Abstract
Weak transcranial direct current stimulation (tDCS) is known to affect corticospinal excitability and enhance motor skill acquisition, whereas its effects on spinal reflexes in actively contracting muscles are yet to be established. Thus, in this study, we examined the acute effects of Active and Sham tDCS on the soleus H-reflex during standing. In fourteen adults without known neurological conditions, the soleus H-reflex was repeatedly elicited at just above M-wave threshold throughout 30 min of Active (N = 7) or Sham (N = 7) 2-mA tDCS over the primary motor cortex in standing. The maximum H-reflex (Hmax) and M-wave (Mmax) were also measured before and immediately after 30 min of tDCS. The soleus H-reflex amplitudes became significantly larger (by 6%) ≈1 min into Active or Sham tDCS and gradually returned toward the pre-tDCS values, on average, within 15 min. With Active tDCS, the amplitude reduction from the initial increase appeared to occur more swiftly than with Sham tDCS. An acute temporary increase in the soleus H-reflex amplitude within the first minute of Active and Sham tDCS found in this study indicates a previously unreported effect of tDCS on the H-reflex excitability. The present study suggests that neurophysiological characterization of Sham tDCS effects is just as important as investigating Active tDCS effects in understanding and defining acute effects of tDCS on the excitability of spinal reflex pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Weak transcranial direct current stimulation (tDCS, 1–4 mA) alters corticospinal excitability (Nitsche and Paulus 2000) and can enhance motor skill acquisition and retention (Devanathan and Madhavan 2016; Buch et al. 2017; Foerster et al. 2018a; Rostami et al. 2020). For example, tDCS over the motor cortex improved a fine motor skill of the hand in healthy participants (Reis et al. 2009), resulted in better balance in older, high fall-risk participants (Yosephi et al. 2018), and improved unique skills of ankle tracking and toe-pinch force tasks in healthy controls (Xiao et al. 2020) and people with stroke (Madhavan et al. 2011; Yamaguchi et al. 2016). At the same time, the mechanisms of tDCS as an adjuvant therapy for enhancing sensorimotor rehabilitation are not well understood. Some have reported that even when tDCS changes corticospinal excitability, it may have no effects on behavioral outcomes (Horvath et al. 2016; Aneksan et al. 2021). Possible explanations for this disconnect between induction of corticospinal plasticity and motor skill improvement (which may or may not be produced by tDCS) may be found in inter-study differences in experimental paradigms, outcome measures, study populations, and individual physiological differences (Ridding and Ziemann 2010; Bikson et al. 2018).
The impact of tDCS on the function and excitability of spinal cord pathways has not been well established. The Hoffmann-reflex (H-reflex), the electrical analog of the spinal stretch reflex, is a measure of spinal reflex excitability. Its amplitude reflects the excitability of its pathway, which is influenced by spinal and supraspinal input (Zehr 2002; Knikou 2008). For example, electrocorticographic activity over the sensorimotor cortex is correlated with H-reflexes in the beta and gamma bands in rodents, and modulation of the electroencephalographic sensorimotor rhythm is related to H-reflex amplitude modulation in people (Boulay et al. 2015; Jarjees and Vuckovic 2016; Thompson et al. 2018). Thus, tDCS that affects cortical activity (Roy et al. 2014; Hordacre et al. 2018) and corticospinal excitability (Nitsche and Paulus 2000; Quiles et al. 2022) may affect the excitability of spinal reflex pathways. However, the effects of tDCS on spinal reflex excitability are not apparent or consistent across currently available literatures. In the lower extremity, a recent study reported that a single-dose of “excitatory” tDCS (anode placed over the leg area of the primary motor cortex M1) increased the submaximal soleus H-reflex amplitude at rest in power athletes (Grospretre et al. 2021). Another study reported that a single-dose of “inhibitory” tDCS (cathode over M1) applied during standing had no effect on the soleus submaximal H-reflexes (Baudry and Duchateau 2014). Yet other studies reported that the soleus maximum H-reflex (Hmax) amplitude was not affected by single-doses of excitatory or inhibitory tDCS in seated individuals (Nitsche et al. 2003; Roche et al. 2011; Grospretre et al. 2021). Studies that examined the effects of tDCS on spinal interneuronal pathways are limited; one study found a single-dose of excitatory tDCS produced no change in reciprocal inhibition of the soleus H-reflex by common peroneal nerve stimulation at the end of the dose (Yamaguchi et al. 2016), while another found it decreased during the dose but not after (Roche et al. 2011). Two studies found presynaptic inhibition of the soleus H-reflex by common peroneal nerve stimulation unchanged after a single-dose of tDCS (Roche et al. 2011; Yamaguchi et al. 2016). In sum, a limited number of studies have examined the effects of tDCS on the excitability of lower extremity spinal cord pathways, and the findings are inconsistent. Again, discrepancies in tDCS applications and/or methods to measure spinal reflex excitability (e.g., in active vs. resting muscles) may have contributed to such variable findings. Thus, in this study, we aimed to examine the effects of tDCS on a spinal pathway that is actively engaged in the simple motor task of standing. Specifically, Active (excitatory) or Sham tDCS was applied during repeated elicitation of the soleus H-reflex while the participant maintained a stable level of soleus EMG activity in standing. The reflex measurements were made before, during, and after 30 min of Active or Sham tDCS. To increase confidence in the findings (in expectation of potentially small effects), the same experiment was repeated four times in each participant.
Spinal reflexes contribute to normal and impaired sensorimotor functions in lower extremity (Zehr and Stein 1999; Thompson and Sinkjaer 2021). Thus, elucidating the impact of tDCS on the excitability a spinal reflex pathway during an active motor task (e.g., standing) is essential for defining its potential therapeutic utility in enhancing lower extremity sensorimotor rehabilitation in neuromuscular disorders.
Methods
Participants
Fourteen young adults (9 females and 5 males, mean age = 26.1 years, range 21–36) with no known neurological impairments completed the protocol, which consisted of four sessions of tDCS (Active or Sham) + repeated soleus H-reflex elicitation. Prior to participation, all participants provided written informed consent to the study protocol as approved by the Medical University of South Carolina and the University of Rhode Island Institutional Review Boards and completed a tDCS and transcranial magnetic stimulation (TMS) compatibility screening. They were randomly assigned to the Active (n = 7) and Sham (n = 7) tDCS groups.
