Abstract
The mycotoxins aflatoxin B1 (AFB1) and deoxynivalenol (DON) are found worldwide in crops and dietary staples. The prevalence and levels of these contaminants can vary greatly, and data in Bangladeshi food commodities are scarce. To characterize human exposure, we have conducted biomonitoring, analyzing AFM1 (a metabolite of AFB1) and DON levels in urines of adult cohorts in Bangladesh. Yet, AFM1 and DON occurrence has not been studied in the very young population of this country. Thus, the same methods, HPLC-FD for AFM1 and LC–MS/MS for DON analysis, were now applied to determine these biomarkers in urines of infants (n = 49) and young children (n = 105) in Rajshahi and Dhaka district. Overall, AFM1 and DON detection frequency was 43.5% and 33.4%, with 34.7% and 11.5% in infant and 47.6% and 39.4% in children urines, respectively. The mean AFM1 levels in all infants (9.1 ± 14.3, max 55.6 pg/mL) and children (8.8 ± 12.9, max 75.3 pg/mL) were not significantly different. The AFM1 mean level was slightly higher in Dhaka (9.4 ± 12.4) compared to Rajshahi (8.5 ± 13.9 pg/mL) district. The average DON level was about 2-fold higher in infant (3.8 ± 2.9, max 6.8 ng/mL) than children urines (1.6 ± 1.8, max 8.6 ng/mL), and higher in Rajshahi (2.1 ± 2.3 ng/mL) than Dhaka (1.4 ± 1.6 ng/mL) district. The biomarker-based estimated average daily DON intake (29.6 ± 108.3 ng/kg bw in infants and 36.4 ± 81.8 ng/kg bw in children) or the maximum exposure (560 ng/kg bw) do not exceed the current maximum provisional tolerable daily intake value of 1 µg/kg bw for DON, although DON exposure in infants and children is higher than that of Bangladeshi adults. The AFM1 urine levels in young children are somewhat lower than those found previously in adult cohorts in Bangladesh, but the frequent detection of this biomarker for AFB1 exposure raises further concerns, also for this vulnerable part of the population. Therefore, continuous surveillance for aflatoxins in Bangladeshi food commodities is clearly required, first to identify major sources of intake and then to reduce exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins and deoxynivalenol, secondary fungal metabolites produced by various Aspergillus and Fusarium species, are important contaminants of food commodities including dietary staples (EFSA 2017, 2020; Rushing and Selim 2019; Mishra et al. 2020). Mycotoxin exposure cannot be completely avoided, but it is essential to protect the population against acute and chronic effects. To limit exposures appropriate regulatory standardsFootnote 1 for these food contaminants are set that consider the hazardous properties of a given mycotoxin and its occurrence.
Aflatoxin B1 (AFB1), the most potent mycotoxin, exerts strong hepatotoxic and carcinogenic activity in several animal species. Exposure to aflatoxins, mainly AFB1, has been implicated in severe diseases in some parts of Africa and in Southeast Asia (Wild and Turner 2002; Williams et al. 2004; Groopman et al. 2008). Epidemiological studies have demonstrated a strong correlation between chronic AFB1 exposure and risk of developing hepatocellular carcinoma, alone or in tandem with hepatitis B virus infection (IARC 2012; Liu and Wu 2010; Sun et al. 2013). In addition, chronic exposure to aflatoxins has been linked to growth impairment (Gong et al. 2004; Turner 2013) and immune suppression in children (Turner et al. 2003). For aflatoxins known to act as mutagenic carcinogens, maximal levels are set, most strictly for infant food, and enforced in developed countries to minimize exposure of the population (van Egmond et al. 2007; Escola et al. 2019). But, in many developing countries, even when such regulation exists on paper, there are problems to achieve this goal, as described elsewhere in detail (IARC 2015).
Deoxynivalenol (DON) exposure of animals results in a number of adverse effects, including gastroenteritis, growth inhibition, and immunologic dysregulation (Pestka 2010; Alizadeh et al. 2015). Although evidence for health effects in humans related to chronic DON exposure is lacking, given the adverse effects in animals and its frequent occurrence as food contaminant, human exposure to DON is considered as a significant food safety issue (Sudakin 2003; Mishra et al. 2020). Moreover, recent food and/or biomarker-based assessments found that the mean attributed dietary exposure of children and adolescents often exceeds the tolerable daily intake of 1 µg/kg bw. set for DON and its modified forms (JECFA 2011; EFSA 2017).
The prevalence and levels of mycotoxin contamination are known to vary greatly between types of crops, regions, and season. This and variable dietary habits in different regions of the world make exposure assessments for aflatoxins and DON a rather complex task (FAO/WHO 2018; JECFA 2011). In the last decades, biological monitoring has been established as complementary approach to characterize human exposure to mycotoxins, early on for aflatoxins, then for other mycotoxins. The analysis of suitable biomarkers (parent compounds and/or metabolites) has a key role in investigating health concerns related to mycotoxin exposure (Turner et al. 2012), and biomarker analysis in human body fluids covers mycotoxin intake from all dietary sources and exposure by other routes (Degen 2011).
A recent review on biomarker results in human samples (blood, urine, breast milk) documents the variable patterns of mycotoxin exposure in different parts of the world (Al-Jaal et al. 2019). For the developing country Bangladesh data on contaminant levels in food commodities are scarce (Dawlatana et al. 2002; Bhuyian et al. 2013; Roy et al. 2013), and regulatory standards for aflatoxins were only recently established (BFSA 2017). In this context, biomonitoring can provide useful insights into mycotoxin exposure of the Bangladeshi population. Thus, we have conducted biomarker analysis in urines from adult residents of urban and rural areas in Raishahi and in Dhaka district: the results indicate low exposure to the Fusarium toxin DON (Ali et al. 2015a, b, 2016a). But AFM1, a biomarker of exposure to the Aspergillus toxin AFB1, has been detected in many of the urines in all adult cohorts, and at significant levels which raise health concerns (Ali et al. 2016b, 2017).
