Abstract
Introduction
To prospectively compare of the diagnostic value of digital subtraction angiography (DSA) and time-of-flight magnetic resonance angiography (TOF-MRA) in the follow-up of intracranial aneurysms after endovascular treatment.
Methods
Seventy-two consecutive patients were examined 3 months after the embolization. The index tests included: two-dimensional DSA (2D-DSA), three-dimensional DSA (3D-DSA), and TOF-MRA. The reference test was a retrospective consensus between 2D-DSA images, 3D-DSA images, and source rotational DSA images. The evaluation included: detection of the residual flow, quantification of the flow, and validity of the decision regarding retreatment. Intraobserver agreement and interobserver agreement were determined.
Results
The sensitivity and specificity of residual flow detection ranged from 84.6 % (2D-DSA and TOF-MRA) to 92.3 % (3D-DSA) and from 91.3 % (TOF-MRA) to 97.8 % (3D-DSA), respectively. The accuracy of occlusion degree evaluation ranged from 0.78 (2D-DSA) to 0.92 (3D-DSA, Cohen’s kappa). The 2D-DSA method presented lower performance in the decision on retreatment than 3D-DSA (P < 0.05, ROC analysis). The intraobserver agreement was very good for all techniques (κ = 0.80–0.97). The interobserver agreement was moderate for TOF-MRA and very good for 2D-DSA and 3D-DSA (κ = 0.72–0.94).
Conclusion
Considering the invasiveness of DSA and the minor difference in the diagnostic performance between 3D-DSA and TOF-MRA, the latter method should be the first-line modality for follow-up after aneurysm embolization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many centers, endovascular embolization has become a method of choice for the treatment of intracranial aneurysms [1, 2]. However, coiled aneurysms present a significant rate of recanalization, which occurs in approximately 20 % of patients [3, 4]. Due to the possibility of recanalization and the availability of relatively safe endovascular retreatment [5], follow-up of coiled aneurysms is recommended [6, 7].
Despite the invasiveness, need for hospitalization and relatively high cost, intra-arterial digital subtraction angiography (DSA) is still the standard follow-up method after aneurysm embolization [7–9]. Recently, several reports have indicated that DSA can be replaced by magnetic resonance angiography (MRA), which is less invasive and presents very good accuracy in detecting residual flow in the aneurysm [6, 10–12]. However, several authors have indicated that in some cases of aneurysm recanalization, MRA may be more sensitive than the commonly used two-dimensional DSA (2D-DSA) [11, 13–15]. Some other authors have suggested that three-dimensional DSA (3D-DSA) may be more sensitive in detecting residual flow than 2D-DSA [16, 17]. This raised a question of contemporary standard for follow-up imaging of embolized aneurysms.
The purpose of the study was a prospective comparison of the diagnostic value of 2D-DSA, 3D-DSA and time-of-flight MRA (TOF-MRA) at follow-up regarding the determination of aneurysm occlusion and the decision-making process regarding possible retreatment.
Materials and methods
Population
The study was approved by our university’s review board and was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent. The sample size calculation was based on a meta-analysis by Kwee and Kwee [18], who estimated the pooled sensitivity and specificity for TOF-MRA in detecting aneurysmal flow at 83.3 % (95 % confidence interval [CI], 70.3–91.3 %) and 90.6 % (95 % CI, 80.4–95.8 %), respectively. Using the method of Flahault et al. [19] and assuming a possible 5 % rate of non-evaluable cases, the sample size was estimated at 74 aneurysms.
We included patients who were treated for subarachnoid hemorrhage due to aneurysm rupture, that were scheduled for the first follow-up imaging at 3 months after the procedure. Patients were excluded for the following reasons: (a) age under 18 years, (b) contraindications to MR imaging, including severe claustrophobia, ferromagnetic foreign bodies or electronic implants, (c) the presence of neurosurgical clips, (d) estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2.
