.
Microscopy and high throughput sequencing approaches have been employed to characterize seed microbiome, especially endophytic bacteria in some leguminous plants [118]. Sánchez-López et al. [119] reported dominant endophytic bacteria phyla, Proteobacteria, Firmicutes, Chlamydiales, and Bacteroidetes while investigating endophytic bacteria in the seed of Low Rattlebox. Information on the microbial community structure of endophytic bacteria in the seed of soybean and other leguminous food crops using high-throughput sequencing are scanty in literature. Consequently, differentiating seed endophyte and soil microbiome are still less understood. Also, seed endophytes can be found in other plant parts via infiltration from the rhizosphere to the above ground level. Interestingly, the synergistic cooperation between the soil microbes and seed endophytes has contributed to plant health and nutrition [120].
Root and Shoot Microbiome
The root and shoot form a key component in the study of the plant microbiome [71]. The microbes found in the root and shoot can be less in number compared to the higher microbial profiling in the rhizosphere due to the nutrients and exudate secretion attributes [96, 121]. The detection of genes involved in bacteria attachment due to specialized cell organelles, such as fimbriae, flagella, and pili in the plant surface, assisted bacteria adhesion to the cell surfaces to form a biofilm [122]. The plant-bacteria interaction and transient within the plant tissue can result due to a rise in water flux during transpiratory processes in plants. Across the plant parts, the presence of the targeted microorganisms may be influenced by the organ location and accessibility to plant nutrients [123].
For microbes to efficiently colonize the host plant, the line of mode of actions involved includes (i) adherence to the root surface, (ii) multiplication, (iii) invasion from the external root environment, and (iv) colonization [124]. After the colonization process, the movement of endophytic microbe from the belowground to the shoot through microbial networking can be achieved. The type and quantity of nutrients available in the plant endosphere can modulate the extent of bacteria diversity. Adeleke et al. [61] and Jie et al. [125] reported diverse endophytic bacteria phyla Chloroflexi, Nitrospirae, Planctomycetes, Palescibacteria, Acidobacteria, Actinobacteria, Cyanobacteria, Saccharibacteria, Firmicutes, Gemmatimonadetes, Bacteroidetes, and Proteobacteria from the root of sunflower and soybean. Recently, endophytic bacteria genera Bacillus, Staphylococcus, Serratia, Stenotrophomonas, Pseudomonas, Enterobacteria, and Erwinia from healthy rapeseed has been reported as part of the shoot microbiome [25]. Endophytic bacteria in the external root environment are usually higher compared to the internal part of roots. In the findings by Adeleke et al. [61], the authors reported a dominant and high bacteria population in the root of growing sunflower compared to the stem due to the agricultural practices, geographical locations, plant type, organ location, etc., which contribute to the bacterial diversity.
The reason for microbial differences in the rhizosphere, endosphere, and phyllosphere can be biological, chemical, or physical factors, which may exert selective pressure on endophytic bacteria to infiltrate the root endosphere [126]. The endophytic microbiome tends to adjust to a plant environment with stable biomass, while the rhizosphere microbiome may vary due to niche complexity. Acknowledging the fact that plants harbor a multifunctional microbiome in the root and shoot can be a pointer to understanding factors that modulate the shape of the microbiome in plants.
Plant Growth Stimulation Attributes of Endophytic Bacteria
Beneficial plant microbiome helps in sustaining the ecosystem [100]. The ecological services range from plant growth promotion, pathogen control, phytoremediation, biofertilization, and abiotic stress mitigation to human safety [86, 127]. In recent times, the multifunctional attributes of endophytic microbes as plant growth stimulators and bioinoculants promise to revolutionize agriculture without negative ecological effects [128]. Also, the role of endophytic microbes in agricultural biotechnology has been focused on; yet, research is still ongoing to meet zero ecological threats for maximum food production [129]. Exploration of endophytic resources to provide alternative measures in ensuring a safe environment and sustainable agricultural productivity have been emphasized due to the negative impact of chemical fertilizers on the ecosystem [130].
From the multifaceted application perspective, the mechanisms employed by endophytic microbes immensely contribute to plant growth and health [22]. Microbes employ direct or indirect mechanisms in sustaining plant growth and health [131]. The core attributes of endophytic microbes in enhancing plant growth include nutrient acquisition and mineralization, phosphate solubilization, nitrogen fixation, siderophore and enzyme production, and synthesis of growth hormones, such as indole-3-acetic acid, gibberellic acid, and abscisic acid, while indirectly, ACC deaminase, exopolysaccharide, and hydrogen cyanide production by endophytic microbes contribute to plants survival under drought stress [132]. All the aforementioned processes, specifically, have been screened from endophytic microbes associated with sunflower and soybean [15, 40]. In addition, the suppression of phytopathogens through the induction of systemic resistance and antibiosis activities of endophytic microbes boost plant immunity against soil and host invading pathogens [133]. Also, findings by Zhao, Xu, and Lai [9] reported high inhibitory activity of soybean nodule endophytic bacterium Acinetobacter calcoaceticus against pathogenic fungus Phytophthora sojae due to their close association with the root of the plants.
Endophytic microbes are said to deliver effectively in enhancing plant growth due to their close interaction, colonization, less composition in plants, and non-exposure to harsh environmental conditions [134]. These attributes make endophytic studies interesting compared to the rhizosphere microbes. The synergistic effect of nodule endophytic bacteria, Pseudomonas aeruginosa (LSE-2) and Bradyrhizobium sp. (LSBR-3) from soybean, has been investigated as a source of bioinoculants and biofertilizers due to their root colonization potential through molecular crosstalk, which supports plant growth and nutrition [135].
Some endophytic microbes solubilize phosphate in natural form by producing organic acids, which lower soil pH and chelate iron for easy phosphate assimilation by plants in soluble form [136]. The ability of endophytic bacteria to produce phosphatases also helps in the mineralization of organic phosphorus [137]. In vitro screening of phosphate-solubilizing endophytic bacteria has been investigated from soybean, sunflower, and rapeseed [138,139,140]. For example, Acinetobacter calcoaceticus, Ochrobactrum haematophilum, B. panacihum, Bacillus subtilis, B. australimaris, B. thuringiensis, B. zhangzhouensis, and Lysinibacillus pakistanensis have been isolated from leguminous crops [9, 18, 30]. Kenasa, Nandeshwar, and Assefa [141] reported the identification of cowpea root endophytic bacteria, Pseudomonas putida, and Bacillus subtilis phosphate producers in their study. Also, a study by Yasmeen and Bano [142] reported an increase in soybean yield co-inoculated with phosphate-solubilizing bacteria, Rhizobium and Enterobacter.
The rhizobacteria in the root nodule of leguminous plants naturally fix nitrogen in the soil, which is needed for plant nutrition [18]. The nitrogen fixation potential of endophytic bacteria in the root nodules of leguminous crops, effectively, has enhanced the nitrogen pool in soil deficient in nitrogen supply [143]. The nitrogen fixation by endophytic bacteria may differ compared to rhizobacteria found in the root of legumes [144, 145]. Interestingly, exploration of the endophytic bacterium Gluconacetobacter diazotrophicus with exceptional nitrogen fixation in plants has long been reported in reclaiming nitrogen loss in the soil [146].
The ability of endophytic bacteria to produce siderophores also plays a major role in plant health sustainability [147]. For instance, biocontrol activity which limits iron supply to the pathogens, heavy metal reduction, and induction of systemic resistance can be linked to the siderophore compounds, i.e., catecholate and hydroxamate, produced by endophytic bacteria [148]. Diverse endophytic bacteria associated with soybean have been reported as siderophore producers [15]. Bhutani et al. [18] and Maheshwari et al. [149] reported siderophore-producing endophytic bacteria strains from legumes. The suppressive and biocontrol activity of endophytic Burkholderia contaminans against Macrophomina phaseolina causing root rot, stem rot, seedling blight, damping off, and charcoal rot in jute due to siderophore biosynthesis has been reported [150]. Since the presence of nitrogen-fixing and siderophore-producing bacteria has been established in soybean, other functions of these bacteria should be further studied.