However, little is known so far about mycotoxin exposure in Bangladeshi children: two studies investigated AFB1 exposure in young children, one in a rural site in the North of the country, the other in an urban slum in Dhaka (Groopman et al. 2014; Mahfuz et al. 2019). Both have analyzed AFB1-lysin albumin in blood, a biomarker which integrates exposure during the course of several weeks. Yet, blood sampling requires medical personal whilst sampling of urine is noninvasive, easier to perform in field studies. Analysis of urinary AFM1 has been used in many studies as biomarker of recent AFB1 exposure (e.g. Polychronaki et al. 2008; Mitchell et al. 2013; Ayelign et al. 2017; Chen et al. 2018; Ezekiel et al. 2018). Urine is widely used for biomonitoring of mycotoxins which are readily excreted, for example DON: it is found in human urine as parent compound (free DON), but a far larger part are DON-glucuronides (DON-GlcA), mostly DON-15-GlcA and DON-3-GlcA (Turner et al. 2008, 2011; Brera et al. 2015). Many studies therefore apply enzymatic hydrolysis of the conjugated forms and determine’total DON’ (sum of free DON and DON-GlcA) as biomarker of exposure (e.g. Turner et al. 2008, 2010, 2011; Ali et al. 2015b, 2016a; Brera et al. 2015; Papageorgiou et al. 2018; Sarkanj et al. 2018; Wang et al. 2019). The metabolite de-epoxy-DON or DOM-1 is also found in human samples, yet less frequently, and at far lower levels than DON. Although DOM-1 is not a biomarker of exposure, an analysis of this metabolite is of some interest as indicator for detoxication of DON by the gut microbiome (Ali et al. 2016a; Wang et al. 2019).
The present study aimed to assess AFB1 and DON exposure in infants and children, vulnerable groups in the population, using the same methods for biomarker analysis as applied previously for adult cohorts in two districts of Bangladesh. As young children are known to ingest more food than adults on a kg body weight basis, the intake of contaminants may be higher. Moreover, as both AFB1 and DON may exert adverse effects on growth and immune function, co-exposure to these mycotoxins is also of interest.
Methods
Chemicals and reagents
Methanol (LC–MS gradient grade) was from Merck (Darmstadt, Germany). HPLC grade methanol and acetonitrile were purchased from Promochem (Wesel, Germany). Standard for AFM1 solution was from Sigma-Aldrich (Taufkirchen, Germany). Isotope labeled standard ([13C15] DON), deoxynivalenol (DON) and de-epoxy DON (DOM-1) were obtained from Romer Labs Diagnostics GmbH (Tulln, Austria). The β-glucuronidase/arylsulfatase from Helix pomatia (with specific activity 5.5 U/mL β-glucuronidase, 2.6 U/mL arylsulfatase at 37 ℃) was purchased from Roche Diagnostics (Mannheim, Germany) and used with 10-fold hydrolysis buffer (13.6 g sodium acetate hydrate, 1.0 g ascorbic acid, 0.1 g EDTA in 100 mL deionised water, adjusted to pH 5.0 with acetic acid 96%) for enzymatic pretreatment of urines. Immunoaffinity columns AflaTest® WBSR and DONTest™ (Vicam®, from Ruttmann, Hamburg, Germany) were used for sample clean-up and enrichment of the target analytes.
Study areas and study subjects
In total, 154 urine samples were collected from Bangladeshi infants and children in Rajshahi (33 infants and 55 children) and Dhaka (16 infants and 50 children) district. Urine samples were collected between January and February 2014, a winter period in Bangladesh. In Rajshahi district, urines were obtained in rural areas (Mongol Para, Bhatpara, Habibpur and Jahubona) under Puthia Upazila. In Dhaka district, urines were obtained in rural and suburban areas (Nalam and Dhamsona) under Savar Upazila. Infants aged 1–12 months and children aged 1–6 years were included in the study if they were in good health. Demographic (age, sex) and anthropometric (height, weight) data were recorded in a brief questionnaire form. Parents or guardians were informed about the study aims and signed the consent form on behalf the participants. Urine collection containers (pots of 30 mL) and written instructions were given to the participants (parents/guardians) before the day of sample collection. On the next day, morning urine samples were collected from the participant’s house. Some urine samples for which information was incomplete were excluded from the study. The collected urine samples were first stored at −20 ℃ at the laboratory of Biochemistry Department of Gonoshasthaya Samaj Vittik Medical College, Dhaka and sent on dry ice to IfADo, Dortmund for subsequent analysis. The Institute of Biological Sciences of Rajshahi University, Bangladesh and the institutional Internal Review Board of IfADo approved the study.
Sample preparation
Sample preparation of all urines for AFM1 biomarker analysis was done as described earlier (Ali et al. 2017). In brief, after centrifugation, 5 mL urine aliquots were adjusted to a pH between 5.5 and 7.0 with 1 N hydrochloric acid or 1 M sodium hydroxide. Then the urine was loaded on a AflaTest® WBSR column at a flow rate of 1 drop/s. The columns were washed twice with 5 mL of distilled H2O, then AFM1 was eluted (flow rate 1 drop/s) with 2 mL of methanol. Then eluates were evaporated to dryness under a stream of nitrogen at 45 ℃, and the residue was reconstituted in 250 µL of acetonitrile/water (25:75). Thus, the analyte enrichment factor was 20.
Due to limited volumes available, the remaining 120 urines (of 26 infants and 94 children) were prepared for DON biomarker analysis. Urine clean-up and enrichment of analytes were done by a slight modification of the procedure used previously (Ali et al. 2015a, b, 2016a). Briefly, 1.5 mL of each urine aliquot was hydrolyzed to cleave DON and DOM-1 conjugates by adding 125 µL of hydrolysis buffer and 20 µL of β-Gluc/ArylS enzyme and incubated overnight at 37 ℃ before sample extraction by immunoaffinity columns. Each column was rinsed with 1 mL of water and the hydrolyzed urine sample was loaded onto a DONTest™ column at a flow rate of 1 drop/sec. Then, the column was washed with 3 mL of distilled water and aglycone analytes were eluted (flow rate 1 drop/sec) from the column with 2 mL methanol. Elutes were evaporated to dryness under a stream of nitrogen at 45 ℃; the residues were dissolved in 250 µL water/methanol (90:10), vortexed and filtered through 0.45 µm pore size PTFE syringe filters before LC–MS/MS analysis. Thus, the enrichment factor was 6.
