Abstract
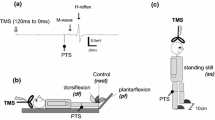
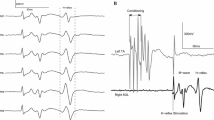
Modulation of a Hoffmann (H)-reflex following transcranial magnetic stimulation (TMS) has been used to assess the nature of signals transmitted from cortical centers to lower motor neurons. Further characterizing the recruitment and time-course of the TMS-induced effect onto the soleus H-reflex adds to the discussion of these pathways and may improve its utility in clinical studies. In 10 healthy controls, TMS was used to condition the soleus H-reflex using TMS intensities from 65 to 110% of the resting motor threshold (RMT). Early facilitation [− 5 to − 3 ms condition-test (C-T) interval] was evident when TMS was 110% of RMT (P < 0.05). By comparison, late facilitation (+ 10 to + 20 ms C-T interval) was several times larger and observed over a wider range of TMS intensities, including 65–110% of RMT. The early inhibition (− 3 to − 1 ms C-T interval) had a low TMS threshold and was elicited over a wide range of intensity from 65% to 95% of RMT (all P < 0.05). A second inhibitory phase was seen ~ 4 ms later (+ 1 to + 4 ms C-T intervals) and was only observed for a TMS intensity of 95% of RMT (P < 0.05). The present findings reaffirm that subthreshold TMS strongly modulates soleus motor neurons and demonstrates that distinct pathways can be selectively probed at discrete C-T intervals when using specific TMS intensities.


Similar content being viewed by others
Availability of data and materials
Data available upon reasonable request.
References
Alvarez R, Valls-Solé J, Valldeoriola F (1995) Abnormal modulation of the soleus H reflex by transcranial cortical magnetic stimuli in patients with Parkinson’s disease. Electroencephalogr Clin Neurophysiol Electromyogr Motor Control 4:S194
Andrews JC, Stein RB, Roy FD (2015a) Post-activation depression in the human soleus muscle using peripheral nerve and transcutaneous spinal stimulation. Neurosci Lett 589:144–149
Andrews JC, Stein RB, Roy FD (2015b) Reduced postactivation depression of soleus H reflex and root evoked potential after transcranial magnetic stimulation. J Neurophysiol 114:485–492
Andrews JC, Stein RB, Jones KE, Hedden DM, Mahood JK, Moreau MJ, Huang EM, Roy FD (2016) Intraoperative spinal cord monitoring using low intensity transcranial stimulation to remove post-activation depression of the H-reflex. Clin Neurophysiol 127:3378–3384
Andrews JC, Roy FD, Stein RB, Ba F, Sankar T (2020) Effects of deep brain stimulation and dopaminergic medication on excitatory and inhibitory spinal pathways in Parkinson’s disease. J Clin Neurophysiol (Online ahead of print)
Bawa P, Chalmers GR, Stewart H, Eisen AA (2002) Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol 88:124–132
Brouwer B, Ashby P (1992) Corticospinal projections to lower limb motoneurons in man. Exp Brain Res 89:649–654
Costa J, Guzmán J, Valldeoriola F, Rumià J, Tolosa E, Casanova-Molla J, Valls-Solé J (2011) Modulation of the soleus H reflex by electrical subcortical stimuli in humans. Exp Brain Res 212:439–448
Cowan JM, Day BL, Marsden C, Rothwell JC (1986) The effect of percutaneous motor cortex stimulation on H reflexes in muscles of the arm and leg in intact man. J Physiol (Lond) 377:333–347
Delwaide PJ, Pepin JL, Maertens de Noordhout A (1991) Short-latency autogenic inhibition in patients with Parkinsonian rigidity. Ann Neurol 30:83–89
Delwaide PJ, Pepin JL, De Pasqua V, de Noordhout AM (2000) Projections from basal ganglia to tegmentum: a subcortical route for explaining the pathophysiology of Parkinson’s disease signs? J Neurol 247(Suppl 2):II7–II1181
Di Lazzaro V, Ziemann U, Lemon RN (2008) State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul 1:345–362
Eccles JC (1964) The physiology of synapses. Springer, Berlin, p 316
Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H (1996) On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res 108:450–462
Iles JF (1996) Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491:197–207
Iles JF, Pisini JV (1992) Cortical modulation of transmission in spinal reflex pathways of man. J Physiol 455:425–446
Jankowska E, Lund S, Lundberg A, Pompeiano O (1968) Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol 106:124–140
Kurz A, Xu W, Wiegel P, Leukel C, Baker SN (2019) Non-invasive assessment of superficial and deep layer circuits in human motor cortex. J Physiol 597(12):2975–2991
Kuypers HGJM (1981) Anatomy of the descending pathways. In: Handbook of physiology, sect. I the nervous system. American Physiology Society, Bethesda, MD, Wiley-Blackwell, pp 597–666