Study protocol
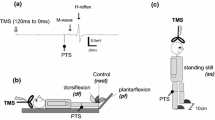
After a preliminary session, in which the presence of the soleus H-reflex was confirmed, each participant completed four approximately one-hour sessions that were separated by 1–18 days (mean = 3.3 ± 3.6 SD days). Prior to the first experimental session, each participant was randomly assigned to either the Active or Sham tDCS group and remained in the same tDCS condition group for the entire study. The session protocol is summarized in Fig. 1A. At the beginning of each session, EMG recording, tibial nerve stimulation, and tDCS electrodes were placed over the study leg and scalp as optimized during the preliminary session. Then, first, the soleus Hmax and Mmax were measured in standing prior to turning on the tDCS. Second, after a 1 min of seated rest, 10 submaximal H-reflex trials were administered in standing while the tDCS remained off (T0). Third, after the T0 (pre-tDCS) block of H-reflex measurement was completed, the participant sat in a chair and the tDCS was turned on. Fourth, when tDCS current had been ramped up to 2 mA, and at least one minute had passed since the T0 reflex block, the participant stood up and received 20 submaximal H-reflex trials (T1). After the T1 block of H-reflex measurement was completed, the participant was seated for at least one minute. Fifth, 225 submaximal H-reflex trials were administered in three blocks of 75 trials each (i.e., T4, T13, and T22 blocks of H-reflexes). At least one minute of seated rest was taken between blocks. These H-reflex trial blocks occurred similarly to the baseline phase of H-reflex operant conditioning studies (Thompson et al. 2009, 2013; Makihara et al. 2014). Upon completing the last block of 75 H-reflex trials (i.e., T22), the participant sat back in the chair and waited until the prescheduled 30 min of tDCS was done, including the full ramps, which was 1 min for Sham (30 s ramp-up to 2 mA, then ramp-down for 30 s) and 30 s for the Active condition. Sixth, when at least one minute had passed since the tDCS was completely turned off, the participant stood up again and the post-tDCS Hmax and Mmax measurements were made.
A Session protocol. The maximum H-reflex (Hmax) and the maximum M-wave (Mmax) were measured in the soleus before and after 30 min of tDCS. During tDCS, submaximal soleus H-reflexes were repeatedly elicited at a stimulus intensity just above M-wave threshold over four blocks (i.e., T1, 4, 14, and 22). In addition, 10 H-reflex trials were obtained prior to turning on the tDCS (i.e., T0). The same protocol was repeated on four different days. B Experimental setup. tDCS and tibial nerve stimulation were delivered while the participant stood and maintained his/her natural standing posture and corresponding level of soleus and TA EMG activity
Note that the above blocks of submaximal H-reflex measurements (i.e., T1–T22 blocks) were named according to their specific measurement onset within the 30 min of tDCS; on average, T1 started at 0.9 min, T4 at 4.4 min, T13 at 13.5 min, and T22 at 22.1 min after tDCS was turned on. T0 measurement occurred before the tDCS was turned on.
In addition, to increase confidence in the findings, the same experiment was repeated four times in each participant, and all sessions were held at the same time of day to eliminate a possibility of diurnal rhythm affecting the H-reflex measurements (Wolpaw and Seegal 1984; Chen and Wolpaw 1994; Carp et al. 2006; Lagerquist et al. 2006).
tDCS
Each participant was randomly assigned to the Active or Sham tDCS group prior to the first experimental session without the participant’s knowledge. They remained in the same group for all 4 study sessions (parallel, single-blinded). The tDCS was applied at the “M1 hotspot”, which was determined for each participant in a separate preliminary session. In the preliminary session, transcranial magnetic stimulation (TMS) was applied to elicit the soleus MEP during standing, using Magstim 200–2 and a 110 mm double-cone coil or a custom-made bat-wing coil with radii of 9 cm (Jali Medical Inc., Woburn, MA). The coil was held over the scalp such that the induced current flowed in the posterior–anterior direction in the brain. The hotspot was determined for each individual by systematically moving the TMS coil around the vertex (Cz) (typically within 0–3 cm lateral and 0–3 cm anterior or posterior to the vertex) at the TMS intensity that produced about a half-maximum MEP (Devanne et al. 1997; Knash et al. 2003; Kido-Thompson and Stein 2004; Thompson et al. 2011) which was estimated at the tentative hot spot (typically 1 cm lateral to the vertex). The minimum interstimulus interval was 5 s. The TMS location that produced the largest soleus MEP through this process was deemed as the hotspot and measured in relation to Cz for future sessions. Across all participants, hotspot was found contralateral to the tested leg, typically 2 cm lateral to the vertex.
Bipolar tDCS was delivered using a Soterix MXN-9 stimulator (Soterix Medical Inc., New York, NY) at 2 mA with rubber electrodes encased in 5 × 7 cm saline soaked sponges (0.06 mA/cm2 current density, 0.10 C/cm2 total charge). The anode was centered over the M1 soleus hotspot, and the cathode was placed over the supraorbital ridge ipsilateral to the EMG recording and nerve stimulation side (Nitsche and Paulus 2000; Patel and Madhavan 2019). The long edge of the anode was placed in the anterior–posterior direction over the hotspot and the cathode placed transversely over the opposite forehead.
For Active tDCS, 30 s of current ramp-up and 30 s of ramp-down were incorporated in the first and thirtieth minutes of the 30 min of tDCS (i.e., 29 min of constant 2 mA stimulation between the ramp-up and ramp-down periods). For Sham tDCS, the current was ramped up to 2 mA over 30 s and then immediately ramped down to 0 mA over the next 30 s, in the first and thirtieth minutes (i.e., 28 min of no stimulation between ramps). These Sham parameters have been shown to have no significant effect on corticospinal excitability while replicating the sensations of Active stimulation (Gandiga et al. 2006; Woods et al. 2016; Dissanayaka et al. 2018). Electrode adjustments were occasionally made based on real-time current delivery reporting on the stimulator to ensure the consistency of tDCS current intensity over 30 min. The tDCS parameters used in this study were within established safety limits (Antal et al. 2017), have been shown to excite the leg motor cortex (Jeffery et al. 2007; Ghosh et al. 2019), and have been used in previous studies in which a spinal reflex was also measured (Bastani and Jaberzadeh 2014; Sriraman et al. 2014; Agboada et al. 2019). Subjective perception of tDCS condition (i.e., Active or Sham tDCS) and sensations were noted in each session for each participant. Every participant reported some sensations at the anode; most reported itchiness and heat, a few reported pinching, and none reported the stimulation as painful. Across all participants from both Active and Sham tDCS groups, 78.6% of the sessions were perceived as Active tDCS sessions (92.8% of Active and 64.3% of Sham participants) when only 50% were Active in reality; two participants with prior tDCS experience also occasionally guessed the condition incorrectly. Thus, we are confident that masking was successful.