Biomarker analysis
AFM1 was determined in urine by HPLC-FD following our previously established method (Ali et al. 2017) on an HPLC Shimadzu system consisting of two LC-10AS pumps, RF-10Axl fluorescence detector, SIL-10AD, Vp auto injector, CBM-20A communication module, and Shimadzu LC solution software. A C18 Microsorb-MV100 column (150 × 4.6 mm, 5 µm, from Agilent Technologies, Waldbronn, Germany) fitted with a C18 Metaguard column (10 × 4.6 mm, Microsorb A104MG) was used. The injection volume was 80 µL, and chromatographic separation was achieved by isocratic elution with mobile phase 25% acetonitrile and 75% water at a column temperature of 25 ℃ and a flow rate of 1 mL/min. The fluorescence detector was set at 360 nm excitation and 440 nm emission wavelengths; the retention time of AFM1 was 7.6 min. The limit of detection (LOD) was 1.7 pg/mL and limit of quantification (LOQ) was 5 pg/mL for AFM1. Recovery of the analyte from urine was about 90%.
Urinary levels of DON and its metabolite DOM-1 were determined by liquid chromatography with tandem mass spectrometry with a previously in-house validated method (Ali et al. 2015b, 2016a). In brief, chromatographic separation was carried out at 25 ℃ on a Nucleosil® C18 column (100–5 material, 125 × 3 mm) with water (mobile phase A) and methanol (mobile phase B) as eluents. LC–MS/MS analysis was done on a Varian 1200-L Quadrupole MS/MS equipped with an electrospray ionization (ESI) source and a Prostar® Varian HPLC system and Varian MS Workstation. DON was monitored by the transitions of m/z 295.1 → 265.1 and 295.1 → 138.1 and DOM-1 of m/z 279.1 → 248.9 and 279.1 → 231.1. The isotope labeled internal standard ([13C15] DON) was used in all quantification steps. The LODs were 0.16 ng/mL and 0.10 ng/mL for DON and DOM-1, and LOQs were 0.30 ng/mL and 0.20 ng/mL for DON and DOM-1. Recoveries for DON and DOM-1 from urine were about 92% and 85%, respectively.
Creatinine analysis
Urinary creatinine levels were measured by a modified Jaffe method on a 96 well plate reader from TecanGenios (Blaszkewicz and Liesenhoff-Henze 2012) to account for variability in urine dilution between individual samples. Biomarker levels determined in pg/mL (for AFM1) or ng/mL (for DON) were adjusted for creatinine in urines and their concentrations expressed as pg/mg creatinine or ng/mg creatinine, respectively, to facilitate the comparison with some biomarker data published previously.
Exposure assessment
The dietary DON intake was estimated based on results of the urinary DON analysis. The following equation was used to assess the probable daily intake (PDI) of DON among the participants
With C = biomarker concentration (DON µg/L), V = daily urine excretion (L), W = body weight (kg) and E = excretion rate (%). In the calculation, 24 h urinary output was assumed to be 0.5 L for children aged up to 6 years (Gong et al. 2015; Wang et al. 2019). The daily urinary DON excretion rate of 68% (Warth et al. 2013) was used, a value slightly lower than that used by others (Turner et al. 2010). The DON intake estimates (PDI) were then compared to the provisional tolerable daily intake (PMTDI) value of 1 µg/kg bw set by scientific advisory committees (JECFA 2011; EFSA 2017) to assess the risk of DON exposure.
Statistical analysis
The software IBM SPSS version 23 was used to analyses the data. Descriptive analysis was done to determine mean, median and interquartile ranges of the analytes. Urines containing the analyte levels ≥ LOD were used in determining the mean and median values. Differences in biomarker concentrations between the infant and children cohorts, or regions were analyzed by independent sample t-test. Pearson’s correlation coefficient test (two-tailed) was applied to evaluate the correlation between biomarker levels with age and anthropometric variables. The box plot represents the distribution of central data where upper and lower limits of the box indicate 25th and 75th percentiles, respectively, and the line inside the box indicate the median value. The p-value lower than 0.05 is considered statistically significant.
Results
Characteristics of the study subjects
The basic characteristics of the study subjects are shown in Table 1. In total, 49 infants (35 males and 14 females) and 105 children (59 males and 46 females) were enrolled in the present study. The mean ages were 7.1 ± 3.7 and 37.5 ± 16.5 months for infants and children, respectively. The average height and weight were 62.4 ± 8.1 cm and 7.4 ± 2.2 kg, respectively for infants. In children, the average height and weight were 87.5 ± 13.9 cm and 12.8 ± 3.5 kg, respectively. The mean creatinine concentration in children urines (0.43 ± 0.29 g/L) was significantly (p < 0.01) higher than in the infant (0.13 ± 0.12 g/L) urines.
AFM1 and DON biomarker levels in urine samples
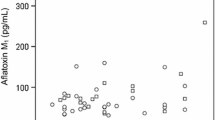
Results of our biomarker analysis in infants and children are given in Table 2 as non-adjusted and creatinine-adjusted urinary concentrations of AFM1 and DON. The overall AFM1 detection frequency was 43.5%, with 34.7% in infants and 47.6% in children urines from two regions. There was no significant difference in the mean level of AFM1 between all infants (9.1 ± 14.3, max 55.6 pg/mL) and children (8.8 ± 12.9, max 75.3 pg/mL) urines. When comparing region, the mean concentration of urinary AFM1 in all (infant and children) samples was slightly higher in Dhaka (9.4 ± 12.4) compared to Rajshahi (8.5 ± 13.9 pg/mL) district, although the difference was not significant. For infant urines, the mean AFM1 level was significantly higher in Dhaka (15.4 ± 19.5 pg/mL) than in Rajshahi (3.6 ± 1.4 pg/mL) district (p < 0.05), but not significant for children (mean 10.1 ± 15.8 in Dhaka and 7.4 ± 8.4 pg/mL in Rajshahi). Yet, the inter-individual variability of AFM1 in all infant and children groups is high, as depicted in Fig. 1 (left panel).
