Abstract
Objective
To determine perioperative changes in circulating (BVI) and central blood volume (CBVI) by a new dye dilution technique using pulse dye densitometry.
Design and setting
Prospective observational study in the cardiac anesthesia and intensive care unit of a university hospital.
Patients
Sixty-six patients undergoing coronary artery bypass surgery.
Measurements and results
Hemodynamic measurements by the dye dilution method using pulse dye densitometry were performed prior to skin incision and 3.3±1.4 h and 17±2.7 h after surgery. Based on conventional monitoring the therapeutic goals of hemodynamic therapy were achieved in all of the patients of this study. Despite a marked positive fluid balance which developed during surgery mean BVI decreased significantly after surgery while CBVI remained unchanged. Postoperative BVI deficits vs. preoperative values were observed in 78% of patients; these BVI deficits were profound in 29% of the cases. In contrast, 65% of the individual patients showed no or only minor postoperative changes in CBVI vs. preoperative values.
Conclusions
Changes in the intravascular volume compartments affected BVI to a greater extent than CBVI. Therefore measuring circulating in addition to central blood volume may be useful to ensure a normal circulating blood volume that can compensate for any change in the central vascular compartment.
Similar content being viewed by others
Introduction
Changes in intravascular blood volume after cardiac surgery using cardiopulmonary bypass are considered a major cause of hemodynamic instability. Since both hypovolemia and hypervolemia have been shown to contribute significantly to perioperative morbidity and mortality [1, 2, 3], methods to determine intravascular volume load are crucial for guiding fluid therapy. Central venous and pulmonary artery occlusion pressure are generally for this. However, in ventilated patients this practice has been under debate recently since no correlation has been established between these preload parameters and cardiac output [4, 5, 6, 7, 8, 9]. In contrast, central blood volume (CBV), which refers to the blood volume of the intrathoracic vascular compartment, has recently been shown to be a better indicator of cardiac preload [4, 5, 6, 7, 8, 9]. Another volume compartment which can be quantified at the bedside is circulating blood volume (BV), which refers to entire circulation. Several studies report that BV is significantly reduced after surgery [1, 10, 11, 12, 13, 14]. Furthermore, Tschaikowsky and coworkers [15] suggest the goal of maintaining BV in the perioperative period to achieve hemodynamic stability after cardiac surgery. However, there are no studies in patients undergoing cardiac surgery addressing the perioperative monitoring of both CBV and BV.
Various indicator dilution techniques are available for measuring intravascular volume compartments. Recently a method for bedside measurement of CBV and BV using a dye dilution technique with indocyanine green (ICG) as indicator has been introduced to the market. Unlike other commercially available devices requiring a specific arterial catheter in the femoral artery [16], the device used in our work detects the ICG blood concentration by pulse dye densitometry (PDD) transcutaneously [17]. In this observational study we report the pattern of perioperative changes in CBV and of BV after coronary bypass surgery using a novel, less invasive monitoring device.
Material and methods
After institutional approval, 66 patients scheduled for coronary bypass surgery gave their written informed consent and were included in the study. Exclusion criteria were: allergy to ICG or contrast media, ejection fraction below 40%, application of an aortal counterpulsation device, renal failure, atrial fibrillation and detection of intracardiac shunting by echocardiography. Five patients in whom the pulsation amplitude of the PDD device was less than 1% were excluded from analysis. The mean characteristics of the remaining 61 patients were: age 66.8±7.8 years, height 170±7.9 cm, weight 81±11.3 kg, body surface area BSA 1.9±0.2 m2, body mass index 28.1±3.2.
On the day of surgery the patients were given 40 mg chloracetate. General anesthesia was induced with 30 µg/kg alfentanil and a target controlled infusion of propofol (target 2.5 µg/ml). Pancuronium bromide (0.1 mg/kg) was used to facilitate tracheal intubation. Anesthesia was maintained by continuous infusion of propofol (target 2–3 µg/ml) and alfentanil (50–100 µg/kg per hour) until the patient was transferred to the intensive care unit (ICU). Patients were ventilated (Kion, Siemens, Erlangen, Germany) using volume-controlled mode. The ventilator was set up to a tidal volume of 10 ml/kg, positive end-expiratory pressure 5 cmH2O and mandatory breath rate to achieve normocapnia. FIO2 was adjusted to maintain a PaO2 of 10 kPa or higher. Analgesia was continued postoperatively using incremental doses of piritramide. Until extubation criteria were met, patients were sedated using propofol infusion, and they were ventilated (Siemens Servo 300, Erlangen, Germany) by synchronized intermittent mandatory ventilation mode. Mean duration of surgery was 53.4±24.8 min.