EMG recording and tibial nerve stimulation
EMG signal was recorded from the soleus and its antagonist tibialis anterior (TA) of the leg stimulated. Pairs of self-adhesive surface Ag–AgCl electrodes (2.2 × 3.5 cm, Nissha Medical Technologies, Buffalo, NY) were placed with their centers ~ 3 cm apart. The pair of soleus electrodes were placed just below the gastrocnemii and the TA pair were placed over the muscle belly. EMG signal was amplified and bandpass filtered at 10–1000 Hz using an AMT-8 EMG amplifier system (Bortec Biomedical Ltd., Calgary, AB, Canada), digitized at 3200 Hz, and stored.
To elicit the soleus H-reflex and M-wave, cathodal stimulation was applied to the tibial nerve in the popliteal fossa using disposable surface self-adhesive Ag–AgCl electrodes (2 × 2 cm for the cathode and 2.2 × 3.5 cm for the anode, Nissha Medical Technologies) and a Digitimer DS8R constant current stimulator (Digitimer Limited, Letchworth Garden City, UK). For each participant, the stimulus electrode locations were carefully selected such that the least amount of current was required to elicit the H-reflex in the soleus while minimizing stimulation of the common peroneal nerve and other unwanted nerves. A single 1-ms square pulse was delivered to the tibial nerve stimulating electrode pair when the standing participant had maintained a pre-defined level of soleus (natural standing level) and TA (resting level) EMG activity for at least 2 s, and at least 5 s had passed since the last trial (Hill et al. 2022). To measure the maximum M-wave (Mmax) and H-reflex (Hmax), tibial nerve stimulus intensity was increased in increments of 0.5–3 mA from below soleus H-reflex threshold, to Hmax, to an intensity just above that needed to elicit Mmax (Thompson et al. 2009; Makihara et al. 2012). At each intensity, four EMG responses were averaged to measure the H-reflex and M-wave; ≈10 different intensities were used to obtain the Hmax and Mmax in each participant. For the subsequent submaximal H-reflex trials, an intensity on the rising portion of the recruitment curve was selected for eliciting reflexes, since the H-reflexes at that stimulus level are sensitive to modulatory (i.e., inhibitory or excitatory) input (Crone et al. 1990). The M-wave size that accompanied these H-reflex trials was typically just above threshold level (≈5–10% Mmax) and was maintained throughout the experimental session by occasionally adjusting stimulus current slightly.
At the end of the preliminary session, a thin, soft cast was fitted for each participant’s lower leg to mark the optimal EMG recording and nerve stimulating electrode locations. This cast was used for all subsequent experimental sessions to ensure consistency in electrode placement across multiple sessions.
Data analysis
A custom MATLAB (MathWorks Inc., Natick, MA) program was used to analyze the EMG data offline. To measure the soleus and TA pre-stimulus (i.e., background: BG) EMG, the EMG signal was full-wave rectified and the mean absolute EMG amplitude was calculated for the 50 ms immediately before stimulation. Soleus H-reflex and M-wave amplitudes were measured as peak-to-peak EMG amplitude, typically in the windows of 32–44 ms post-stimulus for the H-reflex and 6–21 ms post-stimulus for the M-wave.
For analysis of submaximal H-reflexes, the trials with too large or too small M-waves, soleus BG, or TA BG were removed from the statistical analysis to perform adequate comparison of H-reflexes across multiple timepoints. Across all participants, 86 ± 13 SD% of H-reflex trials were included in the final analysis. For each session, soleus, TA, and M-wave values were then normalized to the individual’s pre-tDCS Mmax for that session. For each participant’s submaximal H-reflexes, H-reflex size at T1, T4, T13, T22 (i.e., H-reflex measurements during tDCS), were expressed as a percentage of the T0 value (i.e., pre-tDCS reflex size).
The pre-tDCS and post-tDCS Hmax were the maximum mean values of four trials from the recruitment curve measurement and expressed as a percentage of the Mmax that was obtained from the corresponding recruitment curve.
Statistical analysis
R program by the R Foundation for Statistical Computing (https://www.r-project.org) was used for all statistical analysis. First, in expectation of potentially small effects and to verify such small effects, we repeated the same experimental procedures four times in each participant (i.e., on four separate days), and the mean across four sessions was calculated for each measure to test the acute impact of tDCS (+H-reflex elicitation) in standing. Since the number of days between experimental sessions varied within and across participants (mean days between 3.3 ± 3.6 SD, range 1–18 days), the potential effect of repeated tDCS + H-reflex elicitation over multiple sessions was not evaluated further.
To test for significant predictors of soleus and TA BG, M-wave and H-reflex sizes, and change in submaximal H-reflex size from the pre-tDCS block (T0) to the four during-tDCS measurements (i.e., T1, T4, T13, T22), linear mixed models (LMM) that considered the fixed effects of STIM-CONDITION (i.e., Active vs. Sham tDCS) and TIME (i.e., T1, T4, T13, and T22) with participant as the random effect (intercept) were used.
To examine if Hmax and Mmax were affected by Active or Sham tDCS, pre- and post-tDCS values were compared using paired t test or Wilcoxon Rank-Sum Test (nonparametric data). To assess the stability of EMG and nerve stimulation conditions over the course of the experimental session, the Mmax amplitude change from pre- to post-tDCS was calculated and expressed in % pre-tDCS Mmax for each person.
In 3 of 14 participants (one in the Active and two in the Sham tDCS groups), the post-tDCS Hmax and Mmax measurements were not available for one session. For those, the values from available sessions were averaged together for further analyses.
For reporting of the results, all mean values are reported with standard deviations (± SD) unless indicated. Significance level was set to p = 0.05.