Embolizations were performed using platinum coils (GDC Detachable Coils, Boston Scientific, Natick, MA, USA; Axium and Nexus, ev3 Corporate, Plymouth, USA; MicroPlex, MicroVention, Inc., Tustin, USA), hydrogel coils (HydroCoil and HydroSoft, MicroVention, Inc., Tustin, USA), and intracranial stents (Neuroform3; Boston Scientific). Intracranial stents were used for coiling assistance in patients with wide-neck aneurysms to achieve a better packing density and to prevent coil prolapse into the parent artery. After all of the procedures, the baseline status of the aneurysm was documented by 2D-DSA and 3D-DSA.MR imaging was not routinely performed during the perioperative period.
Follow-up DSA technique
Intra-arterial DSA was performed with a monoplane angiographic unit (Axiom Artis dTA, Siemens Medical Systems, Erlangen, Germany) by means of transfemoral catheterization. Contrast material (iopromide, Ultravist 300 mg-I/ml; Bayer Schering Pharma AG, Berlin, Germany) was administrated with a power injector through a 5 F catheter. The 2D-DSA acquisition consisted of three projections: posteroanterior (LAO/RAO 0 °, CRAN 26 °), oblique (LAO/RAO 26 °, CRAN 26 °), and lateral (LAO/RAO 90 °, CRAN 0 °). Contrast agent was administered at 12 ml (6 ml/s) to common carotid arteries (CCA) and at 10 ml (5 ml/s) to vertebral arteries (VA).
The 3D-DSA imaging included arteries with embolized aneurysms only. The acquisitions consisted of two rotational scans, covering 200 °, resulting in 122 2D-source images in cine mode. Contrast agent was administered at 15 ml (5 ml/s) to CCA and at 8 ml (3 ml/s) to VA. Images were analyzed on a dedicated workstation (Syngo XVP VA72B, Siemens AG, Berlin, Germany) using InSpace 3Dsoftware. The following reconstruction parameters were used: voxel size 0.57 mm, number of slices 220, slice matrix 512 × 512, kernel type EE, reconstruction mode Dual Volume.
Follow-up MRA technique
MR angiography was performed with a 1.5-T Signa Hdx unit, using an eight-channel HD Brain Coil (GE Medical Systems, Waukesha, USA) within 24 h after DSA. A 3D TOF ASSET Multislab technique was used in axial plane to cover the whole intracranial space. The following parameters were used: TE 2.7 ms, TR 30 ms, flip angle 20 °, bandwidth 31.25 kHz, section thickness 1.2 mm, matrix 320 × 224, effective voxel size 0.7 × 0.8 × 0.6 mm.
Angiograms were evaluated with Advantage Workstation 4.4 and Volume Share 8.4.3 software (GE Medical Systems). Analysis included non-reconstructed images, as well as MIP and VR reconstructions.
Image analysis
Examined methods were evaluated as concerns the detection of residual flow in the aneurysm, classification of the flow, i.e., classification of the degree of aneurysm occlusion with a method described by Roy et al. [20], and possibility of retreatment.
Index tests included 2D-DSA, 3D-DSA, and TOF-MRA. Images were independently assessed by two interventional neuroradiologists (Z.S, P.S.) with a 10-year experience in the field. The observers evaluated blinded data, and were unaware of the other imaging results of the patient. Discordant results were solved by means of joint reassessment.
The reference result was established retrospectively when results of index tests were set in all study participants. The reference test was DSA, which constituted a simultaneous analysis of 2D-DSA images, 3D-DSA VR images, and source rotational DSA images. Examinations were evaluated by two observers and all discrepancies between methods were solved by consensus.
Statistical analysis
Test characteristics of 2D-DSA, 3D-DSA, and TOF-MRA versus the reference method were calculated with corresponding 95 % CIs. We tested the ability of the index tests to properly detect residual flow in the aneurysm and to properly define indications for retreatment. The test characteristics included sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic accuracy. We also calculated the areas under the receiver operating characteristic curves (AUCs) with their 95 % CIs. Significance of the AUC values and significance of differences between them was tested with z-test. The ability of the index tests to properly classify the degree of aneurysm occlusion (class 1–3) was estimated using concordance correlation coefficient (CCC) with its 95 % CI and weighted Cohen’s kappa (κ). Intraobserver agreement and interobserver agreement were measured with Cohen’s κ with its 95 % CI. A P value of <0.05 was considered significant. Statistical analyses were performed using MedCalc 11.6.0 (MedCalc Software, Mariakerke, Belgium) and Statistica 9 (StatSoft Inc., Tulsa, OK, USA).