Measurement of intravascular volume compartments
Intravascular volume compartments and cardiac output were determined by a dye dilution technique using indocyanine green (Pulsion, Munich, Germany). After injection of ICG via the distal port of the central venous catheter the blood concentration of ICG in the systemic circulation was determined by pulse dye densitometry (DDG-Analyser 2001, Nihon-Kohden, Tokyo, Japan). The detector cell is a clip attached to the patient’s nasal wing. PDD works similarly to pulse oximetry. It uses three instead of the usual two wavelengths to detect the maximal absorption of ICG in addition to oxyhemoglobin and reduced hemoglobin. As the PDD device detects only the relative ICG blood concentration, separate determination of the actual hemoglobin concentration by blood gas analysis (ABL 625, Radiometer, Copenhagen, Denmark) is needed to calculate absolute ICG blood concentrations. To obtain reliable measurements the pulsatile component of light absorption in pulse oximetry should account for 1–5% of the total signal. If the PDD monitor indicated a pulsation amplitude below 1%, measurements were not performed.
After ICG injection the dye densitometer calculates cardiac output (CO) and mean transit time (MTT) from the dye dilution curve obtained over a period of 90 s. MTT is the time between the first detection of the indicator at the injection-detector attached to the central venous catheter and the mean time of indicator persistence at the detector cell attached to the nasal wing. The product of MTT and CO is the distribution volume of ICG between the injection site (right atrium) and the detection site (arterial vascular system), which is called CBV. In this study CO and MTT were calculated from mean values out of three consecutive dye dilution measurements. For each measurement 5 mg ICG diluted in 1 ml sterile water was filled into an injection line, which was attached to the distal port of the central venous catheter. ICG was injected into the circulation by flushing this line with 9 ml 5% glucose. Since total elimination of ICG would last about 3 half-lives (approx. 12 min), remaining plasma ICG would cause error when measurements were repeated every 2 min during CO determination. Therefore the device automatically calibrates to zero ICG plasma concentration at the time a new measurement starts. This baseline gradually drops during the measurement as a result of the elimination of the remaining ICG. Hence the area under the ICG concentration curve is underestimated, but, as previously reported, the resulting error is negligible [18].
To measure BV the ICG blood concentration was detected over a total period of 15 min following injection, and the resulting concentration vs. time data was fitted to a one compartment model using the following equation: C=A×exp(−Kt), where C=ICG concentration, A=constant factor, K=elimination rate constant. To determine BV the ICG concentration calculated at MTT was applied to: BV=D/CMTT, where D=ICG dose, CMTT=ICG concentration at MTT. A more detailed description of CBV and BV determinations by the PDD method is given elsewhere [19]. In this study BV was measured using a single ICG-bolus of 0.4 mg/kg which was injected via the distal port of the central venous catheter. To avoid indicator loss the central venous line was flushed by 10 ml glucose 5%. Accumulation of ICG was not observed in our patients since in every case ICG plasma concentrations dropped rapidly and reached values below 1.5 mg/l at the end of each CO and BV measurement.
After induction of anesthesia both BV and CBV were measured before skin incision (t0). These values served as control. After surgery two subsequent measurements were performed, one after admission to the intensive care unit (t1) and a second on the first postoperative day (t2). All measurements were performed by a single investigator.
Hemodynamic management
Starting with anesthesia induction 10 ml/kg ringer solution and 6 ml/kg hydroxyethylstarch (Voluven, Fresenius Kabi, Bad Homburg, Germany) were infused in each patient prior to skin incision. During surgery and the postoperative period Ringer’s solution was constantly infused at a rate of 1 ml/kg per hour. When needed, additional amounts of Ringer’s solution were given to compensate for excessive urine output. Intraoperative blood losses and postoperative drainage losses were treated by infusion of equal amounts of hydroxyethylstarch or packed red blood cells. Hemodynamic therapy was guided by criteria such as heart rate, mean arterial blood pressure, central venous pressure, and urine output (intervention triggers: >100 beats/min, <60 mmHg, <8 mmHg, <1 ml/kg). Fluids were substituted to maintain the parameters as specified above. In the case of failure to the clinical standard therapy (n=10), a femoral arterial catheter (Pulsiocath 4F PV2014L16 and PiCCO, Pulsion Medical Systems, Munich, Germany) was introduced to improve hemodynamic therapy according to the results of an extended monitoring [intervention triggers: cardiac index <2 l min−1 m−2, systemic vascular resistance (SVRI) <1200 dyne s−5 m−2). Dobutamine was used for standard inotropic support; dobutamine was replaced by epinephrine in the case of severe cardiac dysfunction. Norepinephrine was the drug of choice in patients with relevant hypotension due to decreased SVRI. Nitroglycerin was used for treatment of hypertension (mean arterial blood pressure >90 mmHg). Physicians involved in patient care were unaware of the study results of determination of the volume compartments.