Results
Stability of experimental (data collection) condition
The soleus Mmax, which was 11.7 ± 3.8 mV, ranged 5.3–20.1 mV across participants in the pre-tDCS measurement, tended to decrease over an experimental session by a small amount (− 4.3% at post-tDCS, expressed as % of pre-tDCS Mmax), equivalent to ≈0.50 mV change in Mmax. For all participants, the pre- and post- Mmax values were not significantly different (p = 0.60, Wilcoxon paired), nor were they different between the groups (p = 0.48, Wilcoxon). The extent of Mmax change with tDCS + H-reflex elicitation did not differ between the Active or Sham tDCS groups (p = 0.62, Wilcoxon test).
Soleus BG EMG (mean of 26 ± 8 μV), TA BG EMG (≈8 μV, corresponding to a resting level) and soleus M-wave size (mean of 0.45 ± 0.02 mV), the stability of which is essential in assessing changes in H-reflex size across multiple measurement time points, remained stable across measurements. For soleus BG, when normalized to the Mmax, the LMM’s total explanatory power was substantial (conditional R2 = 0.97), and the part related to the fixed effects alone was small (marginal R2 = 0.04); the effects of STIM-CONDITION (p = 0.41), TIME (p = 0.40), and their interaction (p = 0.70) were not significant. The results were the same when LMM analysis was applied to the raw soleus BG EMG value; the model’s explanatory power was substantial (conditional R2 = 0.99) and the part related to the fixed effects alone was small (marginal R2 = 0.008); and the effects of STIM-CONDITION (p = 0.76), TIME (p = 0.07), and their interaction (p = 0.85) were not significant. For TA BG (in raw EMG values), the LMM’s total explanatory power was substantial (conditional R2 = 0.99), and the part related to the fixed effects alone was small (marginal R2 = 0.003). The effects of STIM-CONDITION (p = 0.84), TIME (p = 0.18), and their interaction (p = 0.92) did not significantly affect TA BG. For soleus M-wave size that accompanied submaximal H-reflexes, the LMM’s total explanatory power was substantial (conditional R2 = 0.94), and the part related to the fixed effects alone was small (marginal R2 = 0.008). The effects of STIM-CONDITION (p = 0.70), TIME (p = 0.55), and their interaction (p = 0.57) did not significantly affect M-wave size. Combined, these results indicate the consistency in reflex measurement condition within experimental sessions.
Changes in the soleus H-reflex over 30 min of tDCS
To examine if Active or Sham tDCS affected the excitability of the H-reflex pathway over the course of 30 min of tDCS in standing, we fitted a LMM to predict change in submaximal reflex size (expressed as % of the pre-tDCS block, T0) with STIM-CONDITION and TIME. The model's total explanatory power was large (conditional R2 = 0.79), and the part related to the fixed effects alone was moderate (marginal R2 = 0.09). The main effect of TIME was significant (p = 0.002), while STIM-CONDITION (p = 0.89) and the STIM-CONDITION*TIME interaction (p = 0.86) were nonsignificant. The temporal change in H-reflex size from T0 was similar in both Active and Sham tDCS groups; there was an initial increase in H-reflex size from the pre-tDCS block (T0) to the first minute(s) (T1) of 6%, then a decrease over the dosage time back to baseline in both groups (Fig. 2). H-reflex size change from T0 to T1–T22 ranged, on average, from 70 to 143% for the Active tDCS group and 59–147% for the Sham tDCS group and the mean change over all post-tDCS blocks was 100 ± 16% for Active and 101 ± 17% for Sham. The same temporal signature of reflex size change was seen in both groups as a temporary, rapid increase in H-reflex size at T1 from T0 followed by, from T4 on, a decrease in H-reflex size back toward pre-tDCS level (Fig. 2). Figure 2 also shows that the mean return to the baseline reflex size appeared to occur sooner for the Active group, at T4, while the Sham group’s return was not until T13. These rapid changes in the soleus H-reflex were clearly visible in individual EMG sweeps (Fig. 2A).
A Sample H-reflex traces for a Sham (top) and an Active (bottom) participant in the order of elicitation, from left to right, blocks T1, T4, T13, T22. The solid lines plotted in each graph are a pre-tDCS reflex (T0) for each participant and the dashed lines are a reflex trace from the block collected during tDCS. The increase in the T1 H-reflex size from T0, and its return to pre-tDCS size over time, is easily seen for both participants. B, C The 4-session mean percent reflex change from T0, y-axis, is plotted over dosage time, x-axis, for each participant in B and each group’s block average in C (bars are standard error). The dashed gray line at the y-intercept of 100 in B and C represents no change in reflex size post-tDCS. Sham is cyan dashed lines in B and circles in C; Active are orange solid lines in B and square symbols in C. The pre-tDCS block is plotted at − 1.5 min. Post-tDCS blocks are plotted at the midpoint of the tDCS dosage for that block (1.7, 7.8, 16.5, and 25.3 min). Gray bars are mean block durations
Effects of tDCS on the soleus Hmax
Across both groups of participants, the Hmax was 4.8 ± 1.6 mV with a range of 1.3–9.0 mV. To examine if the Hmax collected during standing was affected by Sham or Active tDCS + submaximal H-reflex elicitation, pre-tDCS Hmax and post-tDCS Hmax (both expressed as a % of corresponding Mmax) were compared by paired t test. Pre- and post-tDCS Hmax were not significantly different from each other overall (p = 0.99, Wilcoxon) or per group (Active, p = 0.53, t test; Sham, p = 0.97, Wilcoxon). The mean change in the Hmax was − 3 ± 6% for the Active tDCS group and − 0.7 ± 6% for the Sham tDCS group.
Discussion
Here, we examined the impact of tDCS delivered during an active motor task on the excitability of a spinal reflex pathway. The size of soleus submaximal H-reflex showed some rapid and temporary changes during 30-min of tDCS over the leg M1. During the first minutes of Active and Sham tDCS, H-reflex size increased, and then within the following several minutes it returned to near pre-tDCS size. The return to baseline appeared to occur more rapidly with Active tDCS. Similar to several previous studies (Nitsche et al. 2003; Roche et al. 2011; Grospretre et al. 2021), the soleus Hmax that was measured after tDCS was turned off did not differ from the Hmax measured pre-tDCS, suggesting that there was no immediate offline effect of tDCS on the excitability of this pathway. It is important to note that the changes in submaximal reflex size during Active and Sham tDCS reported here were not seen in other studies that collected multiple soleus H-reflexes with similar timelines but without tDCS (Thompson et al. 2009; Makihara et al. 2014). Below, we discuss the mechanistic and functional implications of these brief tDCS effects on the excitability of a spinal reflex pathway and thereby its behavior.
