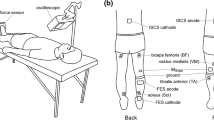
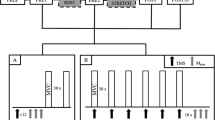
Abstract
Unilateral training involving voluntary contractions, neuromuscular electrical stimulation (NMES), or a combination of the two can increase the excitability of neural circuits bilaterally within the CNS. Many rehabilitation programs are designed to promote such “neuroplasticity” to improve voluntary movement following CNS damage. While much is known about this type of activity-dependent plasticity for the muscles that dorsi-flex the ankle, similar information is not available for the plantar-flexors. Presently, we assessed the excitability of corticospinal (CS) and spinal circuits for both soleus (SOL) muscles before and after voluntary contractions of the right plantar-flexors (VOL; 5 s on–5 s off, 40 min), NMES of the right tibial nerve (tnNMES; 5 s on–5 s off, 40 min), or both together (V + tnNMES). CS excitability for the right (rSOL) and left SOL (lSOL) muscles was assessed by quantifying motor evoked potentials elicited by transcranial magnetic stimulation. Spinal excitability was assessed using measures from the ascending limb of the M-wave versus H-reflex recruitment curve. CS excitability did not change for rSOL (the activated muscle) or lSOL following any condition. In contrast, there was a marked increase in spinal excitability for rSOL, but only following V + tnNMES; the slope of the M-wave versus H-reflex recruitment curve increased approximately twofold (pre = 7.9; post = 16.2) and H-reflexes collected when the M-wave was ~5 % of the maximal M-wave (Mmax) increased by ~1.5× (pre = 19 % Mmax, post = 29 % Mmax). Spinal excitability for lSOL did not change following any condition. Thus, only voluntary contractions that were coupled with NMES increased CNS excitability, and this occurred only in the ipsilateral spinal circuitry. These results are in marked contrast to previous studies showing NMES-induced changes in CS excitability for every other muscle studied and suggest that the mechanisms that regulate activity-dependent neuroplasticity are different for SOL than other muscles. Further, while rehabilitation strategies involving voluntary training and/or NMES of the plantar-flexors may be beneficial for producing movement and reducing atrophy, a single session of low-intensity NMES and voluntary training may not be effective for strengthening CS pathways to the SOL muscle.





Similar content being viewed by others
References
Arienzo D, Babiloni C, Ferretti A, Caulo M, Del Gratta C, Tartaro A, Rossini PM, Romani GL (2006) Somatotopy of anterior cingulate cortex (ACC) and supplementary motor area (SMA) for electric stimulation of the median and tibial nerves: an fMRI study. NeuroImage 33:700–705
Bajd T, Stefancic M, Matjacic Z, Kralj A, Savrin R, Benko H, Karcnik T, Obreza P (1997) Improvement in step clearance via calf muscle stimulation. Med Biol Eng Comput 35:113–116
Bajd T, Kralj A, Stefancic M, Lavrac N (1999) Use of functional electrical stimulation in the lower extremities of incomplete spinal cord injured patients. Artif Organs 23:403–409
Barsi GI, Popovic DB, Tarkka IM, Sinkjaer T, Grey MJ (2008) Cortical excitability changes following grasping exercise augmented with electrical stimulation. Exp Brain Res 191:57–66
Bawa P, Lemon RN (1993) Recruitment of motor units in response to transcranial magnetic stimulation in man. J Physiol 471:445–464
Bawa P, Chalmers GR, Stewart H, Eisen AA (2002) Responses of ankle extensor and flexor motoneurons to transcranial magnetic stimulation. J Neurophysiol 88:124–132
Beekhuizen KS, Field-Fote EC (2005) Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair 19:33–45
Brooke JD, McIlroy WE, Miklic M, Staines WR, Misiaszek JE, Peritore G, Angerilli P (1997) Modulation of H reflexes in human tibialis anterior muscle with passive movement. Brain Res 766:236–239
Cabric M, Appell HJ (1987) Effect of electrical stimulation of high and low frequency on maximum isometric force and some morphological characteristics in men. Int J Sports Med 8:256–260
Capaday C, Lavoie BA, Barbeau H, Schneider C, Bonnard M (1999) Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. J Neurophysiol 81:129–139
Caramia MD, Scalise A, Gordon R, Michalewski HJ, Starr A (2000) Delayed facilitation of motor cortical excitability following repetitive finger movements. Clin Neurophysiol 111:1654–1660
Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC (2006) Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol 101:1514–1522
Carson RG, Riek S, Mackey DC, Meichenbaum DC, Willms K, Forner M, Byblow WD (2004) Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 560:929–940
Charlton CS, Ridding MC, Thompson PD, Miles TS (2003) Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J Neurol Sci 208:79–85