Results
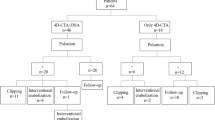
Between November 2009 and March 2011, a total number of 74 patients with 74 aneurysms were prospectively included in the study (Fig. 1). Due to a severe claustrophobia two patients were excluded from the MRA group. Therefore, 72 patients (mean age 51.5 ± 12.4 years) including 24 men and 48 women were finally analyzed (Table 1). During follow-up examinations no adverse reactions to contrast media occurred and no adverse events related to DSA were noted. The mean radiation dose of 2D-DSA in three projections was 97 ± 14 mGy, and the mean radiation dose of 3D acquisition was 102 ± 12 mGy.
Follow-up DSA images were interpretable in all the cases. The reference test presented residual flow in 26 aneurysms (36.1 %): class 2 in eight cases (11.1 %), and class 3 in 18 cases (25.0 %). Technical indications for retreatment were found in 12 patients. When comparing the follow-up results to the immediate post-treatment imaging, we found that: 45 aneurysms remained unchanged and 15 aneurysms recanalized or presented progression of the residual flow. In two cases, the initially observed residual necks occluded spontaneously.
Characteristics of index tests are presented in Tables 2 and 3, and in Fig. 2. The 2D-DSA correctly diagnosed complete occlusion in 43 patients and residual flow in 22 patients, while three diagnoses were false-positive and four were false-negative. Determination of the class of occlusion was incorrect in 12 patients. The decision on retreatment based on 2D-DSA was true-positive in cases, false-positive in three cases, false-negative in three cases, and true-negative in 57 patients. Detection of the residual flow in 3D-DSA was true-positive in 24 cases, false-positive in one case, false-negative in two cases, and true-negative in 45 patients. Estimation of the occlusion class was incorrect in three patients. The 3D-DSA correctly defined indications for retreatment in all but one case, which was false-positive.
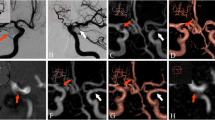
TOF-MRA images were interpretable in all the cases. In two patients, DSA presented a protrusion of a coil loop into the parent artery, which was not visible on TOF-MRA images. There were no cases of branch occlusion and no significant susceptibility artifacts related to the coils. However, in all cases treated with stent-assisted coiling the signal intensity was decreased within the stent lumen, which imitated in-stent stenosis on volume-rendered images (Fig. 3). In such cases, the in-stent stenosis was not confirmed in either 2D-DSA or 3D-DSA(both presented normal flow within the stent and adequate depiction of the stent lumen). TOF-MRA correctly diagnosed complete occlusion in 42 patients and residual flow in 22 patients, while four diagnoses were false-positive and four were false-negative. The determination of the class of occlusion was incorrect in 11 patients. The decision on retreatment based on TOF-MRA was true-positive in 11 cases, false-positive in two cases, false-negative in one case, and true-negative in 58 patients.
Follow-up angiography after stent-assisted coiling of the right internal carotid artery aneurysm. The 2D-DSAimage (a) and 3D-DSAMIP reconstruction (b) properly depict the non-stenosed stent lumen. There is a slight in-stent decrease in the signal intensity on TOF-MRA MIP image (c), which corresponds to false stenosis on TOF-MRA VR image (d)
The determination of the aneurysm occlusion status was the most accurate with 3D-DSA (κ = 0.92; CCC = 0.92, 95 % CI: 0.87–0.95), while 2D-DSA and TOF-MRA presented similar results (κ = 0.78; CCC = 0.85, 95 % CI: 0.77–0.90 and κ = 0.76; CCC = 0.80, 95 % CI: 0.70–0.87, respectively). Comparison of AUC values revealed only one statistically significant difference: 3D-DSA presented a higher diagnostic value than 2D-DSA in the determination of indications for retreatment (P < 0.050).