Rapid increase in H-reflex size with both Active and Sham tDCS
Unexpectedly, we found with both Active and Sham tDCS, submaximal H-reflexes measured at one minute after tDCS onset (i.e., T1, the measurement started 30 s after the ramp-up was completed for the Active tDCS group and at the end of ramp-down for the Sham tDCS group) were, on average, about 6% larger than the pre-tDCS (i.e., T0) H-reflexes. It is unlikely that this temporary increase in H-reflex size was caused by the soleus H-reflex elicitation/measurement procedures used in the present study. In the previous soleus H-reflex and stretch reflex studies in neurologically normal individuals, there was no systematic time-dependent change in the size of soleus reflexes over the course of 225–245 reflex trials over 30–40 min (Thompson et al. 2009; Makihara et al. 2014; Mrachacz-Kersting et al. 2019); during each of 6 baseline sessions in those studies, no systematic rise or fall of reflex sizes was detected (N = 15, 8, and 16 in Thompson, et al. 2009, and Makihara et al. 2014, and Mrachacz-Kersting et al. 2019, respectively). We also analyzed a subset of our existing pool of H-reflex data (without tDCS procedures) from 14 young and healthy individuals (unpublished data); their H-reflex data, sorted to match the T0, T1, T4, and T13 measurement timelines of the present study, indicated no significant effect of time (i.e., H-reflex sizes at T1, T4, and T13 were 103 ± 14, 100 ± 15, and 99 ± 17% of the T0 value). These provide partial support for our interpretation that Active and Sham tDCS procedures could temporarily increase the excitability of soleus H-reflex pathway. This small, rapid increase in reflex size found at T1 dissipated with both Active and Sham tDCS in the following blocks (Fig. 2). Since all H-reflex measures were obtained in the same standing posture with the same background EMG, subthreshold level of changes in the excitability of motoneuron pools or changes in postsynaptic inhibition (e.g., disynaptic reciprocal inhibition) that acts directly onto motoneurons cannot explain these H-reflex changes (Capaday and Stein 1987, 1989; Stein and Capaday 1988). A more probable explanation is that these effects of tDCS were presynaptic, acting at the sensory afferent—motoneuron synapses. That is, presynaptic inhibition over the soleus H-reflex pathway was briefly decreased about a minute into Active and Sham tDCS. In the spinal cord, inhibitory interneurons that presynaptically affect Ia-motoneuron transmission receive excitatory input from cutaneous afferents (mostly indirectly), corticospinal neurons, and other spinal neurons (e.g., other interneurons) (Iles 1996; Pierrot-Deseillingny and Burke 2012), and thus, their excitability decrease could be due to reduction in excitatory input or enhancement in inhibitory input to them. Considering the present experimental paradigm (all measures made in the same static task, posture, and background EMG), increased inhibition or reduced sensory (including cutaneous) afferent excitation of inhibitory interneurons would be an unlikely cause of the temporary reduction of presynaptic inhibition presumably happening then. (Note that, not necessarily a single class of interneurons but potentially different groups of interneurons that exert presynaptic action at the Ia-motoneuron synapses could be involved in the observed temporary changes in the excitability of soleus H-reflex pathway.) In the present paradigm, temporarily decreased corticospinal input to those inhibitory interneurons could be a possible mechanism of the brief H-reflex enhancement, potentially caused by one minute of current injection (as rapid H-reflex changes were observed in both Active and Sham conditions). Such rapid changes in corticospinal excitability with a brief period of tDCS is not surprising; several studies have shown that 4 s of tDCS at 1 mA changes the abductor digiti minimi MEP (Nitsche and Paulus 2000; Nitsche et al. 2005). While the mechanism is as yet unclear, the present findings suggest that one minute of excitatory tDCS (anode over M1) briefly reduces descending influence over presynaptic inhibition from the cortex to the spinal reflex pathway.
Rapid return of H-reflex excitability to the baseline level
The return (i.e., decrease) of H-reflex size from the initial increase was significant over all participants and appeared to occur sooner among the Active tDCS group participants than the Sham group (Fig. 2). Here, we speculate that this minor but clear difference in the time course of H-reflex size change between the Active and Sham tDCS groups may be a potential reflection of how tDCS works. With Active tDCS, the (probable) initial tDCS effect of reduced excitatory descending input onto presynaptic inhibitory interneurons may have been canceled or replaced by opposing effects during continuous positive current injection via tDCS. The apparent faster decrease in H-reflex size with Active tDCS found in this study may be due to an acute increase in corticospinal excitability caused by excitatory tDCS over leg M1, as reported in several other studies in which motor evoked potentials (MEP) to transcranial magnetic stimulation were measured (Jeffery et al. 2007; Madhavan et al. 2016; Foerster et al. 2018b). With Sham tDCS, the excitatory descending input to spinal inhibitory interneurons returned more gradually to its pre-tDCS level, possibly because there was no specific synaptic process that canceled the initial Sham tDCS effect and/or increased corticospinal descending input to inhibitory interneurons.
Interestingly, once the H-reflex decreased back to the pre-tDCS level, with Active tDCS, the continuation of positive current over M1 did not continue to increase the presynaptic inhibition of the soleus H-reflex pathway to decrease reflex size beyond the pre-tDCS level. The absence of further reduction in the Active tDCS group’s H-reflex size from T4 to T22 may be explained by the negotiated equilibrium model of spinal cord plasticity (Wolpaw 2018). In this model, the widely distributed CNS substrates of sensorimotor behaviors (called heksors) are in a constant state of negotiation to maintain the key features of their behaviors, toward keeping them satisfactory (Wolpaw and Kamesar 2022). The soleus H-reflex pathway participates in many important behaviors, including standing. Thus, the reduction of its excitability caused by tDCS would likely disturb many heksors that were already functioning well in the young and healthy individuals studied here. It would particularly disturb the heksor responsible for standing, the behavior being performed when tDCS was administered. Responses by the heksor for standing and other heksors may have counteracted or otherwise limited the reduction in H-reflex excitability caused by tDCS, thereby accounting for the lack of further reduction with continued cortical stimulation.
Implications
The ability of tDCS to induce CNS plasticity, measured with, for example, MEPs, fMRI, EEG, and brain derived neurotrophic factor level (Fritsch et al. 2010; Dayan and Cohen 2011; Stagg et al. 2018), is the basis for its potential therapeutic value. However, the impact of tDCS on spinal reflex pathways is not well defined, despite their known importance for normal motor control and for motor control after CNS injury (Yang et al. 1991; Stein et al. 1993; Zehr and Stein 1999; Sinkjaer et al. 2000; Dietz and Sinkjaer 2007).