Similarly, phytohormones, such as ethylene, IAA, cytokinins, and gibberellin, modulating plant growth via diverse pathways are evident in endophytic microbes [9]. Notably, IAA biosynthesis facilitates root development, which enables plants to absorb nutrients and water from the soil [151]. Tryptophan, which serves as a precursor for IAA production by endophytic microbes in a growth media, helps differentiate IAA-producing bacteria from non-IAA-producing bacteria [152]. Evidence of IAA and other phytohormones, such as gibberellin, and cytokinin production by endophytic bacteria to enhance plant growth, have been documented [63, 153]. Some endophytic bacteria, which produce 1-aminocyclopropane-1-carboxylate (ACC), a precursor for ethylene production, contribute to plant growth and are resilient to drought stress [154]. The ability of endophytic bacteria to circumvent the effect of pathogens by producing jasmonic acid, antibiotics, salicylic acid, volatile compounds, siderophore, and lipopolysaccharide, elicit induced systemic resistance, and abiotic stress amelioration in the host plants [155].
The actual mode of actions employed by endophytic bacteria in oilseed crop soybean is yet to be fully understood. Similarly, the biosynthesis and metabolism of reacting molecules as precursors for the synthesis of novel metabolites or enhancing already identified metabolites are poorly understood. The synthesis of secondary metabolites, such as alkaloids, terpenoids, phenols, organic acids, and flavonoids, which induce antibiosis, can be achieved by endophytic microbes specific to the host plants [156]. Some examples of purified secondary metabolites produced by endophytic bacteria from some economic plants with related biological functions are presented in Table 4. Information relating to secondary metabolites sourced from endophytic bacteria associated with soybean is less documented in the literature. Hence, research focusing on secondary metabolites from endophytic bacteria associated with soybean and their exploration will further reveal their bioprospecting in plant disease management.
The biomolecules sourced from endophytic bacteria stand promising in agriculture, environment, industry, and human safety. Hence, genomic insights into plant microbiome aim to reveal their functions and activity in plant physiology and metabolism. Additionally, it is imperative to unravel soybean-associated endophytic bacteria’s biological functions and physiological attributes, using culture-dependent and culture-independent techniques to identify secondary metabolites in the bacteria genome [24]; making information available on secondary metabolites produced by endophytic bacteria will help find a solution to diverse agricultural problems.
Current Status of Plant Microbiome Collaborative Research
The interdisciplinary synergies among researchers in studying plant–microbe interactions continue to progress. Research efforts to study and explore endophytic bacteria from the leguminous crop as bioinoculants for plant growth and sustainable ecosystem have increased tremendously with driven biotechnological advances and low-cost analysis [124]. Interestingly, the commercialization of endophytic bioinoculants is possible in sustainable agriculture [128]. The computational knowledge about next-generation sequencing and other innovative techniques have informed scientists with accurate information on microbial diversity and related genes [109].
Better still, there is a need to develop robust bioinformatics tools and analytical techniques with the existing technologies to generate microbiome data as a guide for further experiments. Adeleke et al. [40] and Adeleke et al. [157] reported the genomic characterization of plant growth-promoting endophytic bacteria, Bacillus cereus T4S and Stenotrophomonas maltophilia JVB5 as copious sunflower growth enhancers. Furthermore, effort on the use of these endophytic bacteria as biocontrol agent against phytopathogens is expected to be investigated in the future studies. Employing this approach by ecologists, environmental and computational scientists, microbiologists, agriculturists, and industrialists aim to provide insights into plant microbiome research as a reference for further studies. Furthermore, understanding the dynamics and role of endophytic microbes in plants using up-to-date techniques and bioinformatics tools, however, can help develop multiple strategies in understanding their functions in diverse fields, such as agriculture, ecology, medicine, forensics, and exobiology.
The dominant bacteria phyla, Actinobacteria, Firmicutes, Proteobacteria, Bacteroidetes, and Chloroflexi in the root endosphere of food crops, such as maize, cowpea, sorghum, sunflower, soybean, have been reported using culture-independent techniques [61, 158, 159]. Yet, there is a need to investigate further using appropriate techniques to access plant growth-promoting endophytic bacteria in different legumes and other food crops under different climatic conditions. Hence, identifying these bacteria for bioinoculants formulation can serve as a pointer to achieving ecofriendly agriculture sustainably.
Conclusions
This review evaluates endophytic bacteria in soybean and other food crops. The bioprospecting of these bacteria enhances their potential for sustainable yield enhancement. Soybean was discussed as a reference crop for oilseed crops due to its economic importance, high yield, and nutritional value. Soybean harbors some endophytic microbes important in agriculture. Beneficial endophytic microbes inhabiting different parts of the plants can potentially contribute to the growth of soybeans and other food crops. For instance, root nodule bacteria and endophytic bacteria enhanced nitrogen fixation in soybean, which promotes their yield and other yield parameters, enhance immunity, and boost plant defense against diseases. However, the root endophytes are emphasized because of high metabolic activities occurring below ground level due to the high quantity of metabolite secretion, which contributes to plant physiological functions.
Different conventional and molecular techniques have been employed in the past to unravel endophytic microbes in some plants; nevertheless, each method comes with shortcomings. For instance, some endophytes can be difficult to culture on media despite their viability, such that culturing method can only unravel a lesser percentage (i.e., 0.1%) of endophytic populations. Hence, the advancement of endophytic microbes discovery using molecular techniques has proven more promising, although, with diverse challenges. Extracted endophytic bacteria DNA might contain traces of plant DNA, the chloroplast, and mitochondria DNA, which are identical to the targeted endophytic bacteria DNA [160]. Host depletion techniques have been employed to remove a substantial amount of plant DNA that might be present in the DNA extracted from the plant tissues. Conversely, the use of fluorescence in situ hybridization (FISH) is inefficient, because it can only be carried out in a natural habitat.
Oilseed crop soybean is economically important due to their high yield, and nutritional value. The mechanism employed by the endophytes present in the seed, shoot, leaves, roots, and other microbes inhabiting the rhizosphere, bulk soil in plant growth promotion, and disease control still needs to be emphasized, although some research information are available on them. The variation in the diversity and population of microbes inhabiting different plant parts can be due to difference in the geographical locations, cropping system, developmental stage of the plants and the farming practices adopted. These key factors may affect the crop yield, microbial diversity and their ability to produce secondary metabolites. It is therefore very important to understand the mechanisms behind the production of secondary metabolites in soybean as a measure to improve their production, oil content, antioxidant content, seed quality, carbohydrates, chemical composition, and yield in different environments and also as a model to the research of other crops. More research should also be carried out to help understand the use of endophytes in the agriculture, industry, and medical industries, owing to the production of bioproducts. Better still, there is a need to develop robust bioinformatics tools and analytical techniques with the existing technologies to generate microbiome data as a guide for further experiments. Employing this approach by ecologists, environmental and computational scientists, microbiologists, agriculturists, and industrialists aims to provide insights into plant microbiome research as a reference for further studies. Hence, the authors conclude and recommend that the current approaches highlighted in this review will be of help to researchers in understanding the dynamics, prospect, and potential of endophytic microbes in soybeans and other food crops as agricultural bio-input to ensure food security and sustainable agriculture.