The most reproducible results of image analysis by one and two observers were noted in 2D-DSA followed by 3D-DSA and TOF-MRA (Table 4). Intraobserver agreement was very good for all techniques considering both the detection of the residual flow in the aneurysm and the possibility of retreatment (κ = 0.80–0.97). Classification of aneurysm occlusion status gave different results for 2D-DSA, 3D-DSA and TOF-MRA in 1.4 %, 2.8 %, and 6.9 % of cases, respectively. The respective percentages of differing interpretations of indications for retreatment were 1.4 %, 2.8 %, and 5.6 %. Interobserver agreement was moderate for TOF-MRA (κ = 0.72–0.74) and very good for 2D-DSA and 3D-DSA (κ = 0.80–0.94). Diverse results of aneurysm occlusion status evaluation with 2D-DSA, 3D-DSA and TOF-MRA were noted in 2.8 %, 5.6 %, and 12.5 % of cases, respectively. Respective proportions of different decisions on retreatment were 4.2 %, 6.9 %, and 8.3 %.
Discussion
The risk of complications [21] and the high cost of DSA [22] raised the need for alternative imaging methods of follow-up after embolization of intracranial aneurysms. In previous studies, the diagnostic value of MRA has been determined versus various DSA techniques. Reference methods included 2D acquisitions in standard or working projections [10, 11], 3D-DSA [13, 14], or undefined combination of 2D and 3D-DSA [12, 23]. Despite numerous false results of 2D-DSA in the follow-up of embolized aneurysms presented by several authors [13–19], the method was not prospectively validated to our knowledge. In our study, the diagnostic value of 2D-DSA, 3D-DSA and TOF-MRA were tested against a retrospective consensus of 2D-DSA and 3D-DSA. Three-dimensional DSA presented the best diagnostic performance as concerns both the assessment of residual flow in the aneurysm and the decision on retreatment. The 2D-DSA and TOF-MRA had similar diagnostic values. An unexpected observation was the low value of 2D-DSA in the determination of indications for re-embolization.
The consecutive recruitment of patients scheduled for their first follow-up imaging at 3 months after the embolization resulted in collection of a relatively homogenous study population. This might reduce the influence of sample size on the results. The sizes and locations of the aneurysms were similar to those in other series of patients [3, 10]. The only significant difference was the low number of aneurysms in the posterior circulation in our material. This was caused by the system of bleeding aneurysm treatment in our center, where embolization is a method of choice.
In order to quantify the diagnostic value of intra-arterial arteriography we used the retrospective consensus of two observers simultaneously evaluating 2D and 3D-DSA images. In fact, we found no other reasonable method to noninvasively verify the performance of 2D-DSA, which is commonly considered the golden standard for follow-up. Combining multiple test results to construct a reference standard outcome including deterministic predefined rules, consensus procedures and statistical modeling is an accepted approach in cases when the reference test is imperfect [24, 25]. Generally, evaluation of a new modality which would be more sensitive than the contemporary standard usually causes methodological and statistical problems that result in a low specificity of the new method. Good examples of this dilemma are attempts to verify the value of 3D-DSA in follow-up. A recent systematic review revealed only three papers directly comparing 2D-DSA and 3D-DSA [26]. In the article by Kiyosue et al. [16], 3D-DSA presented as virtual endoscopy yielded the sensitivity and specificity of 100 % and 58.3 %, respectively. Buhk et al. [27], who tested 3D-DSA reconstructed as CT-like multiplanar reformations, reported a better diagnostic accuracy but considered this technique only as a supportive tool. The most comprehensive analysis was presented by Zhou et al. [17], who considered 3D-DSA as definitely more accurate and more reproducible than 2D-DSA despite the calculated moderate specificity and sensitivity of 3D-DSA vs. 2D-DSA used as a standard. In the three presented papers, the inappropriateness of classic test characteristics led to application of comparison statistics, including weighted kappa, chi-square test, and Pearson’s correlation coefficient, which are not able to valuate 3D-DSA [16, 17, 27].
