Abstract
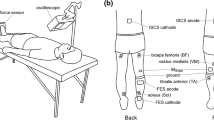
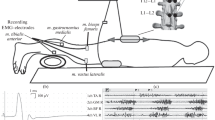
Previously, long-term changes in the motor cortex have been reported after repetitive electrical nerve stimulation (rES) as well as after motor exercise. The purpose of this study was to investigate whether the effects of voluntary motor cortical drive and of rES on the motor cortical output in healthy subjects interact with each other. A 30-min exercise session was performed during the following conditions: rES of the right common peroneal nerve (CPN) during rES at rest (A); voluntary exercise of the right ankle dorsiflexors alone (B); rES combined with voluntary dorsiflexion exercise (C); voluntary exercise of ankle plantar flexors alone (D); and plantar flexion exercise combined with rES (E). Motor evoked potentials (MEPs) were obtained before and after the exercise with a stimulation intensity of 125% of the threshold of the relaxed right tibialis anterior (TA). rES was ON for 1 s and OFF for 2 s in a cycle, and consisted of trains of five pulses, duration 1 ms and frequency 30 Hz, as applied in functional electrical stimulation (FES). MEPs of the TA muscle elicited after the training were increased in A by 38%, in B by 35%, and in C by 66%. In D and E, the MEPs of TA were decreased by 29% and 35%, respectively. The effect was maintained for at least 30 min after the nerve stimulation was completed. Consistent with previous studies (Khaslavskaia et al. (2002) Exp Brain Res 145:309–315), MEPs after the CPN rES are shown to be partly due to increased TA cortical excitability. These results suggest that the effect of FES on motor cortical excitability depends on the concurrent motor cortical drive present at the time of FES, and the combination of these factors modulates neural excitability and probably reorganization. The decrease in motor cortical excitability after plantar flexor exercise probably means that voluntary effort antagonistic to the electrical exercise is stronger and cancels out the effects of rES. Improving FES effects through an agonist voluntary drive implies an enhancement of sensorimotor reorganization through the addition of a voluntary component to a trained movement. Possible mechanisms and implications of these results on the rehabilitation of patients with paralysis and spasticity are discussed.


Similar content being viewed by others
References
Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C (1999) Changes of intracortical inhibition during motor imagery in human subjects. Neurosci Lett 263:113–116
Aimonetti JM, Nielsen JB (2002) Cortical excitability and motor task in man: an investigation of the wrist extensor area. Exp Brain Res 143:431–439
Burridge J, Taylor P, Hagan S, Swain I (1997) Experience of clinical use of the Odstock dropped foot stimulator. Artif Organs 21:254–260
Chen R, Corwell B, Hallett M (1999) Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res 129:77–86
Conforto AB, Kaelin-Lang A, Cohen LG (2002) Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol 51:122–125
Decety J (2002) Is there such a thing as functional equivalence between imagined, observed, and executed action? In: Meltzoff AN, Prinz W (eds) The imitative mind. Cambridge University Press, Cambridge, pp 291–310
Elbert T, Sterr A, Flor H, Rockstroh B, Knecht S, Pantev C, Wienbruch C, Taub E (1997) Input-increase and input-decrease types of cortical reorganization after upper extremity amputation in humans. Exp Brain Res 117(1):161–164
Gaggioli A, Morganti F, Walker R, Meneghini A, Alcaniz M, Lozano JA, Montesa J, Gil JA, Riva G (2004) Training with computer-supported motor imagery in post-stroke rehabilitation. Cyberpsychol Behav 7:327–332
Hummelsheim H, Amberger S, Mauritz KH (1996) The influence of EMG-initiated electrical muscle stimulation on motor recovery of the centrally paretic hand. Eur J Neurol 3:245–254
Hauptmann B, Hummelsheim H (1996) Facilitation of motor evoked potentials in hand extensor muscles of stroke patients; correlation to the level of voluntary contraction. Electroencephalogr Clin Neurophysiol 101:387–394