Christensen LOD, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB (2000) Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol 62:251–272
Conforto AB, Kaelin-Lang A, Cohen LG (2002) Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol 51:122–125
Cramer SC, Finklestein SP, Schaechter JD, Bush G, Rosen BR (1999) Activation of distinct motor cortex regions during ipsilateral and contralateral finger movements. J Neurophysiol 81:383–387
Dragert K, Zehr EP (2011) Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res 208(2):217–227
Enoka RM (1988) Muscle strength and its development: new perspectives. Sports Med 6:146–168
Everaert DG, Thompson AK, Chong SL, Stein RB (2010) Does functional electrical stimulation for footdrop strengthen corticospinal projections? Neurorehabil Neural Repair 24:168–177
Ferretti A, Del Gratta C, Babiloni C, Caulo M, Arienzo D, Tartaro A, Rossini PM, Romani GL (2004) Functional topography of the secondary somatosensory cortex for nonpainful and painful stimulation of median and tibial nerve: an fMRI study. NeuroImage 23:1217–1225
Francis S, Lin X, Aboushoushah S, White TP, Phillips M, Bowtell R, Constantinescu CS (2009) fMRI analysis of active, passive and electrically stimulated ankle dorsiflexion. NeuroImage 44:469–479
Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I et al (2002) Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron 34:831–840
Hagbarth KE (1962) Post-tetanic potentiation of myotatic reflexes in man. J Neurol Neurosurg Psychiatry 25:1–10
Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG (1998) Long term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci 1:64–68
Hauck M, Baumgärtner U, Hille E, Hille S, Lorenz J, Quante M (2006) Evidence for early activation of primary motor cortex and SMA after electrical lower limb stimulation using EEG source reconstruction. Brain Res 1125:17–25
Hauptmann B, Skrotzki A, Hummelsheim H (1997) Facilitation of motor evoked potentials after repetitive voluntary hand movements depends on the type of motor activity. Electroencephalogr Clin Neurophysiol 105(5):357–364
Hayashi H, Kawaguchi M, Yamamoto Y, Inoue S, Koizumi M, Ueda Y, Takakura Y, Furuya H (2008) The application of tetanic stimulation of the unilateral tibial nerve before transcranial stimulation can augment the amplitudes of myogenic motor-evoked potentials from the muscles in the bilateral upper and lower limbs. Anesth Analg 107:215–220
Henneman E, Somjen G, Carpenter DO (1965) Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol 28(3):599–620
Hoffman LR, Field-Fote EC (2007) Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther 87:208–223
Hortobagyi TK (2005) Cross education and the human central nervous system. IEEE Eng Med Biol Mag 24:22–28
Hortobagyi TK, Taylor JL, Petersen NT, Russell G, Gandevia SC (2003) Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90:2451–2459
Jusic A, Baraba R, Bogunovic A (1995) H-reflex and F-wave potentials in leg and arm muscles. Electromyogr Clin Neurophysiol 35:471–478
Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, Binder-Macleod SA (2009) Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: effects on poststroke gait. Stroke 40:3821–3827
Khaslavskaia S, Sinkjaer T (2005) Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res 162:497–502
Khaslavskaia S, Ladouceur M, Sinkjaer T (2002) Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp Brain Res 145:309–315
Kido-Thompson A, Stein RB (2004) Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 159(4):491–500
Kitago T, Mazzocchio R, Liuzzi G, Cohen LG (2004) Modulation of H-reflex excitability by tetanic stimulation. Clin Neurophysiol 115:858–861
Kleim JA (2011) Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord 44:521–528
Knash ME, Kido A, Gorassini M, Chan KM, Stein RB (2003) Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res 153:366–377
Kristeva R, Cheyne D, Deecke L (1991) Neuromagnetic fields and accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr Clin Neurophysiol 81:284–298
Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF (2006a) Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res 170:1–6
Lagerquist O, Zehr EP, Docherty D (2006b) Increased spinal reflex excitability is not associated with neural plasticity underlying the cross-education effect. J Appl Physiol 100:83–90
Latella C, Kidgell DJ, Pearce AJ (2011) Reduction in corticospinal inhibition in the trained and untrained limb following unilateral leg strength training. Eur J Appl Physiol. doi: 10.1007/s00421-011-2289-1 [Epub ahead of print]
Lee M, Hinder MR, Gandevia SC, Carroll TJ (2010) The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol 588:201–212
Liberson WT, Holmquest HJ, Scot D, Dow M (1961) Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil 42:101–105