According to our results, 3D-DSA should replace 2D-DSA as a reference method for follow-up, particularly in the process of decision-making on retreatment. The test characteristics of 2D-DSA for both the detection of the residual flow and the decision-making on possible retreatment were lower than those of 3D-DSA and comparable with those of TOF-MRA. The observed PPV of 2D-DSA indicates that only in 75 % of cases, the decision on retreatment was confirmed by the reference test. The low diagnostic value of 2D-DSA would have probably improved if working projections had been used. Working projections, which are used during embolization, are useful especially in detection of the aneurysm neck remnant [10]. However, a precise reconstruction of those projections at follow-up may be difficult even in center where embolization took place and may lead to an excessive exposition to radiation and contrast media. Still, when a precise 2D working projection is necessary, i.e., for re-embolization, a solution may be the use of 3D-DSA again. Several angiographic systems offer an easy c-arm positioning according to the 3D image presentation set by the user. This option is especially useful in retreatment of lesions located at the tip of the basilar artery or in the distal middle cerebral artery, where branch arteries may obscure the aneurysm neck.
In our series, TOF-MRA presented as an efficient follow-up method. Despite the lower values of test characteristics than that of 3D-DSA, the difference in the diagnostic value was not statistically significant when measured with AUC. Another important observation was only one false-negative result of TOF-MRA in the determination of indications for retreatment, which resulted in high NPV. This reflects very low probability of omitting clinically significant aneurysm remnant when using TOF-MRA. Our results confirm previous reports. In the meta-analysis by Kwee and Kwee [18], the pooled sensitivity and specificity of TOF-MRA in detecting residual flow was 83.3 % and 86.8 %, respectively. In a recent large prospective study by Schaafsma et al. [10], AUC of TOF-MRA was 0.86 (95 % CI, 0.81–0.91).
A significant drawback of TOF-MRA shown in our material was its inability to visualize coil loops protruding into the parent vessel, which was related to the use of 1.5-T field strength for MRA. Such a complication can be visualized with TOF-MRA at 3 T, as presented by Yoneoka et al. [28]. Another observed limitation was susceptibility to artifacts caused by implanted intracranial stents. Similar artifacts had already been observed by several authors [27, 29, 30]. In our patients, these artifacts resulted in false stent lumen narrowing on MR angiograms that might suggest in-stent thrombosis or proliferation of neointima [31]. Stent-related artifacts were most evident on volume-rendered images. In case of a true in-stent stenosis, one may expect a decrease of opacity or a filling defect on 2D-DSA images and the filling defect on 3D-DSA images. More advanced cases of stenosis may present as a difference in contrast medium flow proximally and distally to the stent in 2D-DSA, which reflects a hemodynamically significant blood flow disturbance. In neither of our patients, the stent lumen narrowing was confirmed by the reference test. Therefore, TOF angiograms should always be analyzed including source images and MIP reconstructions. None of the aneurysms treated with stent-assisted coiling presented residual flow. Thus, we may not discuss the possible influence of stent-related artifacts on determination of residual flow. This problem requires further investigation.
Variation in the assessment of aneurysms was reflected by the intraobserver reproducibility and interobserver agreement, which were significant. The 2D-DSA method presented the best agreement, which may be explained by its high resolution and limited possibilities of image adjustment. Since TOF-MRA was analyzed using unreconstructed images, as well as MIP and VR reconstructions, the variability of results was more evident. The observer agreements were at least comparable to previous reports [10, 12, 15, 27].
Study limitations included the mentioned above homogeneity of the sample, which was a result of the applied method of enrollment. We included consecutive patients who were coming for their first scheduled follow-up imaging visit and no active recruitment was carried out. Therefore, patients who died in the early postoperative period and those with severe neurological complications were lost from follow-up. Theoretically, those subjects might have presented a different rate of aneurysm remnants and altered cerebral hemodynamics that might have changed results of the study. Moreover, the method of enrollment limited the study population to patients with ruptured aneurysms only. However, we considered the study population appropriate because test characteristics of imaging modalities appear not to depend on the general patient condition and the preoperative aneurysm status [10]. As mentioned above, the reference test used in the study may be questionable as well. Nevertheless, in our opinion it was the only possible method to test the diagnostic value of 2D-DSA and 3D-DSA. Finally, we performed follow-up DSA examinations by administrating contrast medium into the CCA. At our institution, selective ICA angiograms are not routinely used in diagnostic procedures because we attempt to minimize the patient’s risk as much as possible. Selective catheterization of the ICA may increase the risk of vasospasm in young patients and may result in atherosclerotic plaque fragmentation in the older ones. On the other hand, cerebral vessel opacity might be increased with a selective ICA angiogram. However, in our experience, the used parameters of contrast media flow provide adequate depiction of arteries and of the residual aneurysm flow.
