Abstract
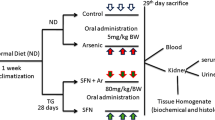
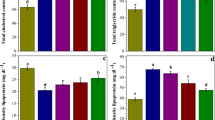
Arsenic trioxide (As2O3) is an environmental toxicant and a potent antineoplastic agent. Exposure to arsenic causes renal cancer. Resveratrol is a well-known polyphenolic compound that is reported to reduce As2O3-induced cardiotoxicity. The present study aimed to investigate the effect of resveratrol on As2O3-induced nephrotoxicity and arsenic metabolism. Chinese Dragon-Li cats were injected with 1 mg/kg As2O3 on alternate days; resveratrol (3 mg/kg) was administered via the forearm vein 1 h before the As2O3 treatment. On the sixth day, the cats were killed to determine the histological renal damage, renal function, the accumulation of arsenic, and antioxidant activities in the kidney. Urine samples were taken for arsenic speciation. In the resveratrol + As2O3-treated group, activities of glutathione peroxidase, catalase, and superoxide dismutase, the ratio of reduced glutathione to oxidized glutathione, the total arsenic concentrations, and the percentage of methylated arsenic in urine were significantly increased. The concentrations of renal malondialdehyde, reactive oxygen species, 8-hydroxydeoxyguanosine, serum creatinine, blood urea nitrogen, and renal arsenic accumulation were significantly decreased and reduced renal morphologic injury was observed compared with the As2O3-treated group. These results demonstrate that resveratrol could significantly scavenge reactive oxygen species, inhibit As2O3-induced oxidative damage, and significantly attenuate the accumulation of arsenic in renal tissues by facilitating As2O3 metabolism. These data suggest that use of resveratrol as postremission therapy for acute promyelocytic leukemia as well as adjunctive therapy in patients with exposure to arsenic may decrease arsenic nephrotoxicity.







Similar content being viewed by others
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- As2O3 :
-
Arsenic trioxide
- As (III):
-
Arsenite
- As (V):
-
Arsenate
- BUN:
-
Blood urea nitrogen
- CAT:
-
Catalase
- CREA:
-
Serum creatinine
- DMA:
-
Dimethylated arsenic
- GPX:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- MDA:
-
Malondialdehyde
- MMA:
-
Monomethylated arsenic
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Aposhian HV (1997) Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol 37:397–419
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5:493–506
Bergquist ER, Fischer RJ, Sugden KD, Martin BD (2009) Inhibition by methylated organo-arsenicals of the respiratory 2-oxo-acid dehydrogenases. J Organomet Chem 694:973–980
Brazy PC, Balaban RS, Gullans SR, Mandel LJ, Dennis VW (1980) Inhibition of renal metabolism. Relative effects of arsenate on sodium, phosphate, and glucose transport by the rabbit proximal tubule. J Clin Invest 66:1211–1221
Cadenas S, Barja G (1999) Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med 26:1531–1537
Chen CJ, Chen CW, Wu MM, Kuo TL (1992) Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 66:888–892
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z (1996) In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood 88:1052–1061
Chen B, Arnold LL, Cohen SM, Thomas DJ, Le XC (2011) Mouse arsenic (+3 oxidation state) methyltransferase genotype affects metabolism and tissue dosimetry of arsenicals after arsenite administration in drinking water. Toxicol Sci 124:320–326
Chen SC, Chen CC, Kuo CY, Huang CH, Lin CH, Lu ZY, Chen YY, Lee HS, Wong RH (2012) Elevated risk of hypertension induced by arsenic exposure in Taiwanese rural residents: possible effects of manganese superoxide dismutase (MnSOD) and 8-oxoguanine DNA glycosylase (OGG1) genes. Arch Toxicol 86:869–878
Chiou HY, Hsueh YM, Hsieh LL, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ (1997) Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res 386:197–207
Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S (1998) Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 92:996–1002
Cojocel C, Thomson MS (2004) Protective effect of resveratrol against 6-hydroxydopamine-induced impairment of renal p-aminohippurate transport. Arch Toxicol 78:525–532
Cui X, Kobayashi Y, Hayakawa T, Hirano S (2004) Arsenic speciation in bile and urine following oral and intravenous exposure to inorganic and organic arsenics in rats. Toxicol Sci 82:478–487
Davison K, Cote S, Mader S, Miller WH (2003) Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 17:931–940
Do Amaral CL, Francescato HD, Coimbra TM, Costa RS, Darin JD, Antunes LM, Bianchi Mde L (2008) Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch Toxicol 82:363–370
Emadi A, Gore SD (2010) Arsenic trioxide—an old drug rediscovered. Blood Rev 24:191–199
Floyd RA, West MS, Eneff KL, Hogsett WE, Tingey DT (1988) Hydroxyl free radical mediated formation of 8-hydroxyguanine in isolated DNA. Arch Biochem Biophys 262:266–272
Fujihara J, Yasuda T, Kato H, Yuasa I, Panduro A, Kunito T, Takeshita H (2011) Genetic variants associated with arsenic metabolism within human arsenic (+3 oxidation state) methyltransferase show wide variation across multiple populations. Arch Toxicol 85:119–125