Change history
09 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00248-022-02141-2
References
Zhou Y, Zhao W, Lai Y, Zhang B, Zhang D (2020) Edible plant oil: global status, health issues, and perspectives. Front Plant Sci 11:1315
Page KL, Dang YP, Martinez C, Dalal RC, Wehr JB, Kopittke PM, Orton TG, Menzies NW (2021) Review of crop-specific tolerance limits to acidity, salinity, and sodicity for seventeen cereal, pulse, and oilseed crops common to rainfed subtropical cropping systems. Land Degrad Develop 32:2459–2480
Raipuria RK, Watts A, Meena NL, Watts A (2021) Genome editing in oilseed crops. Genome Editing in Plants, 1st Edition. CRC Press. Page 109–126
Bagnall DK, Shanahan JF, Flanders A, Morgan CL, Honeycutt CW (2021) Soil health considerations for global food security. Agron J 113:4581–4589
Paul AA, Kumar S, Kumar V, Sharma R (2020) Milk Analog: plant based alternatives to conventional milk, production, potential and health concerns. Crit Rev Food Sci Nutr 60:3005–3023
Velten S, Neumann C, Bleyer M, Gruber-Dujardin E, Hanuszewska M, Przybylska-Gornowicz B, Liebert F (2018) Effects of 50 percent substitution of soybean meal by alternative proteins from Hermetia illucens or Spirulina platensis in meat-type chicken diets with graded amino acid supply. Open J Animal Sci 8:119
Khojely DM, Ibrahim SE, Sapey E, Han T (2018) History, current status, and prospects of soybean production and research in sub-Saharan Africa. The Crop Journal 6:226–235
Siamabele B (2021) The significance of soybean production in the face of changing climates in Africa. Cogent Food Agric 7:1933745
Zhao L, Xu Y, Lai X (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol 49:269–278
Sallam N, Ali EF, Seleim MA, Bagy HMK (2021) Endophytic fungi associated with soybean plants and their antagonistic activity against Rhizoctonia solani. Egypt J Biol Pest Control 31:54
Amrate PK, Shrivastava M, Pancheshwar DK, Sharma S (2020) Charcoal rot and yellow mosaic virus diseases of soybean under hot spot condition: symptoms, incidence and resistance characterization. Int J Biores Stress Manag 11:268–273
Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SE-D (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10:935
Akanmu AO, Babalola OO, Venturi V, Ayilara MS, Adeleke BS, Amoo AE, Sobowale AA, Fadiji AE, Glick BR (2021) Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front Plant Sci 12:1590
Jordaan E, van der Waals JE, McLaren NW (2019) Effect of irrigation on charcoal rot severity, yield loss and colonization of soybean and sunflower. Crop Prot 122:63–69
Dubey A, Saiyam D, Kumar A, Hashem A, Abd_Allah EF, Khan ML (2021) Bacterial root endophytes: characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int J Environ Res Public Health 18:931
Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Etesami H (2022) Root nodules of legumes: a suitable ecological niche for isolating non-rhizobial bacteria with biotechnological potential in agriculture. Curr Res Biotechnol 4:78–86
Bhutani N, Maheshwari R, Kumar P, Suneja P (2021) Bioprospecting of endophytic bacteria from nodules and roots of Vigna radiata, Vigna unguiculata and Cajanus cajan for their potential use as bioinoculants. Plant Gene 28:100326
Mashiane AR, Adeleke RA, Bezuidenhout CC, Chirima GJ (2018) Community composition and functions of endophytic bacteria of Bt maize. South Afr J Sci 114:88–97
Zhao J, Wang S, Zhu X, Wang Y, Liu X, Duan Y, Fan H, Chen L (2021) Isolation and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb 1997 and Serratia plymuthica Sneb2001 for the biological control of root-knot nematode. Appl Soil Ecol 164:103924
Bashir S, Iqbal A, Hasnain S, White JF (2021) Screening of sunflower associated bacteria as biocontrol agents for plant growth promotion. Arch Microbiol 203:4901–4912
Adeleke BS, Babalola OO (2022) Meta-omics of endophytic microbes in agricultural biotechnology. Biocatal Agric Biotechnol 42:102332
Sheteiwy MS, AbdElgawad H, Xiong YC, Macovei A, Brestic M, Skalicky M, Shaghaleh H, Hamoud YA, El-Sawah AM (2021) Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol Plantarum 172:2153–2169
Wu W, Chen W, Liu S, Wu J, Zhu Y, Qin L, Zhu B (2021) Beneficial relationships between endophytic bacteria and medicinal plants. Front Plant Sci 12:758
Schmidt C, Mrnka L, Lovecká P, Frantík T, Fenclová M, Demnerová K, Vosátka M (2021) Bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential for biocontrol of Sclerotinia and Phoma disease. Sci Rep 11:3810
Cui W, He P, Munir S, He P, Li X, Li Y, Wu J, Wu Y, Yang L, He P, He Y (2019) Efficacy of plant growth promoting bacteria Bacillus amyloliquefaciens B9601–Y2 for biocontrol of southern corn leaf blight. Biol Control 139:104080
Bashir I, War AF, Rafiq I, Reshi ZA, Rashid I, Shouche YS (2022) Phyllosphere microbiome: diversity and functions. Microbiol Res 254:126888
Saber M, Andreote F, Kavamura V, Frighetto R, Taketani R, de MELO I (2014) Effect of ultraviolet-B (UV-B) radiation on bacterial community in the soybean phyllosphere. Afr J Microbiol Res 8:2916–2923
Zheng Y, Xu Z, Liu H, Liu Y, Zhou Y, Meng C, Ma S, Xie Z, Li Y, Zhang C-S (2021) Patterns in the microbial community of salt-tolerant plants and the functional genes associated with Salt stress alleviation. Microbiol Spectrum 9:767
Mahgoub HA, Fouda A, Eid AM, Ewais EE-D, Hassan SE-D (2021) Biotechnological application of plant growth-promoting endophytic bacteria isolated from halophytic plants to ameliorate salinity tolerance of Vicia faba L. Plant Biotechnol Reports 15:819–843
Hereira-Pacheco SE, Navarro-Noya YE, Dendooven L (2021) The root endophytic bacterial community of Ricinus communis L. resembles the seeds community more than the rhizosphere bacteria independent of soil water content. Sci Rep 11:2173
Sugiyama A (2019) The soybean rhizosphere: metabolites, microbes, and beyond - a review. J Adv Res 19:67–73
Mina D, Pereira JA, Lino-Neto T, Baptista P (2020) Epiphytic and endophytic bacteria on olive tree phyllosphere: exploring tissue and cultivar effect. Microbial Ecol 80:145–157
de Almeida LK, Carpentieri-Pipolo V, Oro T, Stefani Pagliosa E, Degrassi G (2016) Culturable endophytic bacterial communities associated with field-grown soybean. J Appl Microbiol 120:740–755
Hwang H-H, Chien P-R, Huang F-C, Hung S-H, Kuo C-H, Deng W-L, Chiang E-PI, Huang C-C (2021) A plant endophytic bacterium, Burkholderia seminalis strain 869T2, promotes plant growth in Arabidopsis, Pak Choi, Chinese Amaranth, Lettuces, and other vegetables. Microorganisms 9:1703
Brunda K, Jahagirdar S, Kambrekar D (2018) Antagonistic activity of bacterial endophytes against major soil borne pathogens of soybean. J Entomol Zool Stud 6:43–46
Zhang C, Ma X, Zhu R, Liu Z, Gu M, Zhang J, Li Y, Xu Y, Zhu D (2020) Analysis of the endophytic bacteria community structure and function of Panax notoginseng based on high-throughput sequencing. Curr Microbiol 77:2745–2750
Tangapo AM, Astuti DI, Aditiawati P (2018) Dynamics and diversity of cultivable rhizospheric and endophytic bacteria during the growth stages of cilembu sweet potato (Ipomoea batatas L. var. cilembu). Agric Nat Res 52:309–316