In the present study, we observed that the excitability of soleus H-reflex pathway was briefly increased by both Active and Sham tDCS applied during standing, and then, its excitability significantly decreased from this brief enhancement. These temporary changes in reflex excitability were not observed in previous reflex studies in which 225–245 reflex trials were administered over 30–40 min period without tDCS (Thompson, et al. 2009, and Makihara et al 2014, Mrachacz-Kersting et al. 2019). Studies that use the same type of Sham protocol used in this study should be aware of such acute effects of temporary current injection to the cortex. The present findings suggest that understanding and defining the Sham tDCS effects on spinal pathways may be essential when one considers its application in motor control or motor rehabilitation research. The rapid decrease (return) of H-reflex size to pre-tDCS level with Active tDCS suggests that tDCS indeed affects the excitability of spinal reflex pathways, and it is important to further understand the effects of tDCS on spinal pathways for effectively applying tDCS for therapeutic purposes.
Whether the small effects seen here are enough to change a motor behavior is yet to be determined. Many studies already avoid a possible complication shown here with Sham tDCS (i.e., temporary increase of H-reflex excitability) by not beginning the intervention until after the tDCS is turned off, although evidence exists that applying tDCS during practice of novel hand (pinch force) and leg (pattern tracing) motor skills enhance their acquisition (i.e., accuracy and speed) (Reis et al. 2009; Devanathan and Madhavan 2016).
In sum, future clinical studies that use tDCS as an adjuvant therapy should consider the impact of tDCS on the neural pathway of the targeted behavior (if it is known) and carefully evaluate the effects of their Sham procedures.
Methodological considerations and limitations
Several methodological concerns and limitations in this study warrant discussions. One of the main findings of this study is that during the first minutes of Active and Sham tDCS, H-reflex size increased. Since several prior studies, in which soleus reflexes were repeatedly elicited over the course of 30–40 min, found no systematic changes in soleus reflex size (Thompson et al. 2009; Makihara et al. 2014; Mrachacz-Kersting et al. 2019), we would assume that the presently observed rapid increase in reflex size was due to tDCS. We recognize, however, that this interpretation largely depends on the current choice of sham procedures. Deriving a firm conclusion would require additional tDCS control experiments, such as sham tDCS with zero current injection in naïve participants and delivering current over an “off-target” site (e.g., frontal lobe).
Another methodological concern is that other tDCS montages (other than what we used in this study) may target the leg motor cortex more effectively (Foerster et al. 2018b), and therefore, the findings might have been quite different if other tDCS montages were used. The key mechanism of tDCS is thought to be potentiation of cortical areas related to the target motor behavior, which induces cortical plasticity and improves behavioral outcomes (Bikson et al. 2013; Bortoletto et al. 2015). Currently, how different tDCS dosage parameters (i.e., current density, polarity, electrode montage, duration) may produce different effects on spinal reflex excitability is unknown.
The findings might have also differed if the participants had been walking on a treadmill during tDCS, rather than standing (Fernandez-Lago et al. 2017). They might also have differed in people with CNS injury in whom standing and maintaining balance were more difficult (e.g., difference in functional impact of intervention between neurologically intact individuals and people after spinal cord injury (Thompson et al. 2009, 2022; Makihara et al. 2014)). Further studies are clearly needed to define the utility of tDCS in therapeutic applications.
Conclusion
This study demonstrated that one minute of Active and Sham tDCS temporarily increased spinal reflex excitability and Active tDCS appeared to enhance its decrease back to pre-tDCS levels. These findings could impact motor behavioral outcomes in studies aiming to enhance plasticity with tDCS. Understanding the effects of Active and Sham tDCS on the CNS pathways that involve the targeted motor behavior is essential for developing tDCS applications that can enhance sensorimotor rehabilitation in people with CNS damage.
Data availability
Study data is available upon request.
References
Agboada D, Mosayebi Samani M, Jamil A, Kuo MF, Nitsche MA (2019) Expanding the parameter space of anodal transcranial direct current stimulation of the primary motor cortex. Sci Rep 9:18185. https://doi.org/10.1038/s41598-019-54621-0
Aneksan B, Sawatdipan M, Bovonsunthonchai S et al (2021) Five-session dual-transcranial direct current stimulation with task-specific training does not improve gait and lower limb performance over training alone in subacute stroke: a pilot randomized controlled trial. Neuromodulation. https://doi.org/10.1111/ner.13526
Antal A, Alekseichuk I, Bikson M et al (2017) Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 128:1774–1809. https://doi.org/10.1016/j.clinph.2017.06.001
Bastani A, Jaberzadeh S (2014) Within-session repeated a-tDCS: the effects of repetition rate and inter-stimulus interval on corticospinal excitability and motor performance. Clin Neurophysiol 125:1809–1818. https://doi.org/10.1016/j.clinph.2014.01.010
Baudry S, Duchateau J (2014) Independent modulation of corticospinal and group I afferents pathways during upright standing. Neuroscience 275:162–169. https://doi.org/10.1016/j.neuroscience.2014.06.021
Bikson M, Name A, Rahman A (2013) Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 7:688. https://doi.org/10.3389/fnhum.2013.00688
Bikson M, Brunoni AR, Charvet LE et al (2018) Rigor and reproducibility in research with transcranial electrical stimulation: an NIMH-sponsored workshop. Brain Stimul 11:465–480. https://doi.org/10.1016/j.brs.2017.12.008
Bortoletto M, Pellicciari MC, Rodella C, Miniussi C (2015) The interaction with task-induced activity is more important than polarization: a tDCS study. Brain Stimul 8:269–276. https://doi.org/10.1016/j.brs.2014.11.006
Boulay CB, Chen XY, Wolpaw JR (2015) Electrocorticographic activity over sensorimotor cortex and motor function in awake behaving rats. J Neurophysiol 113:2232–2241. https://doi.org/10.1152/jn.00677.2014