Box plots for urine levels of AFM1 and DON in infants and children from two regions in Bangladesh. Only positive samples (analyte ≥ LOD) are included in the graph. *p < 0.05 when AFM1 level in infant cohort of Dhaka district is compared to Rajshahi district. p-value is obtained from independent sample t-test
The prevalence of the other mycotoxin biomarker was rather low: DON was detected in 11.5% of all infants and in 39.4% of all children urines, whilst DOM-1 was not detectable in any of the samples. The average DON concentration was about 2-fold higher in infants (3.8 ± 2.9, max 6.8 ng/mL) than in children (1.6 ± 1.8, max 8.6 ng/mL) urines. As for region comparison, the mean urinary DON level was higher in Rajshahi (2.1 ± 2.3 ng/mL) than in Dhaka (1.4 ± 1.6 ng/mL) district samples. But, differences among groups were not statistically significant, also due to considerable inter-individual variability in biomarker levels (see Fig. 1, right panel).
Estimated dietary DON intake based on urinary analysis
The probable daily DON intake was calculated for the study subjects based on individual data of urine biomarker analysis and some additional parameters (see Methods section). In the entire study cohort, the mean daily DON intake was 34.8 ± 88.1 ng/kg bw, with 29.6 ± 108.3 ng/kg bw in infants and 36.4 ± 81.8 ng/kg bw in children in both regions (Table 3). Participants in Rajshahi district had a slightly higher calculated daily DON intake (36.5 ± 102.8 ng/kg bw) than those in Dhaka district (32.5 ± 63.0 ng/kg bw), and the highest DON intake reached 560 ng/kg bw. But, none of the subjects had an estimated DON intake that exceeds the provisional maximal tolerable daily intake (PMTDI) of 1 µg/kg bw set by scientific committees (JECFA 2011; EFSA 2017) for DON and its modified forms.
Discussion
Data on the contamination of food commodities with Aspergillus, Penicillium and Fusarium mycotoxins are rather scarce for Bangladesh. To gain some insight into potential risks related to these dietary contaminants, we have previously investigated the occurrence of biomarkers of exposure to major mycotoxins in the adult population of this country (Ali et al. 2014, 2015a, 2016a, 2016b, 2019; b; Gerding et al. 2015). The present study is aimed to explore the exposure of infants and young children to AFB1 and DON in Bangladesh. Children are considered as vulnerable group with increased susceptibility to chemicals, including mycotoxins (Makri et al. 2004; Sherif et al. 2009; Lombard et al. 2014). As outlined in the Introduction, the toxic properties of AFB1 and DON are quite different, the first being primarily known as potent mutagenic carcinogen, and DON (vomitoxin) for adverse effects in the gastrointestinal tract, reduced weight gain and impaired immune function. Child stunting is an emerging topic in the field of aflatoxin-related health outcomes (IARC 2015; FAO/WHO 2018; EFSA 2020); DON exposure may exacerbate this condition, independent of and along with other risk factors (Lombard et al. 2014).
The results of our biomarker analysis, the first for DON in Bangladeshi infants and children, now indicate moderate prevalence of exposure to the trichothecene mycotoxin, whilst the prevalence of AFM1 in their urines indicates quite frequent intake of AFB1 in this vulnerable part of the population (Table 2). Before discussing implications of the new results and possible sources of dietary intake, a remark is appropriate on the approach used to’translate’ biomarker data to exposure-related risks: A high renal excretion rate (68–70% within a day; Warth et al. 2013, Turner et al. 2010) enables biomarker-based estimates for DON exposure, and these values are then compared to the tolerable daily intake value set for this mycotoxin (see Table 3). However, only a small fraction of AFB1 is excreted as AFM1 in urine (1.5–2%; Zhu et al. 1987) which hampers a reliable back-calculation of biomarker levels to dietary AFB1 intake. Yet, one can compare new data on prevalence and urinary levels of AFM1 to results reported from other regions of the world where it served to investigate aflatoxin exposure of children.
Regarding DON biomarker analysis in urine, the detection frequency in the present study (33.3%) is close to that determined in the adult cohort in Rajshahi district (27% in summer and 31% in winter) and a pregnant women cohort in Dhaka (52%) district (Ali et al. 2015b, 2016a). But, the DON concentration in infants and children (mean 1.7, max 8.6) is higher than that found in the adults (mean 0.17, max 1.78 ng/mL in summer and mean 0.16, max 1.21 in winter) and in pregnant women (mean 0.86, max 7.16). The higher DON exposure of young children can be related to higher food consumption per kg body weight than adults and/or children preferring foods such as breads and cookies made from wheat which are more likely contaminated with DON than the typical staple food rice. Yet, no individual of the Bangladesh low age groups exceeds the tolerable daily intake value set for DON (Table 3).
It is also of interest to compare DON biomarker levels in this study to data reported in children from some other countries (Table 4). Exposure to DON in Bangladesh is clearly lower than in two regions of China where 10–73% of the cohorts exceed the TDI (Wang et al. 2019), or in European cohorts: Belgium with 69% above TDI (Heyndrickx et al. 2015), Italy with 25–27.5% above TDI (De Santis et al. 2019), Norway with 20% above TDI (Brera et al. 2015), and the UK, with 33–63% above the TDI (Papageorgiou et al. 2018). In Africa, children in Cameroon have apparently lower DON exposure (Ediage et al. 2013) than those in Tanzania geometric mean 2.5 ng/mL, with 21–54% above TDI in one (Srey et al. 2014) or more in another study (Gong et al. 2015). DON has been detected now in urines of breastfed and non-exclusively breastfed infants from Nigeria (Ezekiel et al 2020). DON was also found in urines from children and adults in Haiti (Gerding et al. 2015), at levels similar to those found in Swedish children (Mitropoulou et al. 2018). DOM-1, a detoxication product of DON, was not detected in our participants, and also not found in children samples from Italy or the UK, but in some urines of Norwegian children (Brera et al. 2015).