Fluid balances
Net fluid balances were obtained at each measurement and at the end of surgery. Crystalloid infusions, cardiopulmonary bypass priming solutions, urine output, gastric tube losses, and perspiratio insensibilis (35 ml/h) referred to the crystalloid balance. The colloid balance was calculated from the amount of colloid infusions, blood and fresh frozen plasma transfusions, shed blood, drainage losses, and the remaining volume in the cardiopulmonary bypass circuit at the end of surgery.
Statistics
Unless otherwise stated, continuous variables are expressed as mean ±SD. The Shapiro-Wilk test was used to test for normal distributions. The mean of physiological and volumetric parameters was analyzed by analysis of variance for repeated measurements. If significant differences were found, post hoc comparisons were performed using Tukey’s honest significant difference test. Statistical significance was set at p≤0.05.
Results
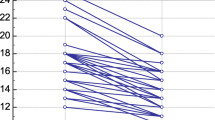
Data were collected 3.3±1.4 h (t1) and 17±2.7 h (t2) after skin closure. At t0 and t1 all patients were artificially ventilated, and at t2 all but four patients (6.1%) were extubated. At t0 no vasoactive drugs were necessary, but infusions of epinephrine, norepinephrine, dobutamine, and nitroglycerin were used in 1, 10, 14, and 12 patients, respectively, after surgery (Table 1). Patients developed significant positive fluid balances intraoperatively, but fluid in- and output was balanced postoperatively (Table 1). During the study period 29% of the patients were transfused by a mean of 1.6±0.6 packed red cells, which were mainly given during surgery. Most of the physiological parameters obtained (Table 2) showed significant differences between pre- and postoperative values. In general both cardiac index and heart rate were significantly increased after surgery while SVRI showed a significant reduction. Only ten patients with decreased SVRI developed hypotension requiring norepinephrine infusions. Mean CBVI remained unchanged throughout the study period, whereas mean BVI was significantly decreased after surgery (Fig. 1).
Postoperative time course of mean indexed central blood volume (open circles) and mean indexed circulating blood volume (open triangles) following admission to the intensive care unit (t1) and on the first postoperative day (t2), presented as percentage change compared to preoperative (t0) values. *p<0.05
Figure 2 presents a more detailed view on the postoperative BVI changes, analyzing the incidence of various degrees of differences between pre- and postoperative BVI. At t1 49% of the patients had a BVI deficit of up to 1000 ml, and another 29% had one greater than 1000 ml. Although there was a shift from large (>1000 ml) to less pronounced BVI deficits (<1000 ml), there was a similar pattern of the differences between pre- and postoperative BVI at t2. Higher postoperative BVI (22% at t1 and 25% at t2) was observed less frequently than BVI deficits (Fig. 2). In contrast, nearly 65% of the patients had an unchanged CBVI or showed differences between pre- and postoperative CBVI less than 100 ml at both t1 and t2 (Fig. 3). Larger postoperative CBVI changes were observed less frequently, and this pattern did not differ between t1 and t2.
Discussion
Measurement of CBV and BV commonly performed using ICG injection via central venous catheter and detection by a reflection densitometer in the descending aorta are regarded as accurate and less sensitive to artifacts [6, 9]. A recent study comparing this techniques with the less invasive one (one catheter, PDD method) also used in this study reported a good correlation between the two methods with respect to the ICG plasma disappearance [20]. The same investigators later found moderate agreement between the methods in comparing corresponding values of CO and CBV in patients with sepsis and acute respiratory distress syndrome [21]. The authors concluded that the PDD method is accurate in measuring relative changes in ICG plasma concentration but less precise in detecting absolute ICG concentrations, mainly due to large intraindividual variability. In contrast, the intraindividual variability was not that large in our study (6.3%). A possible explanation for these different results is that in contrast to our work Sakka and coworkers [21] did not use the mean of a triplet of ICG injections to determine CO and CBV.