Giovannini L, Migliori M, Longoni BM, Das DK, Bertelli AA, Panichi V, Filippi C, Bertelli A (2001) Resveratrol, a polyphenol found in wine, reduces ischemia reperfusion injury in rat kidneys. J Cardiovasc Pharm 37:262–270
Giraudel JM, Diquelou A, Laroute V, Lees P, Toutain PL (2005) Pharmacokinetic/pharmacodynamic modelling of NSAIDs in a model of reversible inflammation in the cat. Br J Pharmacol 146:642–653
Hayakawa T, Kobayashi Y, Cui X, Hirano S (2005) A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol 79:183–191
Holthoff JH, Wang Z, Seely KA, Gokden N, Mayeux PR (2012) Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int 81:370–378
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Kala SV, Neely MW, Kala G, Prater CI, Atwood DW, Rice JS, Lieberman MW (2000) The MRP2/cMOAT transporter and arsenic-glutathione complex formation are required for biliary excretion of arsenic. J Biol Chem 275:33404–33408
Kutuk O, Poli G, Basaga H (2006) Resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-JUN/AP-1 signaling. Toxicol Sci 90:120–132
Lia MS, Hsueh YM, Chen CJ, Shyu MP, Chen SY, Kuo TL, Wu MM, Tai TY (1994) Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol 139:484–492
Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP (2000) Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci 55:460–467
Lo JF, Wang HF, Tam MF, Lee TC (1992) Glutathione S-transferase pi in an arsenic-resistant Chinese hamster ovary cell line. Biochem J 288:977–982
Messarah M, Saoudi M, Boumendjel A, Kadeche L, Boulakoud MS, EI Feki A (2012) Green tea extract alleviates arsenic-induced biochemical toxicity and lipid peroxidation in rats. Toxicol Ind Health. doi:10.1177/0748233711433934
Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S (2002) Mechanisms of action of arsenic trioxide. Cancer Res 62:3893–3903
Morales AI, Buitrago JM, Santiago JM, Fernandez-Tagarro M, Lopez-Novoa JM, Perez-Barriocanal F (2002) Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Sign 4:893–898
Sakurai T, Kojima C, Kobayashi Y, Hirano S, Sakurai MH, Waalkes MP, Himeno S (2006) Toxicity of a trivalent organic arsenic compound, dimethylarsinous glutathione in a rat liver cell line (TRL 1215). Br J Pharmacol 149:888–897
Schläwicke Engström K, Broberg K, Concha G, Nermell B, Warholm M, Vahter M (2007) Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Perspect 115:599–605
Schwerdtle T, Walter I, Mackiw I, Hartwig A (2003) Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA. Carcinogenesis 24:967–974
Shankar S, Singh G, Srivastava RK (2007) Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci 12:4839–4854
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255:67–78
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155:109–117
Stern AH (2012) Assessing the threshold for the duodenal GSH/GSSG ratio in the mouse associated with sodium dichromate dihydrate ingestion reported by Thompson et al. (2011). Toxicol Sci 126:285–286
Takeuchi T, Nakajima M, Ohta Y, Mure K, Takeshita T, Morimoto K (1994) Evaluation of 8-hydroxydeoxyguanosine, a typical oxidative DNA damage, in human leukocytes. Carcinogenesis 15:1519–1523
Tennen RI, Michishita-Kioi E, Chua KF (2012) Finding a target for resveratrol. Cell 148:387–389
Thomas DJ, Styblo M, Lin S (2001) The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharm 176:127–144
Vuky J, Yu R, Schwartz L, Motzer RJ (2002) Phase II trial of arsenic trioxide in patients with metastatic renal cell carcinoma. Invest New Drug 20:327–330
Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ (2012) Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol 86:879–896
Waxman S, Anderson KC (2001) History of the development of arsenic derivatives in cancer therapy. Oncologist 6:3–10
Westervelt P, Brown RA, Adkins DR, Khoury H, Curtin P, Hurd D, Luger SM, Ma MK, Ley TJ, DiPersio JF (2001) Sudden death among patients with acute promyelocytic leukemia treated with arsenic trioxide. Blood 98:266–271
Yamanaka K, Okada S (1994) Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ Health Perspect 102:37–40
Zhang WQ, Xue JD, Ge M, Yu ML, Liu L, Zhang ZG (2013) Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol 51:87–92
Zhao XY, Li GY, Liu Y, Chai LM, Chen JX, Zhang Y, Du ZM, Lu YJ, Yang BF (2008) Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Br J Pharmacol 154:105–113
Acknowledgments
We thank the National Science Foundation Committee of China (31101868), Heilongjiang Province Foundation for Young Scholars (QC2010057), Special Foundation of China. Postdoctoral Science Foundation (2012T50302), Chinese Postdoctoral Science Foundation (20100481040), and Heilongjiang Province Postdoctoral Science Foundation (LBH-Z10256).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meiling Yu and Jiangdong Xue contributed equally to this work.
Northeast Agricultural University and Inner Mongolia University for Nationalities have contributed equally in the study.
Rights and permissions
About this article
Cite this article
Yu, M., Xue, J., Li, Y. et al. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol 87, 1025–1035 (2013). https://doi.org/10.1007/s00204-013-1026-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1026-4