These biomonitoring studies (Table 4) show a wide range of DON exposures in the pediatric population of different countries, but also differences between regions of a country, as in China (Wang et al. 2019). The extent of DON exposure can be explained in part by differences in dietary habits: food items such as wheat bread, pasta, breakfast cereals, bran rolled flakes and baked goods are major sources of DON exposure in European populations (Brera et al. 2015; EFSA 2017), but these foods are far less often consumed in Bangladesh. Biomarker levels not only differ between countries, but also between years and season (Gratz et al. 2014; Ali et al. 2016a). This reflects the quite variable DON contamination of many crops worldwide (Mishra et al. 2020). Thus, one should keep in mind that biomonitoring sheds a light on the DON exposure in a given cohort and sampling season, and should be followed up, in particular when data indicate exceedance of TDI-values in vulnerable groups (Papageorgiou et al. 2018).
Regarding AFM1 analysis, the biomarker has been frequently detected (43.5% in the entire cohort), with 34.7% in infants and 47.6% in children urines from two regions (Table 2), and with a high inter-individual variability in AFM1 levels of all groups (Fig. 1). The new results are first compared with data in Bangladeshi adults and then with findings for children of other countries. The AFM1 urine concentrations in infants (mean 9.1, max 55.6 pg/mL) and children (mean 8.8, max 75.3 pg/mL) are somewhat lower than those measured in the adult (13.5 pg/mL, max 104 pg/mL in summer and 27.7 pg/mL, max 189.9 pg/mL in winter) and pregnant women (13.9 pg/mL, max 141.5 pg/mL) cohorts of this country (Ali et al. 2017). These biomarker data suggest that foods consumed by adults and by children contain notable levels of aflatoxin B1, whilst the sources of mycotoxin intake may differ between both groups.
Urine AFM1 levels in young Bangladeshi children are far lower than levels found in children of some African countries (Table 5), i.e. in Cameroon (Ediage et al. 2013), Guinea (Polychronaki et al. 2008), Nigeria (Ezekiel et al. 2020, 2018; Sarkanj et al. 2018), Sierra Leone (Jonsyn-Ellis 2001) and Tanzania (Chen et al. 2018). However, the AFM1 mean level in our cohort is higher than that reported in children urines in Egypt (Polychronaki et al. 2008), and it approaches levels found in Ethiopia (Ayelign et al. 2017). Also, the average Bangladeshi values are in a similar range as those found in children in Colombia, South America (Sanchez and Diaz 2019). The children data (Table 5), and a previous overview of AFM1 biomarker data for adult cohorts of different continents and countries (Ali et al. 2017) illustrate the wide range of exposure to AFB1. Again, exposure reflects different dietary habits in these populations and also different degrees of aflatoxin contamination in the crops and foods locally produced and consumed. For instance, in Africa maize and groundnuts continue to be the main sources of aflatoxin exposure (Gong et al. 2018; Xu et al. 2018).
So far there are only two studies on aflatoxin occurrence in food commodities in Bangladesh. Roy et al. (2013) analyzed AFB1 in rice, lentils, wheat flour, dates, betelnut, red chilli powder, ginger and groundnuts: mean levels in 5 of these 8 commodities were above EU regulatory limits, and the highest levels were found in dates and groundnuts. Bhuiyan et al. (2013) analyzed total aflatoxins in maize, rice and wheat samples collected in all districts of Bangladesh at six times during a year: the highest incidence and level of contamination was found in maize; the incidence and contaminant level were lower, yet still significant in rice and in wheat, all showing considerable seasonal variability. Both datasets have been used by others to calculate total aflatoxin levels in various food commodities in Bangladesh (Table 1 in Saha Turna and Wu 2019). Then, considering also average dietary consumption data for each of these items, they assessed aflatoxin exposure by dates, groundnuts, lentils, chili/spices, wheat, maize and rice (Table 2 in Saha Turna and Wu 2019). The highest contribution to total aflatoxin exposure was from rice. Rice is the main dietary staple in Bangladesh, and often consumed with curries prepared with several spices. Previously, we noted higher urine AFM1 levels in adult people who consume more rice per day (Ali et al. 2017) which points also to rice as one important source of AFB1 intake (Ali 2019). In their paper Saha Turna and Wu (2019) comment also on the high AFB1 levels in dates, which are consumed mainly during Ramadan in Bangladesh and other Muslim countries. We suggest that along with rice, wheat-based bakery products, also dates may be a relevant source of AFB1 intake as young children prefer sweet types of food. Furthermore, a recent screening of cow milk and milk products by ELISA reveals frequent occurrence of AFM1 (Ali et al. unpublished results). As young children are fond of milk and milk based products, this may have also contributed to AFM1 exposure in our cohort.
AFB1 exposure has been investigated before in another region of Bangladesh (a rural area in Rangpur) by means of the AFB1-lysine albumin adduct analysis in blood samples of pregnant women and later on in their 2-year-old children (Groopman et al. 2014). Median levels of this biomarker were 25.35 and 18.08 pg AFB1-Lys/mg albumin in the first and third trimester, respectively, and 13.79 pg AFB1-Lys/mg albumin in the children. These results, discussed by the authors in the context of biomarker data for cohorts in other countries, document rather high AFB1 exposures of their cohort in the Northwest of Bangladesh between 2008 and 2012. A recent longitudinal study in an urban slum in Dhaka city assessed AFB1 exposure of children at the age of 7, 15, 24 and 36 months and reported a geometric mean of 1.07 pg AFB1-Lys/mg albumin and a range of 0.04–123.5 pg AFB1-Lys/mg albumin (Mahfuz et al. 2019). In this study, a reduction in breastfeeding prevalence, with concomitant introduction of family food, corresponded with an increase in AFB1-lysin adduct detection at 36 months, and 62% of the children were exposed at the end of their 3rd year of life. Of interest is also the seasonal variation in AFB1 biomarker prevalence, with the highest detection observed during and after the monsoon period which provides optimal conditions for fungal growth and aflatoxin contamination (Mahfuz et al. 2019).