BV determination using ICG is based on the dilution principle and depends on the correct measurement of absolute ICG plasma concentrations [22]. Good agreement was reported recently in comparing the PDD method with classical techniques of BV measurement using blood samples to detect the concentration of ICG or 51Cr-labeled red blood cells in cardiac surgical patients [23, 24]. In contrast, Ishihara and coworkers [25] reported BV determination by PDD to be inaccurate early after cardiac surgery. However, in that study all patients but one required vasoactive drugs after surgery, and the authors themselves suggested that poor peripheral circulation, a known problem with the PDD method, related to hypovolemia and vasopressor therapy, was most probably the reason for their results.
We observed profound changes in the hemodynamic profile of our patients after cardiopulmonary bypass surgery, which is in accord with previous reports [5, 6, 26, 27]. The preoperative period was characterized by cardiocirculatory depression due mainly to β-blocking agents and tranquillizers, while decreased SVRI and increased cardiac index and heart rate were observed postoperatively. Reduction in vascular resistance after cardiopulmonary bypass is a well-known phenomenon and most likely the result of a temporary systemic inflammatory response syndrome [28, 29, 30].
Guided by conventional monitoring, the therapeutic goals of hemodynamic therapy as stated above were reached in all of our patients. However, compared to preoperative values we found the average BV to be reduced by about 15% after surgery despite marked positive fluid balances which is well in agreement with other reports [11, 12, 13, 14, 15]. Interestingly, liberal administration of fluids during surgery did not compensate for postoperative BV reduction. Two-thirds of our patients developed moderate to profound BV deficits after surgery compared to preoperative values, a finding which is well in agreement with previous findings by Tschaikowsky and coworkers [15]. These authors found increased plasma lactate concentrations corresponding to the postoperative time course of BV deficits and considered reduced organ perfusion due to hypovolemia to be a likely cause.
In contrast to BV, the average CBV throughout the study period remained unchanged. Major perioperative changes in CBV were both less frequent and less pronounced than the perioperative BV changes. The finding of perioperatively unchanged CBV is interesting because the three time points in our study referred to different pathophysiological conditions, i.e., pre- vs. postoperative status, with and without mechanical ventilation. Schmidt and coworkers [31] studied the effect of mechanical ventilation on CBV and reported that CBV was significantly increased by changing mechanical ventilation to T-piece breathing, and that CBV returned to the values obtained during mechanical ventilation 10 min after extubation. Since we used a different timing that omitted CBV measurement during T-piece breathing, we cannot compare our findings to those of Schmidt et al. However, an unchanged CBV after extubation was found in both studies.
CBV has been reported to be an important indicator of cardiac preload [4, 5, 6, 7, 8, 9, 32]. Hence an unchanged CBV, as found in the majority of our patients, may indicate adequate perioperative volume management. However, Hinder and coworkers [6] recently reported that CBV had to be greater after cardiac surgery than before the incision to maintain a constant left ventricular end diastolic area measured by echocardiography. The authors hypothesized that the cause was an increased pulmonary vascular capacity due to vasodilation related to systemic inflammatory response syndrome in cardiac surgery patients. Strey and coworkers [33] studied pressure-volume relationships and examined compliance of the central and the peripheral low-pressure vascular systems due to hemorrhage in healthy adults. They reported a large distensibility of the central vascular compartment compared to the extrathoracic vasculature. In view of the variable capacitance of the central vascular compartment suggested by the findings of Strey et al. [33] and Hinder et al. [6], a normal CBV may not inevitably allow an adequate preload in cardiac surgery patients. However, normal or even increased BV after surgery could possibly compensate for any change in the central compartment capacitance. Measuring BV in addition to CBV may thereby improve perioperative hemodynamic management.
The pattern of perioperative changes in CBV and BV, especially the relationship between the two parameters, may be affected by changes in the intrathoracic pressure, i.e., initiation or cessation of positive pressure ventilation. In our study the ratio between CBV and BV changed from 25% before skin incision to more than 30% after surgery while the patients were still artificially ventilated. On the first day after surgery, when most of our patients were extubated, we still found CBV to BV ratios of about 30%. Hence a larger fraction of blood volume may have referred to CBV after extubation, suggesting volume shifts from extra- to intrathoracic compartments. In a recent study von Spiegel and coworkers [34] measured the effect of positive pressure ventilation on both CBV and BV. The authors reported a significant blood volume shift from intra- to extrathoracic compartments after onset of positive pressure ventilation during anesthesia induction. Although our findings cannot be compared directly to those of von Spiegel and coworkers, the underlying pathophysiology of volume shifts between intra- to extrathoracic compartments may be similar.