Chlebek D, Pinski A, Żur J, Michalska J, Hupert-Kocurek K (2020) Genome Mining and Evaluation of the biocontrol potential of Pseudomonas fluorescens BRZ63, a new endophyte of oilseed rape (Brassica napus L.) against fungal pathogens. Int J Mol Sci 21:8740
Adeleke BS, Ayangbenro AS, Babalola OO (2021) Genomic analysis of endophytic Bacillus cereus T4S and its plant growth-promoting traits. Plants 10:1776
Lareen A, Burton F, Schäfer P (2016) Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol 90:575–587
Feng N-X, Yu J, Zhao H-M, Cheng Y-T, Mo C-H, Cai Q-Y, Li Y-W, Li H, Wong M-H (2017) Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci Total Environ 583:352–368
Shah S, Shrestha R, Maharjan S, Selosse M-A, Pant B (2019) Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants 8:5
Adeleke BS, Babalola OO (2021) Biotechnological overview of agriculturally important endophytic fungi. Hortic Environ Biotechnols 62:507–520
Ripa FA, Cao W-d, Tong S, Sun J-g (2019) Assessment of plant growth promoting and abiotic stress tolerance properties of wheat endophytic fungi. BioMed Res Int 2019:6105865
Kuźniar A, Włodarczyk K, Wolińska A (2019) Agricultural and other biotechnological applications resulting from trophic plant-endophyte interactions. Agron 9:779
Adeleke BS, Babalola OO (2021) The plant endosphere-hidden treasures: a review of fungal endophytes. Biotechnol Genet Eng Rev 37:154–177
El-Bialy HA, El-Bastawisy HS (2020) Elicitors stimulate paclitaxel production by endophytic fungi isolated from ecologically altered Taxus baccata. J Radiation Res Appl Sci 13:79–87
Wu Y-Y, Zhang T-Y, Zhang M-Y, Cheng J, Zhang Y-X (2018) An endophytic fungi of Ginkgo biloba L. produces antimicrobial metabolites as potential inhibitors of FtsZ of Staphylococcus aureus. Fitoterapia 128:265–271
Hou X, Guo S (2014) Screening and identification of endophytic fungi with growth promoting effect on Dendrobium officinale. China J Chin Mater Med 39:3232–3237
Zhang Q, Zhang J, Yang L, Zhang L, Jiang D, Chen W, Li G (2014) Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biol Control 72:98–108
Yu J, Wu Y, He Z, Li M, Zhu K, Gao B (2018) Diversity and antifungal activity of endophytic fungi associated with Camellia oleifera. Mycobiol 46:85–91
Kumar S, Kaushik N (2013) Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS ONE 8:e56202
da Costa Stuart AK, Stuart RM, Pimentel IC (2018) Effect of agrochemicals on endophytic fungi community associated with crops of organic and conventional soybean (Glycine max L. Merril). Agric Natural Res 52:388–392
Dos Santos IR, Abdel-Azeem AM, Mohesien MT, Piekutowska M, Sheir DH, da Silva LL, da Silva CC, Carvalho DDC, Bezerra JDP, Saad HA (2021) Insights into the bioprospecting of the endophytic fungi of the medicinal plant Palicourea rigida Kunth (Rubiaceae): Detailed biological activities. J Fungi 7:689
Xiao J-l, Sun J-G, Pang B, Zhou X, Gong Y, Jiang L, Zhang L, Ding X, Yin J (2021) Isolation and screening of stress-resistant endophytic fungus strains from wild and cultivated soybeans in cold region of China. Appl Microbiol Biotechnol 105:755–768
Ancheeva E, Daletos G, Proksch P (2020) Bioactive secondary metabolites from endophytic fungi. Curr Med Chem 27:1836–1854
Skiada V, Faccio A, Kavroulakis N, Genre A, Bonfante P, Papadopoulou KK (2019) Colonization of legumes by an endophytic Fusarium solani strain FsK reveals common features to symbionts or pathogens. Fungal Genet Biol 127:60–74
Munshi M, Sohrab M, Begum M, Rony SR, Karim M, Afroz F, Hasan M (2021) Evaluation of bioactivity and phytochemical screening of endophytic fungi isolated from Ceriops decandra (Griff.) W. Theob, a mangrove plant in Bangladesh. Clin Phytosci 7:81
Thomas P, Swarna GK, Roy PK, Patil P (2008) Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tissue Organ Culture 93:55–63
Adeleke BS, Ayangbenro AS, Babalola OO (2021) Bacterial community structure of the sunflower (Helianthus annuus) endosphere. Plant Signaling Behav 16:1974217
Bolivar-Anillo HJ, González-Rodríguez VE, Cantoral JM, García-Sánchez D, Collado IG, Garrido C (2021) Endophytic bacteria Bacillus subtilis, isolated from Zea mays, as potential biocontrol agent against Botrytis cinerea. Biol 10:492
Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res 221:36–49
Alain K, Querellou J (2009) Cultivating the uncultured: limits, advances and future challenges. Extremophiles 13:583–594
Torsvik V, Ovresas L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5:240–245
Garcias-Bonet N, Arrieta JM, de Santana CN, Duarte CM, Marbà N (2012) Endophytic bacterial community of a Mediterranean marine angiosperm (Posidonia oceanica). Front Microbiol 3:342
Piccolo SL, Ferraro V, Alfonzo A, Settanni L, Ercolini D, Burruano S, Moschetti G (2010) Presence of endophytic bacteria in Vitis vinifera leaves as detected by fluorescence in situ hybridization. Ann Microbiol 60:161–167
Ikeda S, Kaneko T, Okubo T, Rallos LE, Eda S, Mitsui H, Sato S, Nakamura Y, Tabata S, Minamisawa K (2009) Development of a bacterial cell enrichment method and its application to the community analysis in soybean stems. Microbial Ecol 58:703–714
Lundberg DS, Yourstone S, Mieczkowski P, Jones CD, Dangl JL (2013) Practical innovations for high-throughput amplicon sequencing. Nat Methods 10:999–1002
Kaul S, Sharma T, Dhar K, M, (2016) “Omics” tools for better understanding the plant–endophyte interactions. Front Plant Sci 7:955
Adeleke BS, Babalola OO, Glick BR (2021) Plant growth-promoting root-colonizing bacterial endophytes. Rhizosph 20:100433
Dos Santos LF, Olivares FL (2021) Plant microbiome structure and benefits for sustainable agriculture. Curr Plant Biol 26:100198
Hartman K, van der Heijden MG, Roussely-Provent V, Walser J-C, Schlaeppi K (2017) Deciphering composition and function of the root microbiome of a legume plant. Microbiome 5:2
Eldridge DJ, Travers SK, Val J, Ding J, Wang JT, Singh BK, Delgado-Baquerizo M (2021) Experimental evidence of strong relationships between soil microbial communities and plant germination. J Ecol 109:2488–2498
Mishra VK, Passari AK, Leo VV, Singh BP (2017) Molecular diversity and detection of endophytic fungi based on their antimicrobial biosynthetic genes. In: Singh BP, Gupta VK (eds) Molecular markers in mycology. Fungal biology. Springer, Cham, pp 1–35. https://doi.org/10.1007/978-3-319-34106-4_1
Mitter B, Pfaffenbichler N, Flavell R, Compant S, Antonielli L, Petric A, Berninger T, Naveed M, Sheibani-Tezerji R, von Maltzahn G (2017) A new approach to modify plant microbiomes and traits by introducing beneficial bacteria at flowering into progeny seeds. Front Microbiol 8:11
Bodenhausen N, Bortfeld-Miller M, Ackermann M, Vorholt JA (2014) A synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLoS Genet 10:1004283
Dubey RK, Tripathi V, Prabha R, Chaurasia R, Singh DP, Rao CS, El-Keblawy A, Abhilash PC (2020) Methods for exploring soil microbial diversity. In: Dubey RK, Tripathi V, Prabha R, Chaurasia R, Singh DP, Rao CS, El-Keblawy A, Abhilash PC (eds) Unravelling the soil microbiome: perspectives for environmental sustainability. Springer International Publishing, pp 23–32. https://doi.org/10.1007/978-3-030-15516-2_3
İnceoğlu Ö, Salles JF, van Overbeek L, van Elsas JD (2010) Effects of plant genotype and growth stage on the betaproteobacterial communities associated with different potato cultivars in two fields. Appl Environ Microbiol 76:3675–3684