Buch ER, Santarnecchi E, Antal A et al (2017) Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 128:589–603. https://doi.org/10.1016/j.clinph.2017.01.004
Capaday C, Stein RB (1987) A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods 21:91–104
Capaday C, Stein RB (1989) The effects of postsynaptic inhibition on the monosynaptic reflex of the cat at different levels of motoneuron pool activity. Exp Brain Res 77:577–584
Carp JS, Tennissen AM, Chen XY, Wolpaw JR (2006) Diurnal H-reflex variation in mice. Exp Brain Res 168:517–528. https://doi.org/10.1007/s00221-005-0106-y
Chen XY, Wolpaw JR (1994) Circadian rhythm in rat H-reflex. Brain Res 648:167–170
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen JB, Pierrot-Deseillingny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning. Neuron 72:443–454. https://doi.org/10.1016/j.neuron.2011.10.008
Devanathan D, Madhavan S (2016) Effects of anodal tDCS of the lower limb M1 on ankle reaction time in young adults. Exp Brain Res 234:377–385. https://doi.org/10.1007/s00221-015-4470-y
Devanne H, Lavoie BA, Capaday C (1997) Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Dietz V, Sinkjaer T (2007) Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6:725–733. https://doi.org/10.1016/s1474-4422(07)70193-x
Dissanayaka TD, Zoghi M, Farrell M, Egan GF, Jaberzadeh S (2018) Sham transcranial electrical stimulation and its effects on corticospinal excitability: a systematic review and meta-analysis. Rev Neurosci 29:223–232. https://doi.org/10.1515/revneuro-2017-0026
Fernandez-Lago H, Bello O, Mora-Cerda F, Montero-Camara J, Fernandez-Del-Olmo MA (2017) Treadmill walking combined with anodal transcranial direct current stimulation in Parkinson disease: a pilot study of kinematic and neurophysiological effects. Am J Phys Med Rehabil 96:801–808. https://doi.org/10.1097/PHM.0000000000000751
Foerster A, Dutta A, Kuo MF, Paulus W, Nitsche MA (2018a) Effects of anodal transcranial direct current stimulation over lower limb primary motor cortex on motor learning in healthy individuals. Eur J Neurosci 47:779–789. https://doi.org/10.1111/ejn.13866
Foerster AS, Rezaee Z, Paulus W, Nitsche MA, Dutta A (2018b) Effects of cathode location and the size of anode on anodal transcranial direct current stimulation over the leg motor area in healthy humans. Front Neurosci 12:443. https://doi.org/10.3389/fnins.2018.00443
Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B (2010) Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66:198–204. https://doi.org/10.1016/j.neuron.2010.03.035
Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. https://doi.org/10.1016/j.clinph.2005.12.003
Ghosh S, Hathorn D, Eisenhauer J, Dixon J, Cooper ID (2019) Anodal transcranial direct current stimulation over the vertex enhances leg motor cortex excitability bilaterally. Brain Sci. https://doi.org/10.3390/brainsci9050098
Grospretre S, Grandperrin Y, Nicolier M et al (2021) Effect of transcranial direct current stimulation on the psychomotor, cognitive, and motor performances of power athletes. Sci Rep 11:9731. https://doi.org/10.1038/s41598-021-89159-7
Hill NJ, Gupta D, Eftekhar A et al (2022) The evoked potential operant conditioning system (EPOCS): a research tool and an emerging therapy for chronic neuromuscular disorders. J vis Exp. https://doi.org/10.3791/63736
Hordacre B, Moezzi B, Ridding MC (2018) Neuroplasticity and network connectivity of the motor cortex following stroke: a transcranial direct current stimulation study. Hum Brain Mapp 39:3326–3339. https://doi.org/10.1002/hbm.24079
Horvath JC, Carter O, Forte JD (2016) No significant effect of transcranial direct current stimulation (tDCS) found on simple motor reaction time comparing 15 different simulation protocols. Neuropsychologia 91:544–552. https://doi.org/10.1016/j.neuropsychologia.2016.09.017
Iles JF (1996) Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of I a afferents from the human lower limb. J Physiol 491(1):197–207
Jarjees M, Vuckovic A (2016) The effect of voluntary modulation of the sensory-motor rhythm during different mental tasks on H reflex. Int J Psychophysiol 106:65–76. https://doi.org/10.1016/j.ijpsycho.2016.06.005
Jeffery DT, Norton JA, Roy FD, Gorassini MA (2007) Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 182:281–287. https://doi.org/10.1007/s00221-007-1093-y
Kido Thompson A, Stein RB (2004) Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 159:491–500. https://doi.org/10.1007/s00221-004-1972-4
Knash ME, Kido A, Gorassini M, Chan KM, Stein RB (2003) Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res 153:366–377. https://doi.org/10.1007/s00221-003-1628-9
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171:1–12. https://doi.org/10.1016/j.jneumeth.2008.02.012
Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF (2006) Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170:1–6. https://doi.org/10.1007/s00221-005-0172-1
Madhavan S, Weber KA 2nd, Stinear JW (2011) Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res 209:9–17. https://doi.org/10.1007/s00221-010-2511-0
Madhavan S, Sriraman A, Freels S (2016) Reliability and variability of tDCS induced changes in the lower limb motor cortex. Brain Sci. https://doi.org/10.3390/brainsci6030026
Makihara Y, Segal RL, Wolpaw JR, Thompson AK (2012) H-reflex modulation in the human medial and lateral gastrocnemii during standing and walking. Muscle Nerve 45:116–125. https://doi.org/10.1002/mus.22265
Makihara Y, Segal RL, Wolpaw JR, Thompson AK (2014) Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J Neurophysiol 112:1439–1446. https://doi.org/10.1152/jn.00225.2014
Mrachacz-Kersting N, Kersting UG, de Brito SP, Makihara Y, Arendt-Nielsen L, Sinkjaer T, Thompson AK (2019) Acquisition of a simple motor skill: task-dependent adaptation and long-term changes in the human soleus stretch reflex. J Neurophysiol 122:435–446. https://doi.org/10.1152/jn.00211.2019
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(3):633–639
Nitsche MA, Nitsche SS, Klein CC, Tergaua F, Rothwell JC, Paulus W (2003) Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol 114:600–604
Nitsche MA, Seeber A, Frommann K et al (2005) Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568:291–303. https://doi.org/10.1113/jphysiol.2005.092429