Overall, the results of our study with determination of AFM1 metabolite in urine, and the two studies (Groopman et al. 2014; Mahfuz et al. 2019) that measured AFB1-lysin albumin adduct in blood plasma, document widespread exposure of young children in several parts of Bangladesh. This and previous data on frequent AFB1 exposure of adult and pregnant women cohorts (Ali et al. 2017; Groopman et al. 2014) raise concern with regard to dietary intake of this carcinogenic mycotoxin in the Bangladeshi population. Further efforts to analyse aflatoxin contamination are clearly needed to identify major sources of aflatoxin intake, and establish surveillance in food commodities with the aim to protect the population against long-term adverse health effects. Bangladesh has issued in mid 2017 regulation for aflatoxin contamination of certain food items, namely groundnuts, almonds, Brazil nuts, hazelnuts, pistachios and AFM1 in milk (BFSA 2017). Yet, a recent risk assessment of aflatoxin-related liver cancer in Bangladesh concluded that the new regulations are unlikely to significantly reduce the risk of this cancer in the country (Saha Turna and Wu 2019). Indeed, considering food contaminant data available (vide supra), cereal-based commodities including rice, as well as pulses, spices and other items are likely to contribute far more to overall AFB1 exposure than various types of nuts. Thus, we recommend to conduct regular surveys on aflatoxin contamination, at least in major staples (Ali 2019), and consider also further biomonitoring as this integrates human exposure from all sources.
Conclusion
This study applied sensitive biomonitoring methods to assess for the first time aflatoxin and DON exposure among infant and children cohorts in Bangladesh. DON exposure appears to be of low concern, with intake estimates below tolerable levels. But, the prevalence and levels of AFM1 in infant and children urines indicate widespread contamination of the children’s diets with the carcinogenic mycotoxin AFB1, a finding which raises serious health concerns for this vulnerable population. Continuous surveillance of aflatoxins in Bangladeshi food commodities are urgently needed, in order to identify major sources of intake, and then take appropriate steps to further reduce risks from exposure-related adverse health effects.
Notes
Scientific bodies evaluate dose–response relationships for the most critical effect observed in animal studies. Then considering also mode of action they derive tolerable daily intake values by a margin of safety approach (e.g. for DON) whereas a margin of exposure approach is used for mutagenic carcinogens like aflatoxins (details in FAO/WHO 2018; JECFA 2011; EFSA 2017; EFSA 2020).
References
Al-Jaal BA, Jaganjac M, Barcaru A et al (2019) Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: a systematic literature review, 2001–2018. Food Chem Toxicol 129:211–228. https://doi.org/10.1016/j.fct.2019.04.047
Ali N (2019) Aflatoxins in rice: worldwide occurrence and public health perspectives. Toxicol Rep 6:1188–1197. https://doi.org/10.1016/j.toxrep.2019.11.007
Ali N, Degen GH (2019) Biomonitoring of zearalenone and its main metabolites in urines of Bangladeshi adults. Food Chem Res 130:276–283. https://doi.org/10.1016/j.fct.2019.05.036
Ali N, Blaszkewicz M, Manirujjaman M, Perveen R, Al Nahid A, Mahmood S, Rahman M, Hossain K, Degen GH (2014) Biomonitoring of ochratoxin A and exposure assessment of adult students in Bangladesh. Mol Nutr Food Res 58:2219–2225. https://doi.org/10.1002/mnfr.201400403
Ali N, Blaszkewicz M, Degen GH (2015a) Occurrence of the mycotoxin citrinin and its metabolite dihydrocitrinone in urines of German adults. Arch Toxicol 89:573–578. https://doi.org/10.1007/s00204-014-1363-y
Ali N, Blaszkewicz M, Al Nahid A et al (2015b) Deoxynivalenol exposure assessment for pregnant women in Bangladesh. Toxins 7:3845–3857. https://doi.org/10.3390/toxins7103845
Ali N, Blaszkewicz M, Degen GH (2016a) Assessment of deoxynivalenol exposure among Bangladeshi and German adults by a biomarker-based approach. Toxicol Lett 258:20–28. https://doi.org/10.1016/j.toxlet.2016.06.006
Ali N, Blaszkewicz M, Hossain K, Degen GH (2017) Determination of aflatoxin M1 in urine samples indicates frequent dietary exposure to aflatoxin B1 in the Bangladeshi population. Int J Hyg Environ Health 220:271–281. https://doi.org/10.1016/j.ijheh.2016.11.002
Ali N, Hossain K, Blaszkewicz M et al (2016b) Occurrence of aflatoxin M1 in urines from rural and urban adult cohorts in Bangladesh. Arch Toxicol 90:1749–1755. https://doi.org/10.1007/s00204-015-1601-y
Alizadeh A, Braber S, Akbari P et al (2015) Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 7:2071–2095. https://doi.org/10.3390/toxins7062071
Ayelign A, Woldegiorgis AZ, Adish A et al (2017) Assessment of aflatoxin exposure among young children in Ethiopia using urinary biomarkers. Food Addit Contam Part A 34:1606–1616. https://doi.org/10.1080/19440049.2017.1350290
BFSA (2017) Bangladesh Food Safety Authority; Food safety regulations. Available at: https://www.bfsa.gov.bd/sites/default/files/files/bfsa.portal.gov.bd/law/81690675_9a69_483b_8915_1fe8f6b2ddd0/Food-Safety-(Contaminants,-Toxins-and-Harmful-Residues)-Regulations,-2017.pdf. Accessed 8 June 2020
Bhuiyan MNH, Hassan MT, Begum M et al (2013) Occurrence and seasonal trends of aflatoxin in rice, maize and wheat in Bangladesh. Int J Sustain Agril Tech 9:8–14