In summary, the less invasive and easy-to-use method of PDD seems to be a useful tool for determining volume compartments in cardiac surgery patients at the bedside. However, PDD is sometimes difficult to perform due to motion artifacts and poor tissue perfusion after surgery. Readings of ICG blood concentrations should therefore always be checked for plausibility and interpreted carefully. Since CBV is considered to be a measure of cardiac preload, achieving hemodynamic stability accompanied by an overall unchanged CBV after surgery, as seen in our patients, supports the adequacy of fluid therapy. However, CBV may after cardiac surgery vary due to systemic inflammatory response syndrome related vasodilation of the central vasculature. Preservation of a normal BV could possibly compensate for any change in the central vascular capacitance. Thus monitoring BV in addition to CBV may be worthwhile. However, further studies are required to define the therapeutic goals that a hemodynamic therapy based on monitoring of CBV and BV should aim at to maintain organ perfusion perioperatively.
References
Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH (1973) Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg 106:630–636
Shoemaker WC (1986) Resuscitation of the critically ill patient. Use of branched-chain decision trees to improve outcome. Emerg Med Clin North Am 4:655–694
Paret G, Cohen AJ, Bohn DJ, Edwards H, Taylor R, Geary D, Williams WG (1992) Continuous arteriovenous hemofiltration after cardiac operations in infants and children. J Thorac Cardiovasc Surg 104:1225–1230
Brock H, Gabriel C, Bibl D, Necek S (2002) Monitoring intravascular volumes for postoperative volume therapy. Eur J Anaesthesiol 19:288–294
Buhre W, Weyland A, Schorn B, Scholz M, Kazmaier S, Hoeft A, Sonntag H (1999) Changes in central venous pressure and pulmonary capillary wedge pressure do not indicate changes in right and left heart volume in patients undergoing coronary artery bypass surgery. Eur J Anaesthesiol 16:11–17
Hinder F, Poelaert JI, Schmidt C, Hoeft A, Mollhoff T, Loick HM, Van Aken H (1998) Assessment of cardiovascular volume status by transoesophageal echocardiography and dye dilution during cardiac surgery. Eur J Anaesthesiol 15:633–640
Wiesenack C, Prasser C, Keyl C, Rodijg G (2001) Assessment of intrathoracic blood volume as an indicator of cardiac preload: single transpulmonary thermodilution technique versus assessment of pressure preload parameters derived from a pulmonary artery catheter. J Cardiothorac Vasc Anesth 15:584–588
Bindels AJ, van der Hoeven JG, Graafland AD, de Koning J, Meinders AE (2000) Relationships between volume and pressure measurements and stroke volume in critically ill patients. Crit Care 4:193–199
Godje O, Peyerl M, Seebauer T, Lamm P, Mair H, Reichart B (1998) Central venous pressure, pulmonary capillary wedge pressure and intrathoracic blood volumes as preload indicators in cardiac surgery patients. Eur J Cardiothorac Surg 13:533–539–
Lazrove S, Waxman K, Shippy C, Shoemaker WC (1980) Hemodynamic, blood volume, and oxygen transport responses to albumin and hydroxyethyl starch infusions in critically ill postoperative patients. Crit Care Med 8:302–306
Yamazaki M, Shakunaga K, Hirota K (2001) Changes of circulating blood volume in coronary arterial graft surgery with and without cardiopulmonary bypass. Can J Anaesth 48:935
Beattie HW, Evans G, Garnett ES, Webber CE (1972) Sustained hypovolemia and extracellular fluid volume expansion following cardiopulmonary bypass. Surgery 71:891–897
Barta E, Kuzela L, Tordova E, Horecky J, Babusikova F (1980) The blood volume and the renin-angiotensin-aldosterone system following open-heart surgery. Resuscitation 8:137–146
Karanko M (1987) Severely decreased blood volume after coronary bypass surgery. Crit Care Med 15:182
Tschaikowsky K, Neddermeyer U, Pscheidl E, von der Emde J (2000) Changes in circulating blood volume after cardiac surgery measured by a novel method using hydroxyethyl starch. Crit Care Med 28:336–341