Hauptmann B, Skrotzki A, Hummelsheim H (1997) Facilitation of motor evoked potentials after repetitive voluntary hand movements depends on the type of motor activity. Electroencephalogr Clin Neurophysiol 105:357–64
Jackson PL, Lafleur MF, Malouin F, Richards C, Doyon J (2001) Potential role of mental practice using motor imagery in neurologic rehabilitation. Arch Phys Med Rehabil 82:1133–1141
Irlbacher K, Meyer BU, Voss M, Brandt SA, Roricht S (2002) Spatial reorganization of cortical motor output maps of stump muscles in human upper-limb amputees. Neurosci Lett 321:129–132
Khaslavskaia S, Ladouceur M, Sinkjaer T (2002) Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp Brain Res 145:309–315
Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR (2004) Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res 154:450–460
Knash ME, Kido A, Gorassini M, Chan KM, Stein RB (2003) Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res 153:366–377
Ladouceur M, Barbeau H (2000) Functional electrical stimulation-assisted walking for persons with incomplete spinal injuries: longitudinal changes in maximal overground walking speed. Scand J Rehabil Med 32:28–36
Mangold S, Keller T, Curt A, Dietz V (2004) Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord (in press) PMID: 15289804
Merletti R, Zelaschi F, Latella D, Galli M, Angeli S, Sessa M (1978) A control study of muscle force recovery in hemiparetic patients during treatment with functional electrical stimulation. Scand J Rehabil Med 10:147–154
Morganti F, Gaggioli A, Castelnuovo G, Bulla D, Vettorello M, Riva G (2003) The use of technology-supported mental imagery in neurological rehabilitation: a research protocol. Cyberpsychol Behav 6:421–427
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Netto JP, Cammarota A (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 174:1037–1045
Popovic M, Popovic D, Sinkjaer T, Stefanovic A, Schwirtlich L (2002) Restitution of reaching and grasping promoted by functional electrical stimulation. Artif Organs 26:271–275
Popovic M, Popovic D, Sinkjaer T, Stefanovic A, Schwirtlich L (2003) Clinical evaluation of functional electrical therapy in acute hemiplegic subjects. J Rehabil Res Dev 40:443–454
Recanzone GH, Allard TT, Jenkins WM, Merzenich MM (1990) Receptive-field changes induced by peripheral nerve stimulation in SI of adult cats. J Neurophysiol 63:1213–1225
Ridding MC, Rothwell JC (1999) Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res 126:536–544
Ridding MC, Taylor JL, Rothwell JC (1995) The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol 487:541–548
Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD (2000) Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143
Sinkjaer T, Khaslavskaia S (2002) Tibialis anterior motor cortex excitability following repetitive electrical stimulation (rES) of CP nerve depends on the voluntary motor control drive during the time of rES. In: Soc Neurosci 32nd Ann Meeting, 2–7 November 2002, Orlando, FL, No. 562.4 (CD-ROM)
Sinkjaer T, Toft E, Andreassen S, Hornemann BC (1988) Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophys 60:1110–1121
Stevens JA, Stoykov ME (2003) Using motor imagery in the rehabilitation of hemiparesis. Arch Phys Med Rehabil 84:1090–1092
Thompson AK, Stein RB (2004) Short-term effects of functional electrical stimulation on motor-evoked potentials in ankle flexor and extensor muscles. Exp Brain Res 159:491–500
Ziemann U, Corwell B, Cohen LG (1998) Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci 18:1115–1123
Acknowledgements
This work was supported by a grant from the Danish National Research Foundation. The authors also wish to express their gratitude to Mr. Knud Larsen for his technical assistance and the recruitment of subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khaslavskaia, S., Sinkjaer, T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res 162, 497–502 (2005). https://doi.org/10.1007/s00221-004-2153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2153-1