Lauber B, Gollhofer A, Taube W (2018) Differences in motor cortical control of the soleus and tibialis anterior. J Exp Biol 221:1–8
Meunier S (1999) Modulation by corticospinal volleys of presynaptic inhibition to Ia afferents in man. J Physiol Paris 93:387–394
Misiaszek JE (2003) The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 28:144–160
Morita H, Olivier E, Baumgarten J, Petersen N, Christensen LO, Nielsen JB (2000) Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol Scand 170:65–76
Morita H, Shindo M, Morita S, Hashimoto T, Tada T, Ikeda S (2002) Abnormal conditioning effect of transcranial magnetic stimulation on soleus H-reflex during voluntary movement in Parkinson’s disease. Clin Neurophysiol 113:1316–1324
Nielsen J, Petersen N (1995a) Changes in the effect of magnetic brain stimulation accompanying voluntary dynamic contraction in man. J Physiol (Lond) 484(Pt 3):777–789
Nielsen J, Petersen N (1995b) Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. J Physiol (Lond) 486(Pt 3):779–788
Nielsen J, Petersen N, Deuschl G, Ballegaard M (1993) Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol (Lond) 471:223–243
Niemann N, Wiegel P, Kurz A, Rothwell JC, Leukel C (2018) Assessing TMS-induced D and I waves with spinal H-reflexes. J Neurophysiol 119:933–943
Palmieri RM, Ingersoll CD, Hoffman MA (2004) The Hoffmann Reflex: methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train 39(3):268–277
Phillips CG, Porter R (1977) Corticospinal neurones. Their role in movement. Monogr Physiol Soc 34:1–450
Pierrot-Deseilligny E, Mazevet D (2000) The monosynaptic reflex: a tool to investigate motor control in humans. Interests and limits. Clin Neurophysiol 30(2):67–80
Pierrot-Desseilligny E, Burke D (2005) The circuitry of the human spinal cord: Its role in motor control and movement disorders. Cambridge University Press. Cambridge, UK, pp 7–188
Pötter-Nerger M, Ilic TV, Siebner HR, Deuschl G, Volkmann J (2008) Subthalamic nucleus stimulation restores corticospinal facilitation in Parkinson’s disease. Mov Disord 23:2210–2215
Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD (1991) Stimulation of the human motor cortex through the scalp. Exp Physiol 76:159–200
Rudomin P (1990) Presynaptic inhibition of muscle spindle and tendon organ afferents in mammalian spinal cord. Trends Neurosci 13:499–505
Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I (1997) Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 113:24–32
Serranová T, Valls-Solé J, Muñoz E, Genís D, Jech R, Seeman P (2008) Abnormal corticospinal tract modulation of the soleus H reflex in patients with pure spastic paraparesis. Neurosci Lett 437:15–19
Swadlow HA (1994) Efferent neurons and suspected interneurons in motor cortex of the awake rabbit: axonal properties, sensory receptive fields, and subthreshold synaptic inputs. J Neurophysiol 71:437–453
Takakusaki K, Ohta Y, Mori S (1989) Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res 74:11–23
Valls-Solé J, Alvarez R, Tolosa ES (1994) Vibration-induced presynaptic inhibition of the soleus H reflex is temporarily reduced by cortical magnetic stimulation in human subjects. Neurosci Lett 170:149–152
Wiegel P, Kurz A, Leukel C (2020) Evidence that distinct human primary motor cortex circutis control discrete and rhythmic movements. J Physiol 598(6):1235–1251
Acknowledgements
Jennifer Andrews Ph.D. was supported by a Parkinson Association of Alberta post-doctoral fellowship award. This work was supported in part by the University of Alberta Hospital Foundation (Kaye Fund, Dr. Tejas Sankar Research Establishment Grant and the Karen Bain and David Baine Movement Disorder Research Fund), and the Charles H. Backman Fund.
Funding
Parkinson Association of Alberta, University of Alberta Hospital Foundation (Kaye Fund, Dr. Tejas Sankar Research Establishment Grant and the Karen Bain and David Baine Movement Disorder Research Fund), and the Charles H. Backman Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Ethics approval
Research approved by the Health Ethics Board at the University of Alberta.
Consent to participate
All participants provided written informed consent to the experimental protocol approved by the Health Ethics Board at the University of Alberta.
Additional information
Communicated by Winston D. Byblow.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andrews, J.C., Sankar, T., Stein, R.B. et al. Characterizing the effect of low intensity transcranial magnetic stimulation on the soleus H-reflex at rest. Exp Brain Res 238, 2725–2731 (2020). https://doi.org/10.1007/s00221-020-05879-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05879-8