Hayatsu M, Tago K, Saito M (2008) Various players in the nitrogen cycle: diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci Plant Nutr 54:33–45
Qiu P, Feng Z-X, Tian J-W, Lei Z-C, Wang L, Zeng Z-G, Chu Y-W, Tian Y-Q (2015) Diversity, bioactivities, and metabolic potentials of endophytic actinomycetes isolated from traditional medicinal plants in Sichuan, China. Chin J Nat Med 13:942–953
Allan E (2014) Metagenomics: unrestricted access to microbial communities. Virulence 5:397–398
Shahzad R, Khan AL, Bilal S, Waqas M, Kang S-M, Lee I-J (2017) Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ Exp Bot 136:68–77
Pei C, Mi C, Sun L, Liu W, Li O, Hu X (2017) Diversity of endophytic bacteria of Dendrobium officinale based on culture-dependent and culture-independent methods. Biotechnol Biotechnolog Equip 31:112–119
Gupta R, Anand G, Gaur R, Yadav D (2021) Plant–microbiome interactions for sustainable agriculture: a review. Physiol Mol Biol Plants 27:165–179
Aswani R, Thomas R, Radhakrishnan EK (2022) Induction of plant defense response by endophytic microorganisms. In: Radhakrishnan EK, Ajay K, Aswani R (eds) Biocontrol mechanisms of endophytic microorganisms. Academic Press, Cambridge, pp 89–115. https://doi.org/10.1016/B978-0-323-88478-5.00002-X
Samreen T, Naveed M, Nazir MZ, Asghar HN, Khan MI, Zahir ZA, Kanwal S, Jeevan B, Sharma D, Meena VS (2021) Seed associated bacterial and fungal endophytes: diversity, life cycle, transmission, and application potential. Appl Soil Ecol 168:104191
Gaeth VA, Domondon CJ, Podbielski PA, Aswad VX, Wrightstone EA, Wong NH, Burke WH, Melita J, Murray KM, Hudson AO (2021) Whole-genome sequencing and annotation of 10 endophytic and epiphytic bacteria isolated from Lolium arundinaceum. Microbiol Res Announc 10:e00317-00321
Samaras A, Nikolaidis M, Antequera-Gómez ML, Cámara-Almirón J, Romero D, Moschakis T, Amoutzias GD, Karaoglanidis GS (2020) Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI 600 on cucumber. Front Microbiol 11:600393
Adeleke BS, Ayangbenro AS, Babalola OO (2021) Genomic assessment of Stenotrophomonas indicatrix for improved sunflower plant. Curr Genet 67:891–907
Ulrich K, Kube M, Becker R, Schneck V, Ulrich A (2021) Genomic analysis of the endophytic Stenotrophomonas strain 169 reveals features related to plant-growth promotion and stress tolerance. Front Microbiol 12:687463
Kaewkla O, Franco CMM (2021) Amycolatopsis pittospori sp. nov., an endophytic actinobacterium isolated from native apricot tree and genome mining revealed the biosynthesis potential as antibiotic producer and plant growth promoter. Antonie Van Leeuwenhoek 114:365–377
Ku Y, Xu G, Tian X, Xie H, Yang X, Cao C (2018) Root colonization and growth promotion of soybean, wheat and Chinese cabbage by Bacillus cereus YL6. PLoS ONE 13:e0200181
Kumawat KC, Singh I, Napgal S, Sharma P, Gupta RK, Sirari A (2022) Co-inoculation of indigenous Pseudomonas oryzihabitans and Bradyrhizobium sp. modulates the growth, symbiotic efficacy, nutrient acquisition, and grain yield of soybean. Pedosph 32:438–451
Nwachukwu BC, Ayangbenro AS, Babalola OO (2021) Elucidating the rhizosphere associated bacteria for environmental sustainability. Agric 11:75
Babalola OO, Emmanuel OC, Adeleke BS, Odelade KA, Nwachukwu BC, Ayiti OE, Adegboyega TT, Igiehon NO (2021) Rhizosphere microbiome cooperations: strategies for sustainable crop production. Curr Microbiol 78:1069–1085
Adedeji AA, Babalola OO (2020) Secondary metabolites as plant defensive strategy: a large role for small molecules in the near root region. Planta 252:61
Chen L, Brookes PC, Xu J, Zhang J, Zhang C, Zhou X, Luo Y (2016) Structural and functional differentiation of the root-associated bacterial microbiomes of perennial ryegrass. Soil Biol Biochem 98:1–10
Wongdee J, Yuttavanichakul W, Longthonglang A, Teamtisong K, Boonkerd N, Teaumroong N, Tittabutr P (2021) Enhancing the efficiency of soybean inoculant for nodulation under multi-environmental stress conditions. Polish J Microbiol 70:257
Bavaresco LG, Osco LP, Araujo ASF, Mendes LW, Bonifacio A, Araujo FF (2020) Bacillus subtilis can modulate the growth and root architecture in soybean through volatile organic compounds. Theor Exp Plant Physiol 32:99–108
Sibponkrung S, Kondo T, Tanaka K, Tittabutr P, Boonkerd N, Teaumroong N, Yoshida K-i (2017) Genome sequence of Bacillus velezensis S141, a new strain of plant growth-promoting rhizobacterium isolated from soybean rhizosphere. Microbiol Res Announc 5:e01312-01317
Preece C, Penuelas J (2016) Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 409:1–17
Schlatter DC, Hansen JC, Schillinger WF, Sullivan TS, Paulitz TC (2019) Common and unique rhizosphere microbial communities of wheat and canola in a semiarid Mediterranean environment. Appl Soil Ecol 144:170–181
Qiao Q, Wang F, Zhang J, Chen Y, Zhang C, Liu G, Zhang H, Ma C, Zhang J (2017) The variation in the rhizosphere microbiome of cotton with soil type, genotype and developmental stage. Sci Rep 7:3940
Xing M, Zhang Y, Guan C, Guan M (2021) Effects of nitrogen application rate on rhizosphere microbial diversity in oilseed Rape (Brassica napus L.). Agron 11:1539
Liu Y, Gao J, Bai Z, Wu S, Li X, Wang N, Du X, Fan H, Zhuang G, Bohu T (2021) Unraveling mechanisms and impact of microbial recruitment on oilseed rape (Brassica napus L.) and the rhizosphere mediated by plant growth-promoting rhizobacteria. Microorganisms 9:161
Sohn S-I, Ahn J-H, Pandian S, Oh Y-J, Shin E-K, Kang H-J, Cho W-S, Cho Y-S, Shin K-S (2021) Dynamics of bacterial community structure in the rhizosphere and root nodule of soybean: Impacts of growth stages and varieties. Int J Mol Sci 22:5577
Cordero Elvia J, de Freitas RJ, Germida J (2021) Bacterial microbiome associated with the rhizosphere, root interior and aboveground plant organs of wheat and canola at different growth stages. Phytobiomes J 5:442–451
Mavrodi OV, McWilliams JR, Peter JO, Berim A, Hassan KA, Elbourne LD, LeTourneau MK, Gang DR, Paulsen IT, Weller DM (2021) Root exudates alter the expression of diverse metabolic, transport, regulatory, and stress response genes in rhizosphere Pseudomonas. Front Microbiol 12:651282
Soleymani A (2019) Safflower (Carthamus tinctorius L.) seed vigor tests for the prediction of field emergence. Ind Crops Products 131:378–386
Vandana U, Rajkumari J, Singha L, Satish L, Alavilli H, Sudheer P, Chauhan S, Ratnala R, Satturu V, Mazumder P (2021) The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biol 10:101
Rahman MDM, Flory E, Koyro H-W, Abideen Z, Schikora A, Suarez C, Schnell S, Cardinale M (2018) Consistent associations with beneficial bacteria in the seed endosphere of barley (Hordeum vulgare L.). Syst Appl Microbiol 41:386–398
Ghorbanpour M, Hatami M (2014) Biopriming of salvia officinalis seed with growth promoting rhizobacteria affects invigoration and germination indices. J Biol Environ Sci 8:29–36
Omomowo OI, Babalola OO (2019) Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 7:481
Khan AL, Gilani SA, Waqas M, Al-Hosni K, Al-Khiziri S, Kim Y-H, Ali L, Kang S-M, Asaf S, Shahzad R (2017) Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J Zhejiang University-Sci B 18:125–137