Blaszkewicz M, Liesenhoff-Henze K (2012) Creatinine in urine [Biomonitoring Methods, 2010]. In: Deutsche Forschungsgemeinschaft, Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (eds) The MAK-Collection for Occupational Health and Safety. Wiley-VCH Verlag GmbH and Co. KGaA, Weinheim, Germany 169–184
Brera C, de Santis B, Debegnach F et al (2015) Experimental study of deoxynivalenol biomarkers in urine. EFSA Support Publ EN-818. https://doi.org/10.2903/sp.efsa.2015.EN-818
Chen G, Gong YY, Kimanya ME et al (2018) Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for assessing aflatoxin exposure in Tanzanian children. Biomarkers 23:131–136. https://doi.org/10.1080/1354750X.2017.1285960
Dawlatana M, Coker RD, Nagler MJ, Wild CP, Hassan MS, Blunden G (2002) The occurrence of mycotoxins in key commodities in Bangladesh: surveillance results from 1993 to 1995. J Nat Toxins 11:379–386
De Santis B, Debegnach F, Miano B et al (2019) Determination of deoxynivalenol biomarkers in Italian urine samples. Toxins 11:441. https://doi.org/10.3390/toxins11080441
Degen G (2011) Tools for investigating workplace-related risks from mycotoxin exposure. World Mycotoxin J 4:315–327. https://doi.org/10.3920/WMJ2011.1295
Ediage EN, Di Mavungu JD, Song S et al (2013) Multimycotoxin analysis in urines to assess infant exposure: a case study in Cameroon. Environ Int 57:50–59
EFSA (2017) CONTAM Panel: scientific opinion on the risk to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J 15:4718. https://doi.org/10.2903/j.efsa.2017.4718
EFSA (2020) CONTAM Panel: scientific opinion—risk assessment of aflatoxins in food. EFSA J 18(3):6040. https://doi.org/10.2903/j.efsa.2020.6040
Elzupir AO, Alamer AS, Dutton MF (2015) The occurrence of aflatoxins in rice worldwide: a review. Toxin Reviews 34:37–42. https://doi.org/10.3109/15569543.2014.984229
Escola M, Kos G, Elliott CT et al (2019) Worldwide contamination of food crops with mycotoxins: validity of the widely cited ‘FAO estimate’ of 25%. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2019.1658570
Ezekiel CN, Abia WA, Braun D, Šarkanj B et al (2020) Comprehensive mycotoxin exposure biomonitoring in breastfed and non-exclusively breastfed Nigerian children. medRxiv. https://doi.org/10.1101/2020.05.28.20115055
Ezekiel CN, Oyeyemi OT, Oyedele OA et al (2018) Urinary aflatoxin exposure monitoring in rural and semi-urban populations in Ogun state, Nigeria. Food Addit Contam Part A 35:1565–1572. https://doi.org/10.1080/19440049.2018.1475752
FAO/WHO (2018) Food and Agriculture Organization of the United Nations/World Health Organization. Aflatoxins. Safety evaluation of certain contaminants in food: prepared by the 83rd meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Additives Series, No 74: 2–280; available at: https://apps.who.int/iris/bitstream/handle/10665/276868/9789241660747-eng.pdf?ua=1. Accessed 8 June 2020
Gerding J, Ali N, Schwartzbord J et al (2015) A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res 31:127–136. https://doi.org/10.1007/s12550-015-0223-9
Gong Y, Hounsa A, Egal S et al (2004) Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ Health Perspect 112:1334–1338. https://doi.org/10.1289/ehp.6954
Gong YY, Shirima CP, Srey C et al (2015) Deoxynivalenol and fumonisin exposure in children and adults in a family study in rural Tanzania. World Mycotoxin J 8:553–560. https://doi.org/10.3920/WMJ2015.1878
Gong YY, van Rensburg BJ, van Kimanya M, Egmond HP (2018) Foreword WMJ Special Issue ‘Mycotoxins in Africa’. World Mycotoxin J 11:305–509
Gratz SW, Richardson AJ, Duncan G, Holtrop G (2014) Annual variation of dietary deoxynivalenol exposure during years of different Fusarium prevalence: a pilot biomonitoring study. Food Addit Contam Part A 31:1579–1585
Groopman JD, Egner PA, Schulze KJ et al (2014) Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B1-lysine albumin biomarkers. Food Chem Toxicol 74:184–189
Groopman JD, Kensler TW, Wild CP (2008) Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health 29:187–203. https://doi.org/10.1146/annurev.publhealth.29.020907.090859
Heyndrickx E, Sioen I, Huybrechts B et al (2015) Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environ Int 84:82–89
IARC International Agency for Research on Cancer (2012) Aflatoxins. Chemical agents and related occupations: IARC monographs on the evaluation of the carcinogenic risks of chemicals to humans. IARC Press, Lyon, pp 225–248
IARC (2015) Working Group Report No. 9, Mycotoxin control in low- and middle income countries (Wild CP, Miller JD, Groopman JD., eds); available at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Working-Group-Reports/Mycotoxin-Control-In-Low--And-Middle-income-Countries-2015. Accessed 8 June 2020
JECFA (2011) Evaluation of certain contaminants in food. 72nd report of the joint FAO/WHO expert committee on food additives. WHO technical report series. WHO, Geneva
Jonsyn-Ellis FE (2001) Seasonal variation in exposure frequency and concentration levels of aflatoxins and ochratoxins in urine samples of boys and girls. Mycopathologia 152:35–40
Liu Y, Wu F (2010) Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ Health Perspect 118:818–824. https://doi.org/10.1289/ehp.0901388
Lombard MJ (2014) Mycotoxin exposure and infant and young child growth in Africa: what do we know? Ann Nutr Metab 64:42–52. https://doi.org/10.1159/000365126
Mahfuz M, Alam MA, Fahim SM et al (2019) Aflatoxin exposure in children living in Mirpur, Dhaka: data from MAL-ED companion study. J Expo Sci Environ Epidemiol 29:655–662. https://doi.org/10.1038/s41370-018-0066-5
Makri A, Goveia M, Balbus J, Parkin R (2004) Children’s susceptibility to chemicals: a review by developmental stage. J Toxicol Environm Health B Crit Rev 7:417–435