In conclusion, our results indicate that 3D-DSA is the contemporary golden standard for follow-up after aneurysm embolization. On the other hand, the relatively high diagnostic value of TOF-MRA favors this non-invasive method instead of intra-arterial DSA for the routine outpatient follow-up. The use of 3D-DSA should be limited to selected cases, which include patients with contraindications to MR, and those with uncertain MRA results after stent-assisted coiling or with coil protrusion. The 3D-DSA method should be also used to confirm indications for retreatment.
References
Qureshi AI, Vazquez G, Tariq N, Suri MF, Lakshminarayan K, Lanzino G (2010) Impact of International Subarachnoid Aneurysm Trial results on treatment of ruptured intracranial aneurysms in the United States. J Neurosurg 114:834–841
Gnanalingham KK, Apostolopoulos V, Barazi S, O'Neill K (2006) The impact of the international subarachnoid aneurysm trial (ISAT) on the management of aneurysmal subarachnoid haemorrhage in a neurosurgical unit in the UK. Clin Neurol Neurosurg 108:117–123
Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, Sluzewski M, Majoie CB (2009) Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke 40:e523–e529
Ries T, Siemonsen S, Thomalla G, Grzyska U, Zeumer H, Fiehler J (2007) Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. Am J Neuroradiol 28:1755–1761
Ringer AJ, Rodriguez-Mercado R, Veznedaroglu E, Levy EI, Hanel RA, Mericle RA, Lopes DK, Lanzino G, Boulos AS (2009) Defining the risk of retreatment for aneurysm recurrence or residual after initial treatment by endovascular coiling: a multicenter study. Neurosurgery 65:311–315
van Rooij WJ, Sluzewski M (2009) Opinion: imaging follow-up after coiling of intracranial aneurysms. Am J Neuroradiol 30:1646–1648
Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, Yarnold JA, Rischmiller J, Byrne JV (2007) Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 38:1538–1544
Pierot L, Cognard C, Ricolfi F, Anxionnat R, Investigators CLARITY (2010) Immediate anatomic results after the endovascular treatment of ruptured intracranial aneurysms: analysis in the CLARITY series. Am J Neuroradiol 31:907–911
Pierot L, Spelle L, Vitry F, Investigators ATENA (2010) Immediate anatomic results after the endovascular treatment of unruptured intracranial aneurysms: analysis of the ATENA series. Am J Neuroradiol 31:140–144
Schaafsma JD, Velthuis BK, Majoie CB, van den Berg R, Brouwer PA, Barkhof F, Eshghi O, de Kort GA, Lo RT, Witkamp TD, Sprengers ME, van Walderveen MA, Bot JC, Sanchez E, Vandertop WP, van Gijn J, Buskens E, van der Graaf Y, Rinkel GJ (2010) Intracranial aneurysms treated with coil placement: test characteristics of follow-up MR angiography – multicenter study. Radiology 256:209–218
Wikström J, Ronne-Engström E, Gal G, Enblad P, Tovi M (2008) Three-dimensional time-of-flight (3D TOF) magnetic resonance angiography (MRA) and contrast-enhanced MRA of intracranial aneurysms treated with platinum coils. Acta Radiol 49:190–196
Kaufmann TJ, Huston J 3rd, Cloft HJ, Mandrekar J, Gray L, Bernstein MA, Atkinson JL, Kallmes DF (2010) A prospective trial of 3T and 1.5T time-of-flight and contrast-enhanced MR angiography in the follow-up of coiled intracranial aneurysms. Am J Neuroradiol 31:912–918
Yamada N, Hayashi K, Murao K, Higashi M, Iihara K (2004) Time-of-flight MR angiography targeted to coiled intracranial aneurysms is more sensitive to residual flow than is digital subtraction angiography. Am J Neuroradiol 25:1154–1157
Okahara M, Kiyosue H, Hori Y, Yamashita M, Nagatomi H, Mori H (2004) Three-dimensional time-of-flight MR angiography for evaluation of intracranial aneurysms after endosaccular packing with Guglielmi detachable coils: comparison with 3D digital subtraction angiography. Eur Radiol 14:1162–1168