Abdelfattah A, Wisniewski M, Schena L, Tack AJ (2021) Experimental evidence of microbial inheritance in plants and transmission routes from seed to phyllosphere and root. Environ Microbiol 23:2199–2214
Lamichhane JR, Debaeke P, Steinberg C, You MP, Barbetti MJ, Aubertot J-N (2018) Abiotic and biotic factors affecting crop seed germination and seedling emergence: a conceptual framework. Plant Soil 432:1–28
Dhole A, Shelat H, Vyas R, Jhala Y, Bhange M (2016) Endophytic occupation of legume root nodules by nifH-positive non-rhizobial bacteria, and their efficacy in the groundnut (Arachis hypogaea). Annals Microbiol 66:1397–1407
Sánchez-López AS, Thijs S, Beckers B, González-Chávez MC, Weyens N, Carrillo-González R, Vangronsveld J (2018) Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 422:51–66
Mukherjee A, Gaurav AK, Patel AK, Singh S, Chouhan GK, Lepcha A, Pereira APdA, Verma JP (2021) Unlocking the potential plant growth-promoting properties of chickpea (Cicer arietinum L.) seed endophytes bio-inoculants for improving soil health and crop production. Land Degradation Develop 32:4362–4374
Kelly C, Haddix M, Byrne P, Cotrufo MF, Schipanski M, Kallenbach C, Wallenstein M, Fonte SJ (2021) Divergent belowground carbon allocation patterns of winter wheat shape rhizosphere microbial communities and nitrogen cycling activities. Soil Biol Biochem 165:108518
Nascimento FX, Hernández AG, Glick BR, Rossi MJ (2020) Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol Rep 25:e00406
Rana KL, Kour D, Kaur T, Sheikh I, Yadav AN, Kumar V, Suman A, Dhaliwal HS (2020) Endophytic microbes from diverse wheat genotypes and their potential biotechnological applications in plant growth promotion and nutrient uptake. Proceed National Acad Sci 90:969–979
Krishnamoorthy A, Gupta A, Sar P, Maiti MK (2021) Metagenomics of two gnotobiotically grown aromatic rice cultivars reveals genotype-dependent and tissue-specific colonization of endophytic bacterial communities attributing multiple plant growth promoting traits. World J Microbiol Biotechnol 37:59
Jie W-G, Yao Y-X, Guo N, Zhang Y-Z, Qiao W (2021) Effects of Rhizophagus intraradices on plant growth and the composition of microbial communities in the roots of continuous cropping soybean at maturity. Sustainability 13:6623
Van Bruggen A, Finckh M (2016) Plant diseases and management approaches in organic farming systems. Ann Rev Phytopathol 54:25–54
Tyagi J, Chaudhary P, Mishra A, Khatwani M, Dey S, Varma A (2022) Role of endophytes in abiotic stress tolerance: with special emphasis on Serendipita indica. Int J Environ Res 16:1–21
Orozco-Mosqueda M, Flores A, Rojas-Sánchez B, Urtis-Flores CA, Morales-Cedeño LR, Valencia-Marin MF, Chávez-Avila S, Rojas-Solis D, Santoyo G (2021) Plant growth-promoting bacteria as bioinoculants: attributes and challenges for sustainable crop improvement. Agron 11:1167
Müller CA, Obermeier MM, Berg G (2016) Bioprospecting plant-associated microbiomes. J Biotechnol 235:171–180
Mahanty T, Bhattacharjee S, Goswami M, Bhattacharyya P, Das B, Ghosh A, Tribedi P (2017) Biofertilizers: a potential approach for sustainable agriculture development. Environ Sci Poll Res 24:3315–3335
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8:467
Latz MA, Jensen B, Collinge DB, Jørgensen HJ (2018) Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Diversity 11:555–567
Rojas EC, Jensen B, Jørgensen HJ, Latz MA, Esteban P, Ding Y, Collinge DB (2020) Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol Control 144:104222
Marag PS, Suman A, Gond S (2018) Prospecting endophytic bacterial colonization and their potential plant growth promoting attributes in hybrid maize (Zea mays L.). Int J Curr Microbiol Appl Sci 7:1292–1304
Kumawat K, Sharma P, Sirari A, Singh I, Gill B, Singh U, Saharan K (2019) Synergism of Pseudomonas aeruginosa (LSE-2) nodule endophyte with Bradyrhizobium sp. (LSBR-3) for improving plant growth, nutrient acquisition and soil health in soybean. World J Microbiol Biotechnol 35:1–17
Liu Y-Q, Wang Y-H, Kong W-L, Liu W-H, Xie X-L, Wu X-Q (2020) Identification, cloning and expression patterns of the genes related to phosphate solubilization in Burkholderia multivorans WS-FJ9 under different soluble phosphate levels. AMB Express 10:108
Behera BC, Singdevsachan SK, Mishra RR, Dutta SK, Thatoi HN (2014) Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove - a review. Biocatal Agric Biotechnol 3:97–110
Valetti L, Iriarte L, Fabra A (2018) Growth promotion of rapeseed (Brassica napus) associated with the inoculation of phosphate solubilizing bacteria. Appl Soil Ecol 132:1–10
Shahid M, Hameed S, Tariq M, Zafar M, Ali A, Ahmad N (2015) Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Annals Microbiol 65:1525–1536
Lucero CT, Lorda GS, Ludueña LM, Anzuay MS, Taurian T (2020) Motility and biofilm production involved in the interaction of phosphate solubilizing endophytic strains with peanut, maize and soybean plants. Rhizosph 15:100228
Kenasa G, Nandeshwar B, Assefa F (2021) In vitro inorganic phosphate solubilization tests of cowpea root nodule bacteria from Ethiopia. Agric Sci Digest 41:195–198
Yasmeen S, Bano A (2014) Combined effect of phosphate-solubilizing microorganisms, Rhizobium and Enterobacter on root nodulation and physiology of soybean (Glycine max L.). Comm Soil Sci Plant Analysis 45:2373–2384
Tariq M, Hameed S, Yasmeen T, Zahid M, Zafar M (2014) Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J Microbiol Biotechnol 30:719–725
Sánchez-Cruz R, Tpia Vázquez I, Batista-García RA, Méndez-Santiago EW, Sánchez-Carbente MdR, Leija A, Lira-Ruan V, Hernández G, Wong-Villarreal A, Folch-Mallol JL (2019) Isolation and characterization of endophytes from nodules of Mimosa pudica with biotechnological potential. Microbiol Res 218:76–86
Chaudhary A, Chaudhary P, Upadhyay A, Kumar A, Singh A (2021) Effect of Gypsum on plant growth promoting rhizobacteria. Environ Ecol 39:1248–1256
Bertalan M, Albano R, de Pádua V, Rouws L, Rojas C, Hemerly A, Teixeira K, Schwab S, Araujo J, Oliveira A (2009) Complete genome sequence of the sugarcane nitrogen-fixing endophyte Gluconacetobacter diazotrophicus Pal5. BMC Genomics 10:1–17
El Attar I, Taha K, El Bekkay B, El Khadir M, Thami Alami I, Aurag J (2019) Screening of stress tolerant bacterial strains possessing interesting multi-plant growth promoting traits isolated from root nodules of Phaseolus vulgaris L. Biocatal Agric Biotechnol 20:101225
Ferreira CMH, Soares HMVM, Soares EV (2019) Promising bacterial genera for agricultural practices: an insight on plant growth-promoting properties and microbial safety aspects. Sci Total Environ 682:779–799
Maheshwari R, Bhutani N, Suneja P (2019) Screening and characterization of siderophore producing endophytic bacteria from Cicer arietinum and Pisum sativum plants. J Applied Biol Biotechnol 7:7–14
Zaman NR, Chowdhury UF, Reza RN, Chowdhury FT, Sarker M, Hossain MM, Akbor MA, Amin A, Islam MR, Khan H (2021) Plant growth promoting endophyte Burkholderia contaminans NZ antagonizes phytopathogen Macrophomina phaseolina through melanin synthesis and pyrrolnitrin inhibition. PLoS ONE 16:e0257863