Patel R, Madhavan S (2019) Comparison of transcranial direct current stimulation electrode montages for the lower limb motor cortex. Brain Sci. https://doi.org/10.3390/brainsci9080189
Pierrot-Deseillingny E, Burke D (2012) The circuitry of the human spinal cord. Cambridge University Press, Cambridge, United Kingdom
Quiles V, Ferrero L, Iáñez E, Ortiz M, Azorín JM (2022) Review of tDCS configurations for stimulation of the lower-limb area of motor cortex and cerebellum. Brain Sci. https://doi.org/10.3390/brainsci12020248
Reis J, Schambra HM, Cohen LG et al (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. PNAS 106:1590–1595
Ridding MC, Ziemann U (2010) Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 588:2291–2304. https://doi.org/10.1113/jphysiol.2010.190314
Roche N, Lackmy A, Achache V, Bussel B, Katz R (2011) Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J Physiol 589:2813–2826. https://doi.org/10.1113/jphysiol.2011.205161
Rostami M, Mosallanezhad Z, Ansari S et al (2020) Multi-session anodal transcranial direct current stimulation enhances lower extremity functional performance in healthy older adults. Exp Brain Res 238:1925–1936. https://doi.org/10.1007/s00221-020-05827-6
Roy A, Baxter B, He B (2014) High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: a simultaneous tDCS-EEG study. IEEE Trans Biomed Eng 61:1967–1978. https://doi.org/10.1109/TBME.2014.2311071
Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB (2000) Major role for sensory fedback in soleus EMG activity in the stance phase of walking man. J Physiol 523(3):817–827
Sriraman A, Oishi T, Madhavan S (2014) Timing-dependent priming effects of tDCS on ankle motor skill learning. Brain Res 1581:23–29. https://doi.org/10.1016/j.brainres.2014.07.021
Stagg CJ, Antal A, Nitsche MA (2018) Physiology of transcranial direct current stimulation. J ECT 34:1–9
Stein RB, Capaday C (1988) Modulation of human reflexes during functional motor tasks. Trends Neurosci 11:328–332
Stein RB, Yang JF, Bélanger M, Pearson KG (1993) Chapter 18 Modification of reflexes in normal and abnormal movements. Natural and artificial control of hearing and balance. Elsevier, pp 189–196
Thompson AK, Sinkjaer T (2021) Features and physiology of spinal stretch reflexes in people with chronic spinal cord injury. In: Rajendram R, Preedy VR, Martin C (eds) Neuroscience of spinal injury book 2. Spinal cord injury cellular mechanisms, physiology and behavior. Elsevier
Thompson AK, Chen XY, Wolpaw JR (2009) Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29:5784–5792. https://doi.org/10.1523/JNEUROSCI.4326-08.2009
Thompson AK, Lapallo B, Duffield M, Abel BM, Pomerantz F (2011) Repetitive common peroneal nerve stimulation increases ankle dorsiflexor motor evoked potentials in incomplete spinal cord lesions. Exp Brain Res 210:143–152. https://doi.org/10.1007/s00221-011-2607-1
Thompson AK, Pomerantz FR, Wolpaw JR (2013) Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33:2365–2375. https://doi.org/10.1523/JNEUROSCI.3968-12.2013
Thompson AK, Carruth H, Haywood R et al (2018) Effects of sensorimotor rhythm modulation on the human flexor carpi radialis H-Reflex. Front Neurosci 12:505. https://doi.org/10.3389/fnins.2018.00505
Thompson AK, Gill CR, Feng W, Segal RL (2022) Operant down-conditioning of the soleus H-reflex in people after stroke. Front Rehabil Sci 3:859724. https://doi.org/10.3389/fresc.2022.859724
Wolpaw JR (2018) The negotiated equilibrium model of spinal cord function. J Physiol 596:3469–3491. https://doi.org/10.1113/JP275532
Wolpaw JR, Kamesar A (2022) Heksor: the central nervous system substrate of an adaptive behaviour. J Physiol 600:3423–3452. https://doi.org/10.1113/JP283291
Wolpaw JR, Seegal RF (1984) Diurnal rhythm in the spinal stretch reflex. Brain Res 244:365–369
Woods AJ, Antal A, Bikson M et al (2016) A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 127:1031–1048. https://doi.org/10.1016/j.clinph.2015.11.012
Xiao S, Wang B, Zhang X, Zhou J, Fu W (2020) Systematic review of the impact of transcranial direct current stimulation on the neuromechanical management of foot and ankle physical performance in healthy adults. Front Bioeng Biotechnol 8:587680. https://doi.org/10.3389/fbioe.2020.587680
Yamaguchi T, Fujiwara T, Tsai YA et al (2016) The effects of anodal transcranial direct current stimulation and patterned electrical stimulation on spinal inhibitory interneurons and motor function in patients with spinal cord injury. Exp Brain Res 234:1469–1478. https://doi.org/10.1007/s00221-016-4561-4
Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H (1991) H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci 18:443–452. https://doi.org/10.1017/s0317167100032133
Yosephi MH, Ehsani F, Zoghi M, Jaberzadeh S (2018) Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: primary motor cortex versus cerebellar stimulation. Brain Stimul 11:1239–1250. https://doi.org/10.1016/j.brs.2018.07.044
Zehr EP (2002) Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol 86:455–468. https://doi.org/10.1007/s00421-002-0577-5
Zehr EP, Stein RB (1999) What functions do reflexes serve during human locomotion. Prog Neurobiol 58:185–205
Acknowledgements
The authors wish to thank Roland Cote, Blair Dellenbach, Markus Melvin, Michelle McLeod, Alan Phipps and Bridgette Pouliot for their support and encouragement during challenging times. The authors also wish to thank Kirstin-Friederike Heise for valuable statistical advice.
Funding
This work was supported in part by the National Institute of Neurological Disorders and Stroke [NS114279 to AKT], the National Institute of Biomedical Imaging and Bioengineering [P41-EB018783 to JRW], the Eunice Kennedy Shriver National Institute of Child Health and Human Development [P2C HD086844 to Kautz], the New York State Spinal Cord Injury Research Trust [C32236GG & C33279GG to JRW] and the Stratton VA Medical Center.
Author information
Authors and Affiliations
Contributions
LMM conceived the study. LMM, JRW and AKT were responsible for the design. LMM performed the experiments and the analysis. LMM, JRW and AKT interpreted the results. LMM drafted the manuscript. LMM, JRW and AKT edited the manuscript. All authors approved the final version submitted and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McCane, L.M., Wolpaw, J.R. & Thompson, A.K. Effects of active and sham tDCS on the soleus H-reflex during standing. Exp Brain Res 241, 1611–1622 (2023). https://doi.org/10.1007/s00221-023-06624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06624-7