Maertens de Noordhout AM, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ (1999) Corticomotoneuronal synaptic connections in normal man: an electrophysiological study. Brain 122:1327–1340
Mang CS, Lagerquist O, Collins DF (2010) Changes in corticospinal excitability evoked by common peroneal nerve stimulation depend on stimulation frequency. Exp Brain Res 203:11–20
Mang CS, Clair JM, Collins DF (2011) Neuromuscular electrical stimulation has a global effect on corticospinal excitability for leg muscles and a focused effect for hand muscles. Exp Brain Res 209:355–363
McDonnell MN, Ridding MC (2006) Afferent stimulation facilitates performance on a novel motor task. Exp Brain Res 170:109–115
Morita H, Baumgarten J, Petersen N, Christensen LOD, Nielsen J (1999) Recruitment of extensor-carpi-radialis motor units by transcranial magnetic stimulation and radial-nerve stimulation in human subjects. Exp Brain Res 128:557–562
Muellbacher W, Facchini S, Boroojerdi B, Hallett M (2000) Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111:344–349
Muellbacher W, Ziemann U, Boroojerdi B, Cohen LG, Hallett M (2001) Role of the human motor cortex in rapid motor learning. Exp Brain Res 136:431–438
Nadeau S, Gravel D, Arsenault AB, Bourbonnais D (1999) Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech 14:125–135
Neptune RR, Kautz SA, Zajac FE (2001) Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34:1387–1398
Nielsen J, Morita H, Baumgarten J, Petersen N, Christensen LOD (1999) On the comparability of H-reflexes and MEPs. Electroenchepalogr Clin Neurophysiol Suppl 51:93–101
Pascual-Leone A, Dang N, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74(3):1037–1045
Pascual-Leone A, Amedi A, Fregni F, Merabet LB (2005) The plastic human brain cortex. Annu Rev Neurosci 28:377–401
Perez MA, Lungholt BKS, Nyborg K, Nielsen JB (2004) Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159:197–205
Powell J, Pandya AD, Granat M, Cameron M, Stott DJ (1999) Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke 30:1384–1389
Ridding MC, Rothwell JC (1995) Reorganization in human motor cortex. Can J Physiol Pharmacol 73:218–222
Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD (2000) Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143
Ridding MC, McKay DR, Thompson PD, Miles TS (2001) Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol 112(8):1461–1469
Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG (2009) Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol 107:1874–1883
Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD (1991) Stimulation of the human motor cortex through the scalp. Exp Physiol 76:159–200
Sabut SK, Lenka PK, Kumar R, Mahadevappa M (2010a) Effect of functional electrical stimulation on the effort and walking speed, surface electromyography, and metabolic responses in stroke patients. J Electromyogr Kinesiol 20(6):1170–1177
Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M (2010b) Restoration of gait and motor recovery by functional electrical stimulation in persons with stroke. Disabil Rehabil 32(19):1594–1603
Stedman A, Davey NJ, Ellaway PH (1998) Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Musc Nerve 21:1033–1039
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123:572–584
Taborikova H, Sax DS (1968) Motoneurone pool and the H-reflex. J Neurol Neurosurg Psychiatry 31:354–361
Tamm AS, Lagerquist O, Ley AL, Collins DF (2009) Chronotype influences diurnal variations in the excitability of the human motor cortex and the ability to generate torque during a maximum voluntary contraction. J Biol Rhythms 24:211–224
Thompson AK, Stein RB (2004) Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 159:491–500
Thompson AK, Lapallo B, Duffield M, Abel BM, Pomerantz F (2011) Repetitive common peroneal nerve stimulation increases ankle dorsiflexor motor evoked potentials in incomplete spinal cord lesions. Exp Brain Res 210:143–152
Trinastic JP, Kautz SA, McGregor K, Gregory C, Bowden M, Benjamin MB, Kurtzman M, Chang YL, Conway T, Crosson B (2010) An fMRI study of the differences in brain activity during active ankle dorsiflexion and plantarflexion. Brain Imag Behav 4:121–131
Walton C, Kalmar J, Cafarelli E (2003) Caffeine increases spinal excitability in humans. Muscle Nerve 28:359–364
Winter DA (1983) Energy generation and absorption at the ankle and knee during fast, natural, and slow cadences. Clin Ortho Rel Res 175:147–154
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. The authors also thank Mr. Alejandro Ley for his technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lagerquist, O., Mang, C.S. & Collins, D.F. Changes in spinal but not cortical excitability following combined electrical stimulation of the tibial nerve and voluntary plantar-flexion. Exp Brain Res 222, 41–53 (2012). https://doi.org/10.1007/s00221-012-3194-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3194-5