Gao J-l, Sun P, Sun Y-c, Xue J, Wang G, Wang L-w, Du Y, Zhang X, Sun J-g (2021) Caulobacter endophyticus sp. nov., an endophytic bacterium harboring three lasso peptide biosynthetic gene clusters and producing indoleacetic acid isolated from maize root. Antonie Van Leeuwenhoek 114:1213–1224
Ahmad E, Sharma SK, Sharma PK (2020) Deciphering operation of tryptophan-independent pathway in high indole-3-acetic acid (IAA) producing Micrococcus aloeverae DCB-20. FEMS Microbiol Lett 367:fnaa190
Pérez-Montaño F, Alías-Villegas C, Bellogín RA, del Cerro P, Espuny MR, Jiménez-Guerrero I, López-Baena FJ, Ollero FJ, Cubo T (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–336
Sofy MR, Aboseidah AA, Heneidak SA, Ahmed HR (2021) ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ Sci Poll Res 28:40971–40991
Montejano-Ramírez V, García-Pineda E, Valencia-Cantero E (2020) Bacterial compound N, N-dimethylhexadecylamine modulates expression of iron deficiency and defense response genes in Medicago truncatula independently of the jasmonic acid pathway. Plants 9:624
Conti R, Chagas FO, Caraballo‐Rodriguez AM, Melo WGdP, do Nascimento AM, Cavalcanti BC, de Moraes MO, Pessoa C, Costa‐Lotufo LV, Krogh R (2016) Endophytic actinobacteria from the Brazilian medicinal plant Lychnophora ericoides Mart. and the biological potential of their secondary metabolites. Chem Biodiversity 13:727-736
Adeleke BS, Ayangbenro A, Babalola OO (2022) Effect of endophytic bacterium, Stenotrophomonas maltophilia JVB5 on sunflowers. Plant Protection Sci 58:185–198
Fadiji AE, Ayangbenro AS, Babalola OO (2020) Metagenomic profiling of the community structure, diversity, and nutrient pathways of bacterial endophytes in maize plant. Antonie Van Leeuwenhoek 113:1559–1571
Maropola MKA, Ramond J-B, Trindade M (2015) Impact of metagenomic DNA extraction procedures on the identifiable endophytic bacterial diversity in Sorghum bicolor (L. Moench). J Microbiol Methods 112:104–117
Bullington LS, Lekberg Y, Larkin BG (2021) Insufficient sampling constrains our characterization of plant microbiomes. Sci Rep 11:3645
Lipková N, Medo J, Artimová R, Maková J, Petrová J, Javoreková S, Michalko J (2021) Growth promotion of rapeseed (Brassica napus L.) and blackleg disease (Leptosphaeria maculans) suppression mediated by endophytic bacteria. Agron 11:1966
Ribeiro IDA, Bach E, da Silva Moreira F, Müller AR, Rangel CP, Wilhelm CM, Barth AL, Passaglia LMP (2021) Antifungal potential against Sclerotinia sclerotiorum (Lib.) de Bary and plant growth promoting abilities of Bacillus isolates from canola (Brassica napus L.) roots. Microbiol Res 248:126754
Martínez-Hidalgo P, Flores-Félix JD, Sánchez-Juanes F, Rivas R, Mateos PF, Santa Regina I, Peix Á, Martínez-Molina E, Igual JM, Velázquez E (2021) Identification of Canola roots endophytic bacteria and analysis of their potential as biofertilizers for Canola crops with special emphasis on sporulating bacteria. Agron 11:1796
Jiménez-Gómez A, Saati-Santamaría Z, Kostovcik M, Rivas R, Velázquez E, Mateos PF, Menéndez E, García-Fraile P (2020) Selection of the root endophyte Pseudomonas brassicacearum CDVBN10 as plant growth promoter for Brassica napus L. crops. Agron 10:1788
Cheng Z, Park E, Glick BR (2007) 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Canad J Microbiol 53:912–918
Trivedi G, Patel P, Saraf M (2020) Synergistic effect of endophytic selenobacteria on biofortification and growth of Glycine max under drought stress. South Afr J Bot 134:27–35
Egamberdieva D, Jabborova D, Berg G (2016) Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 405:35–45
Subramanian P, Kim K, Krishnamoorthy R, Sundaram S, Sa T (2015) Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Reg 76:327–332
Kim M-J, Radhakrishnan R, Kang S-M, You Y-H, Jeong E-J, Kim J-G, Lee I-J (2017) Plant growth promoting effect of Bacillus amyloliquefaciens H-2-5 on crop plants and influence on physiological changes in soybean under soil salinity. Physiol Mol Biol Plants 23:571–580
Archana T, Rajendran L, Manoranjitham S, Krishnan VS, Paramasivan M, Karthikeyan G (2020) Culture-dependent analysis of seed bacterial endophyte, Pseudomonas spp. EGN 1 against the stem rot disease (Sclerotium rolfsii Sacc.) in groundnut. Egypt J Biol Pest Control 30:119
Puri A, Padda KP, Chanway CP (2016) Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b–2R. Biol Fertility Soils 52:119–125
Ghavami N, Alikhani HA, Pourbabaei AA, Besharati H (2017) Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J Plant Nutr 40:736–746
Li L, Zhang Z, Pan S, Li L, Li X (2019) Characterization and metabolism effect of seed endophytic bacteria associated with peanut grown in south China. Front Microbiol 10:2659
Chen L, Shi H, Heng J, Wang D, Bian K (2019) Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol Res 218:41–48
Preyanga R, Anandham R, Krishnamoorthy R, Senthilkumar M, Gopal N, Vellaikumar A, Meena S (2021) Groundnut (Arachis hypogaea) nodule Rhizobium and passenger endophytic bacterial cultivable diversity and their impact on plant growth promotion. Rhizosph 17:100309
Wang X, Liang G (2014) Control efficacy of an endophytic Bacillus amyloliquefaciens strain BZ6-1 against peanut bacterial wilt, Ralstonia solanacearum. BioMed Res Int 2014:465435
Lucero CT, Lorda GS, Anzuay MS, Ludueña LM, Taurian T (2021) Peanut endophytic phosphate solubilizing bacteria increase growth and P content of soybean and maize plants. Curr Microbiol 78:1961–1972
Kumar A, Voropaeva O, Maleva M, Panikovskaya K, Borisova G, Rajkumar M, Bruno LB (2021) Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosph 266:128983
Qadir M, Hussain A, Hamayun M, Shah M, Iqbal A, Irshad M, Ahmad A, Lodhi MA, Lee I-J (2021) Phytohormones producing Acinetobacter bouvetii P1 mitigates chromate stress in sunflower by provoking host antioxidant response. Antioxidants 10:1868
Selim HM, Gomaa NM, Essa AM (2017) Application of endophytic bacteria for the biocontrol of Rhizoctonia solani (Cantharellales: ceratobasidiaceae) damping-off disease in cotton seedlings. Biocontrol Sci Technol 27:81–95
Yang P, Sun Z-x, Liu S-y, Lu H-x, Zhou Y, Sun M (2013) Combining antagonistic endophytic bacteria in different growth stages of cotton for control of Verticillium wilt. Crop Prot 47:17–23
Pawlik M, Płociniczak T, Thijs S, Pintelon I, Vangronsveld J, Piotrowska-Seget Z (2020) Comparison of two inoculation methods of endophytic bacteria to enhance phytodegradation efficacy of an aged petroleum hydrocarbons polluted soil. Agron 10:1196
Wang Q, Ma L, Zhou Q, Chen B, Zhang X, Wu Y, Pan F, Huang L, Yang X, Feng Y (2019) Inoculation of plant growth promoting bacteria from hyperaccumulator facilitated non-host root development and provided promising agents for elevated phytoremediation efficiency. Chemosph 234:769–776
Diaz PAE, Baron NC, Rigobelo EC (2019) Bacillus spp. as plant growth-promoting bacteria in cotton under greenhouse conditions. Austr J Crop Sci 13:2003–2014
Rana KL, Kour D, Kaur T, Devi R, Yadav A, Yadav AN (2021) Bioprospecting of endophytic bacteria from the Indian Himalayas and their role in plant growth promotion of maize (Zea mays L.). J Appl Biol Biotechnol 9:41–50
Pal G, Kumar K, Verma A, Verma SK (2022) Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol Res 255:126926