Ferré JC, Carsin-Nicol B, Morandi X, Carsin M, de Kersaint-Gilly A, Gauvrit JY, Desal HA (2009) Time-of-flight MR angiography at 3T versus digital subtraction angiography in the imaging follow-up of 51 intracranial aneurysms treated with coils. Eur J Radiol 72:365–369
Kiyosue H, Okahara M, Tanoue S, Nakamura T, Nagatomi H, Mori H (2002) Detection of the residual lumen of intracranial aneurysms immediately after coil embolization by three-dimensional digital subtraction angiographic virtual endoscopic imaging. Neurosurgery 50:476–484
Zhou B, Li MH, Wang W, Xu HW, Cheng YD, Wang J (2010) Three-dimensional volume-rendering technique in the angiographic follow-up of intracranial aneurysms embolized with coils. J Neurosurg 112:674–680
Kwee TC, Kwee RM (2007) MR angiography in the follow-up of intracranial aneurysms treated with Guglielmi detachable coils: systematic review and meta-analysis. Neuroradiology 49:703–713
Flahault A, Cadilhac M, Thomas G (2005) Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol 58:859–862
Roy D, Milot G, Raymond J (2001) Endovascular treatment of unruptured aneurysms. Stroke 32:1998–2004
Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ, Coley SC (2007) Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology 49:753–759
Schaafsma JD, Koffijberg H, Buskens E, Velthuis BK, van der Graaf Y, Rinkel GJ (2010) Cost-effectiveness of magnetic resonance angiography versus intra-arterial digital subtraction angiography to follow-up patients with coiled intracranial aneurysms. Stroke 41:1736–1742
Kau T, Gasser J, Celedin S, Rabitsch E, Eicher W, Uhl E, Hausegger KA (2009) MR angiographic follow-up of intracranial aneurysms treated with detachable coils: evaluation of a blood-pool contrast medium. Am J Neuroradiol 30:1524–1530
Reitsma JB, Rutjes AW, Khan KS et al (2009) A review of solutions for diagnosticaccuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62:797–806
Phelps CE, Hutson A (1995) Estimating diagnostic test accuracy using a "fuzzy gold standard". Med Decis Making 15:44–57
Serafin Z, Strzesniewski P, Lasek W, Beuth W (2011) Methods and time schedule for follow-up of intracranial aneurysms treated with endovascular embolization: a systematic review. Neurol Neurochir Pol 45:421–430
Buhk JH, Kallenberg K, Mohr A, Dechent P, Knauth M (2009) Evaluation of angiographic computed tomography in the follow-up after endovascular treatment of cerebral aneurysms – a comparative study with DSA and TOF-MRA. Eur Radiol 19:430–436
Yoneoka Y, Watanabe M, Nishino K, Ito Y, Kwee IL, Nakada T, Fujii Y (2008) Evaluation of post-procedure changes in aneurysmal lumen following detachable coil-placement using multi-planar reconstruction of high-field (3.0T) magnetic resonance angiography. Acta Neurochir 150:351–358
Lubicz B, Neugroschl C, Collignon L, François O, Balériaux D (2008) Is digital substraction angiography still needed for the follow-up of intracranial aneurysms treated by embolisation with detachable coils? Neuroradiology 50:841–848
Wong JH, Mitha AP, Willson M, Hudon ME, Sevick RJ, Frayne R (2007) Assessment of brain aneurysms by using high-resolution magnetic resonance angiography after endovascular coil delivery. J Neurosurg 107:283–289
Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG (2006) Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery 59:34–42
Acknowledgement
The study was supported by a scientific grant from Nicolaus Copernicus University, Toruń, Poland.
Conflict of interest
We declare that we have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Serafin, Z., Strześniewski, P., Lasek, W. et al. Follow-up after embolization of ruptured intracranial aneurysms: A prospective comparison of two-dimensional digital subtraction angiography, three-dimensional digital subtraction angiography, and time-of-flight magnetic resonance angiography. Neuroradiology 54, 1253–1260 (2012). https://doi.org/10.1007/s00234-012-1030-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-012-1030-z