Abstract
Background
Fluid management strongly affects hepatic resection and aims to reduce intraoperative bleeding during living donation. The Pleth Variability Index (PVI) is a tool to assess the fluid responsiveness from the pulse oximeter waveform; we evaluated the efficacy and accuracy of finger PVI compared to pulse pressure variation (PPV) from arterial waveform in predicting the fluid response in donor hepatectomy patients with the guide of non-invasive cardiac output (CO) measurements. We recruited forty patients who were candidates for right lobe hepatectomy for liver transplantation under conventional general anesthesia methods. During periods of intraoperative hypovolemia not affected by surgical manipulation, PVI, PPV, and CO were recorded then compared with definitive values after fluid bolus administration of 3–5 ml/kg aiming to give a 10% increase in CO which classified the patients into responders and non-responders.
Results
Both PPV and PVI showed a significant drop after fluid bolus dose (P < 0.001) leading to an increase of the CO (P < 0.0001), and the area under the curve was 0.934, 0.842 (95% confidence interval, 0.809 to 0.988, 0.692 to 0.938) and the standard error was 0.0336, 0.124, respectively. Pairwise comparison of PPV and PVI showed non-significant predictive value between the two variables (P = 0.4605); the difference between the two areas was 0.0921 (SE 0125 and 95% CI − 0.152 to 0.337).
Conclusions
PVI is an unreliable indicator for fluid response in low-risk donors undergoing right lobe hepatectomy compared to PPV. We need further studies with unbiased PVI monitors in order to implement a non-invasive and safe method for fluid responsiveness.
Similar content being viewed by others
Background
Egypt is one of the leading countries in living donor liver transplantation in the Middle East; in our center, we performed more than 400 cases till November 2019. Our first successful case has been done in 2001, and we still did not establish a cadaveric program for liver transplantation in neither any other centers all over the country although we have an established law for organ transplant in 2010; therefore, we usually try to optimize the donors’ condition to avoid any form of complications. Liver transplant is performed mainly for end-stage liver failure arising mainly from chronic liver disease due to hepatitis C virus inoculation.
Egypt has higher rates of HCV relative to the neighboring countries as well as other countries in the world with comparable socioeconomic conditions and hygienic standards for invasive medical, dental, or paramedical procedures (Beavers et al., 2001; Broelsch et al., 2000).
Fluid optimization and monitoring are crucial in the intraoperative period of hepatic donor resection; several researches discussed the tools used for fluid management like the high stroke volume variation (SVV) method and pulse pressure variation (PPV). Due to several financial constraints in our center, we usually use the last two methods by minimizing the CVP in order to decrease the right side of the heart pressures, minimizing the IVC pressure and leading to a soft and decongested liver, thus reducing blood loss and controlling hepatic venous hemorrhage. During the past year, we stopped inserting internal jugular vein line (IJV) and basically depend on arterial waveform variation, i.e., PPV.
The strategy of respiratory variation of SV is widely used to assess the cyclic fluid changes, the method of PPV allows the prediction of fluid responsiveness, and several studies relied on the reliability of PPV to demonstrate fluid responsiveness as a simple, non-invasive, and safe method (Chhibber et al., 2007; Marik et al., 2009). But still, PPV needs a continuous arterial line insertion and continuous invasive blood pressure measurement, which is an invasive method for the assessment of fluid optimization. On the other hand, respiratory variations in the pulse oximeter plethysmographic waveform amplitude have been shown to be useful in perioperative fluid management in the operating room (Cannesson et al., 2007). Depending on this principle, the Pleth Variability Index (PVI) is a novel algorithm allowing for automated and continuous calculation of the respiratory variations in the pulse oximetry waveform amplitude and can also predict fluid responsiveness non-invasively in mechanically ventilated patients (Feissel et al., 2007). Using this method, we can measure the perfusion index (PI) dynamic changes in a complete respiratory cycle. PVI is described as the fraction of light absorbed due to arterial pulsation (AC) in relation to the total amount of light absorbed (DC). PVI = [(PImax − PImin)/PImax] × 100.
In our study, we are trying to validate the variation of the plethysmographic pulse during respiration or PVI as an indicator for fluid responsiveness in donors undergoing right lobe hepatectomy as a part of living donor liver transplantation procedure by comparing the PVI with an established predictor of fluid responsiveness like the PPV and their ability to increase the cardiac output (CO) of the hypovolemic patients by 10%.
Methods
The liver transplantation program in Egypt relies on related living donors. Cautious attention during anesthetic management of the donor is warranted because of clear ethical considerations.