Mishra S, Srivastava S, Dewangan J, Divakar A, Rath SK (2020) Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: a survey. Crit Rev Food Sci Nutr 60(8):1346–1374. https://doi.org/10.1080/10408398.2019.1571479
Mitchell NJ, Kumi J, Johnson NM et al (2013) Reduction in the urinary aflatoxin M1 biomarker as an early indicator of the efficacy of dietary interventions to reduce exposure to aflatoxins. Biomarkers 18:391–398
Mitropoulou A, Gambacorta L, Warrensjö Lemming E, Solfrizzo M, Olsen M (2018) Extended evaluation of urinary multi-biomarker analyses of mycotoxins in Swedish adults and children. World Mycotoxin J 11:647–659
Papageorgiou M, Wells L, Williams C et al (2018) Assessment of urinary deoxynivalenol biomarkers in UK children and adolescents. Toxins 10:50. https://doi.org/10.3390/toxins10020050
Pestka JJ (2010) Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84(9):663–679
Polychronaki N, Wild CP, Mykkänen H et al (2008) Urinary biomarkers of aflatoxin exposure in young children from Egypt and Guinea. Food Chem Toxicol 46:519–526. https://doi.org/10.1016/j.fct.2007.08.034
Roy M, Harris J, Afreen S et al (2013) Aflatoxin contamination in food commodities in Bangladesh. Food Addit Contam Part B 6:17–23
Rushing BR, Selim MI (2019) Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxication methods. Food Chem Toxicol 124:81–100
Saha Turna N, Wu F (2019) Risk assessment of aflatoxin-related liver cancer in Bangladesh. Food Addit Contam Part A 36:320–326. https://doi.org/10.1080/19440049.2019.1567941
Sanchez EM, Diaz GJ (2019) Frequency and levels of aflatoxin M1 in urine of children in Bogota, Colombia. Mycotoxin Res 35:271–278
Sarkanj B, Ezekiel C, Turner PC et al (2018) Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal Chim Acta 1019:84–92
Sherif SO, Salama EE, Abdel-Wahaab MA (2009) Mycotoxins and child health: the need for health risk assessment. Int J Hyg Environ Health 212:347–368
Solfrizzo M, Gambacorta L, Warth B et al (2013) Comparison of single and multi-analyte methods based on LC-MS/MS for mycotoxin biomarker determination in human urine. World Mycotoxin J 6:355–366. https://doi.org/10.3920/WMJ2013.1575
Srey C, Kimanya ME, Routledge MN et al (2014) Deoxynivalenol exposure assessment in young children in Tanzania. Mol Nutr Food Res 58:1574–1580
Sudakin DL (2003) Trichothecenes in the environment: relevance to human health. Toxicol Lett 143:97–107. https://doi.org/10.1016/s0378-4274(03)00116-4
Sun Z, Chen T, Thorgeirsson SS et al (2013) Dramatic reduction of liver cancer incidence in young adults: 28-year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 34:1800–1805. https://doi.org/10.1093/carcin/bgt007
Turner PC (2013) The molecular epidemiology of chronic aflatoxin driven impaired child growth. Scientifica 2013:1–21. https://doi.org/10.1155/2013/152879
Turner PC, Flannery B, Isitt C, Ali M, Pestka J (2012) The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev 25:162–179
Turner PC, Hopton RP, White KLM et al (2011) Assessment of deoxynivalenol metabolite profiles in UK adults. Food Chem Toxicol 49:132–135. https://doi.org/10.1016/j.fct.2010.10.007
Turner PC, Moore SE, Hall AJ et al (2003) Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ Health Perspect 111:217–220. https://doi.org/10.1289/ehp.5753
Turner PC, Rothwell JA, White KLM et al (2008) Urinary deoxynivalenol is correlated with cereal intake in individuals from the United Kingdom. Environ Health Perspect 116:21–25. https://doi.org/10.1289/ehp.10663
Turner PC, White KL, Burley VJ et al (2010) A comparison of deoxynivalenol intake and urinary deoxynivalenol in UK adults. Biomarkers 15:553–562
Van Egmond HP, Schothorst RC, Jonker MA (2007) Regulations relating to mycotoxins in food: perspectives in a global and European context. Ann Bioanal Chem 389:147–157
Wang X, Liang J, Cao P et al (2019) Biomonitoring study of deoxynivalenol exposure in Chinese inhabitants. Int J Environ Res Public Health 16:2169. https://doi.org/10.3390/ijerph16122169
Warth B, Sulyok M, Berthiller F et al (2013) New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol Lett 220:88–94
Warth B, Sulyok M, Fruhmann P et al (2012) Assessment of human deoxynivalenol exposure using an LC–MS/MS based biomarker method. Toxicol Lett 211:85–90. https://doi.org/10.1016/j.toxlet.2012.02.023
Wild CP, Turner PC (2002) The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 17:471–481. https://doi.org/10.1093/mutage/17.6.471
Williams JH, Phillips TD, Jolly PE et al (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 80:1106–1122. https://doi.org/10.1093/ajcn/80.5.1106
Xu Y, Gong YY, Routledge M (2018) Aflatoxin exposure assessed by aflatoxin albumin adduct biomarker in populations from six African countries. World Mycotoxin J 11:411–419
Zhu JQ, Zhang LS, Hu X et al (1987) Correction of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res 47:1848–1852
Acknowledgements
This work was supported by a DAAD scholarship to Nurshad Ali. The authors are grateful to all caretakers of children for participating in this study. We also thank Iris Glaeser and Gabi Baumhoer for their excellent technical assistance.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, N., Manirujjaman, M., Rana, S. et al. Determination of aflatoxin M1 and deoxynivalenol biomarkers in infants and children urines from Bangladesh. Arch Toxicol 94, 3775–3786 (2020). https://doi.org/10.1007/s00204-020-02857-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02857-5