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Khan M, Asaf S, Khan A, Adhikari A, Jan R, Ali S, Imran M, Kim KM, Lee IJ (2020) Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol 22:850–862
Lu L, Chang M, Han X, Wang Q, Wang J, Yang H, Guan Q, Dai S (2021) Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J Appl Microbiol 131:1919–1931
Hossain MT, Khan A, Chung EJ, Rashid MH-O, Chung YR (2016) Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. The Plant Pathol J 32:228
Pan D, Mionetto A, Tiscornia S, Bettucci L (2015) Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotox Res 31:137–143
Shah D, Khan MS, Aziz S, Ali H, Pecoraro L (2022) Molecular and biochemical characterization, antimicrobial activity, stress tolerance, and plant growth-promoting effect of endophytic bacteria isolated from wheat varieties. Microorganisms 10:21
Govindasamy V, George P, Kumar M, Aher L, Raina SK, Rane J, Annapurna K, Minhas PS (2020) Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench]. 3 Biotech 10:13
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, Abd_Allah EF (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol 8:1887
Liu Y, Yan H, Zhang X, Zhang R, Li M, Xu T, Yang F, Zheng H, Zhao J (2020) Investigating the endophytic bacterial diversity and community structures in seeds of genetically related maize (Zea mays L.) genotypes. Biotechnol 10:27
Kuramae EE, Derksen S, Schlemper TR, Dimitrov MR, Costa OY, da Silveira AP (2020) Sorghum growth promotion by Paraburkholderia tropica and Herbaspirillum frisingense: Putative mechanisms revealed by genomics and metagenomics. Microorganisms 8:725
Tian B-Y, Cao Y, Zhang K-Q (2015) Metagenomic insights into communities, functions of endophytes and their associates with infection by root-knot nematode, Meloidogyne incognita, in tomato roots. Sci Rep 5:1–15
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Mitter B, Hauberg-Lotte L, Friedrich F, Rahalkar M (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant-Microbe Interact 25:28–36
Ali M, Ali Q, Sohail MA, Ashraf MF, Saleem MH, Hussain S, Zhou L (2021) Diversity and taxonomic distribution of endophytic bacterial community in the rice plant and its prospective. Int J Mol Sci 22:10165
Yang R, Liu P, Ye W (2017) Illumina-based analysis of endophytic bacterial diversity of tree peony (Paeonia Sect. Moutan) roots and leaves. Braz J Microbiol 48:695–705
Hakim S, Mirza BS, Imran A, Zaheer A, Yasmin S, Mubeen F, Mclean JE, Mirza MS (2020) Illumina sequencing of 16S rRNA tag shows disparity in rhizobial and non-rhizobial diversity associated with root nodules of mung bean (Vigna radiata L.) growing in different habitats in Pakistan. Microbiol Res 231:126356
Brígido C, Singh S, Menéndez E, Tavares MJ, Glick BR, Félix MdR, Oliveira S, Carvalho M (2019) Diversity and functionality of culturable endophytic bacterial communities in chickpea plants. Plants 8:42
Li Y, Wang M, Chen S (2021) Application of N2-fixing Paenibacillus triticisoli BJ-18 changes the compositions and functions of the bacterial, diazotrophic, and fungal microbiomes in the rhizosphere and root/shoot endosphere of wheat under field conditions. Biol Fertility Soils 57:347–362
Iqrar I, Numan M, Khan T, Shinwari ZK, Ali GS (2021) LC–MS/MS-based profiling of bioactive metabolites of endophytic bacteria from Cannabis sativa and their anti-Phytophthora activity. Antonie Van Leeuwenhoek 114:1165–1179
Gorai PS, Ghosh R, Mandal S, Ghosh S, Chatterjee S, Gond SK, Mandal NC (2021) Bacillus siamensis CNE6-a multifaceted plant growth promoting endophyte of Cicer arietinum L. having broad spectrum antifungal activities and host colonizing potential. Microbiol Res 252:126859
Taechowisan T, Chanaphat S, Ruensamran W, Phutdhawong WS (2014) Antibacterial activity of new flavonoids from Streptomyces sp. BT01; an endophyte in Boesenbergia rotunda (L.) Mansf. J Appl Pharm Sci 4:8–13
Rajivgandhi G, Ramachandran G, Maruthupandy M, Vaseeharan B, Manoharan N (2019) Molecular identification and structural characterization of marine endophytic actinomycetes Nocardiopsis sp. GRG 2 (KT 235641) and its antibacterial efficacy against isolated ESBL producing bacteria. Microb Pathog 126:138–148
Peng C, An D, Ding W-X, Zhu Y-X, Ye L, Li J (2020) Fungichromin production by Streptomyces sp. WP-1, an endophyte from Pinus dabeshanensis, and its antifungal activity against Fusarium oxysporum. Appl Microbiol Biotechnol 104:10437–10449
Sun L, Lu Z, Bie X, Lu F, Yang S (2006) Isolation and characterization of a co-producer of fengycins and surfactins, endophytic Bacillus amyloliquefaciens ES-2, from Scutellaria baicalensis Georgi. World J Microbiol Biotechnol 22:1259–1266
Dowarah B, Agarwal H, Krishnatreya DB, Sharma PL, Kalita N, Agarwala N (2021) Evaluation of seed associated endophytic bacteria from tolerant chilli cv. Firingi Jolokia for their biocontrol potential against bacterial wilt disease. Microbiol Res 248:126751
Liang Y, Wei G, Ning K, Zhang G, Liu Y, Dong L, Chen S (2021) Contents of lobetyolin, syringin, and atractylolide III in Codonopsis pilosula are related to dynamic changes of endophytes under drought stress. Chin Med 16:1–16
Pachaiyappan A, Sadhasivam G, Kumar M, Muthuvel A (2021) Biomedical potential of Astaxanthin from novel endophytic pigment producing bacteria Pontibacter korlensis AG6. Waste Biomass Valorization 12:2119–2129
Spina R, Saliba S, Dupire F, Ptak A, Hehn A, Piutti S, Poinsignon S, Leclerc S, Bouguet-Bonnet S, Laurain-Mattar D (2021) Molecular identification of endophytic bacteria in Leucojum aestivum in vitro culture, NMR-based metabolomics study and LC-MS analysis leading to potential Amaryllidaceae alkaloid production. Int J Mol Sci 22:1773
Liu T-h, Zhang X-m, Tian S-z, Chen L-g, Yuan J-l (2020) Bioinformatics analysis of endophytic bacteria related to berberine in the Chinese medicinal plant Coptisteeta Wall. 3 Biotech 10:1–12
Abdelshafy Mohamad OA, Ma J-B, Liu Y-H, Zhang D, Hua S, Bhute S, Hedlund BP, Li W-J, Li L (2020) Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front Plant Sci 11:47
Acknowledgements
MSA is grateful to North-West University, South Africa, for the postgraduate bursary. BSA thanked the National Research Foundation and The World Academy of Science for the NRF-TWAS African Renaissance Doctoral scholarship (UID: 116100). OOB recognizes National Research Foundation for grants (UID: 123634; 132595) that support work in her research group.
Funding
This study was funded by the National Research Foundation, South Africa (UID: 123634; 132595).
Author information
Authors and Affiliations
Contributions
MSA, BSA, and OOB had the idea for the article and suggested the review topic. MSA and BSA performed the literature search and wrote the first draft. MSA and MSA revised the manuscript. OOB made substantial technical and intellectual contributions to the structure of the various drafts of the manuscript. All authors approved the article for publication.
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ayilara, M.S., Adeleke, B.S. & Babalola, O.O. Bioprospecting and Challenges of Plant Microbiome Research for Sustainable Agriculture, a Review on Soybean Endophytic Bacteria. Microb Ecol 85, 1113–1135 (2023). https://doi.org/10.1007/s00248-022-02136-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-022-02136-z