Buhre W, Bendyk K, Weyland A, Kazmaier S, Schmidt M, Mursch K, Sonntag H (1998) Assessment of intrathoracic blood volume. Thermo-dye dilution technique vs single-thermodilution technique. Anaesthesist 47:51–53
Fuse M, Aoyagi T, Xie CT, Kanemoto M, Tomita H, Hosaka H, Katayama M, Miyasaka K, Ishikawa K, Katori R, Shintani F (1992) Dye dilution curve measurement with principle of the pulse oximeter. Jpn J Med Electronics Biol Eng 30:249
Kobayashi N (Oct 1998) Dye-densitometry-based cardiac output, circulating blood volume and hepatic clearance measurement. Presented at “Computers in Anesthesia XIX,” Society for Computers in Anesthesia, Orlando, Florida
Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H (1997) Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit 13:81–89
Sakka SG, Reinhart K, Meier-Hellmann A (2000) Comparison of invasive and noninvasive measurements of indocyanine green plasma disappearance rate in critically ill patients with mechanical ventilation and stable hemodynamics. Intensive Care Med 26:1553–1556
Sakka SG, Reinhart K, Wegscheider K, Meier-Hellmann A (2002) Comparison of cardiac output and circulatory blood volumes by transpulmonary thermo-dye dilution and transcutaneous indocyanine green measurement in critically ill patients. Chest 121:559–565
Barker SJ (1998) Blood volume measurement: the next intraoperative monitor? Anesthesiology 89:1310–1312
Haruna M, Kumon K, Yahagi N, Watanabe Y, Ishida Y, Kobayashi N, Aoyagi T (1998) Blood volume measurement at the bedside using ICG pulse spectrophotometry. Anesthesiology 89:1322–1328
Imai T, Mitaka C, Nosaka T, Koike A, Ohki S, Isa Y, Kunimoto F (2000) Accuracy and repeatability of blood volume measurement by pulse dye densitometry compared to the conventional method using 51Cr-labeled red blood cells. Intensive Care Med 26:1343–1349
Ishihara H, Okawa H, Iwakawa T, Umegaki N, Tsubo T, Matsuki A (2002) Does indocyanine green accurately measure plasma volume early after cardiac surgery? Anesth Analg 94:781–786
Goedje O, Hoeke K, Lichtwarck-Aschoff M, Faltchauser A, Lamm P, Reichart B (1999) Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: comparison with pulmonary arterial thermodilution. Crit Care Med 27:2407–2412
Hachenberg T, Tenling A, Rothen HU, Nystrom SO, Tyden H, Hedenstierna G (1993) Thoracic intravascular and extravascular fluid volumes in cardiac surgical patients. Anesthesiology 79:976–984
Kristof AS, Magder S (1999) Low systemic vascular resistance state in patients undergoing cardiopulmonary bypass. Crit Care Med 27:1121–1127
Laffey JG, Boylan JF, Cheng DC (2002) The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 97:215–252
Johnson MR (1999) Low systemic vascular resistance after cardiopulmonary bypass: are we any closer to understanding the enigma? Crit Care Med 27:1048–1050
Schmidt H, Rohr D, Bauer H, Bohrer H, Motsch J, Martin E (1997) Changes in intrathoracic fluid volumes during weaning from mechanical ventilation in patients after coronary artery bypass grafting. J Crit Care 12:22–27
Junghans T, Bohm B, Haase O, Fritzmann J, Zuckermann-Becker H (2002) Conventional monitoring and intravascular volume measurement can lead to different therapy after upper gastrointestinal tract surgery. Intensive Care Med 28:1273–1275
Strey W, Richter G, Lange S (1975) [On the pressure-volume relation of the central and the peripheral vascular bed of the low pressure system (author’s transl)]. Anaesthesist 24:157–161
Spiegel T von, Giannaris S, Schorn B, Scholz M, Wietasch GJ, Hoeft A (2002) Effects of induction of anaesthesia with sufentanil and positive-pressure ventilation on the intra- to extrathoracic volume distribution. Eur J Anaesthesiol 19:428–435
Acknowledgement
The authors thank Dr. S. Ensminger for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Bremer and A. Schiele contributed equally to this manuscript
Rights and permissions
About this article
Cite this article
Bremer, F., Schiele, A., Sagkob, J. et al. Perioperative monitoring of circulating and central blood volume in cardiac surgery by pulse dye densitometry. Intensive Care Med 30, 2053–2059 (2004). https://doi.org/10.1007/s00134-004-2445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2445-8