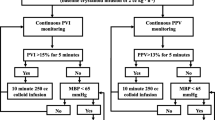
This prospective observational study was performed after obtaining written approval from the ethical committee and consent to participate from the University FWA 000017585, FMASU R 53/2018 and registered in ClinicalTrials.gov with number NCT03921125. A sample of 40 healthy ASA I or II donors who were candidates for right lobe hepatectomy for living donor liver transplantation (LDLT) were included, and consent was taken between April 2019 and December 2019. A conventional form of anesthesia was induced with a left radial arterial line inserted for invasive blood pressure monitoring, frequent blood gases and lactate sampling, and measuring the PPV. No IJV CVL was inserted in all of the patients. Fluid management in the form of ringer acetate 3–5 ml/kg/h recruitment of the patients (hypovolemic donors) depends on the presenting signs that led the anesthesiologist to decide if the patient’s volume status was hypovolemic and in need of fluid replacement without the presence of surgical mechanical compression on the IVC or liver during dissection phase. The diagnostic criteria for volume assessment were heart rate (HR) more than 100 beats/min and systolic arterial blood pressure (SAP) less than 90 mmHg.
Assessment of hemodynamics during periods of hypovolemia
After the exclusion of any surgical mechanical compression after communication with the surgeons, all enrolled hypovolemic patients had left radial arterial line measuring PPV using Drager Infinity Kappa (Drager Medical Systems, Inc., PA, USA) and a pulse co-oximeter probe measuring PVI using Device Masimo Rainbow SET (Masimo Corporation, Irvine, CA, USA) applied over the index finger and covered with drapes to avoid any light interference. This device measures the perfusion index (PI) which is the indicator of the amplitude of the signal of PVI, confirmation of the wave signal should be obtained from any finger, fingers should be kept warm before the test is done, and PI should be above 1 to take our records of PVI. Careful monitoring of the PVI signal is done to avoid hypothermia of the fingers and lost signals till the accurate signal is obtained.
Recruitment of hypovolemic patients based on the above criteria is given a fluid bolus in the form of ringer acetate 5 ml/kg over 10 min, and after volume expansion, another recorded PPV and PVI are done.
Non-invasive cardiac output (CO) measurements
After the induction of anesthesia and supine positioning, CO is monitored using a minimally invasive method device LiDCOunity (LiDCO, Orsman Road, London, UK) monitor using a PulseCO algorithm which converts blood pressure to its constituent parts of flow (cardiac output (CO), stroke volume (SV)); recordings of the measurements of CO were taken during diagnosis of hypovolemia (T0) and 10 min after fluid administration (T10).
Study design
After the diagnosis of hypovolemia despite no surgical intervention such as compression on the IVC or liver manipulation baseline HR, PPV, PVI, and CO were recorded (T0), then 10 min after ringer acetate 5 ml/kg bolus (T10).
Statistical analysis
Forty patients’ data were collected, revised, coded, and entered into the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as means, standard deviations, and ranges when their distribution found parametric and median with inter-quartile range (IQR) when found non-parametric. Patients were classified into responders and non-responders according to an increase of 10% of the CO. The comparison between responders and non-responders paired groups with quantitative data and parametric distribution was done using paired t-test while data with non-parametric distribution were done using the Wilcoxon rank test. Receiver operating characteristics (ROC) curves were done to test the ability of PPV and PVI to predict fluid responsiveness. The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, the P value was considered significant at P < 0.05 and highly significant at P < 0.01. The minimal sample size was calculated to be 35 patients, so we raised the number to 40 patients to avoid any dropouts like loss of signal of the PVI.
Results
We recruited forty ASA I patients who were candidates for donor’s right lobe hepatectomy to the study of 29.39 ± 5.52 mean age, 28 female and 12 male of mean body mass index (BMI) 26.32 ± 5.29 and average surgery duration of 6 h.
After confirming the diagnosis of intraoperative hypovolemia and excluding any surgical interference, the initial bolus of 5 ml/kg Ringer acetate revealed a significant decrease in HR (P < 0.0001) associated with a significant increase in SBP (P < 0.0001) (Table 1). As regards the hemodynamic variables after fluid bolus, our results showed a highly significant decrease in both PPV (P < 0.001) and finger PVI (P < 0.001) with a significant rise in the CO (P < 0.0001) (Table 1).
Among the forty patients, there were 38 (95%) responders to the fluid bolus and 2 (5%) non-responders. No patients received any kind of vasopressors and blood products.
Assessment of fluid responsiveness
A drop of the CO of 10% associated with an increased heart rate over 100 beats/min and drop of SBP less than 90 mmHg with no surgical compression on the IVC required intraoperative fluid bolus of ringer acetate; ROC analysis explained a significant decrease of PPV and PVI. The area under curve was 0.934, 0.842 (95% confidence interval, 0.809 to 0.988, 0.692 to 0.938) and standard error 0.0336, 0.124, respectively (Figs. 1 and 2).
The analysis of ROC for PPV after fluid bolus (Fig. 3) showed AUC 0.934 (SE 0.0336, 95% CI 0.809 to 0.988), Z statistic 12.930) with 89.47% sensitivity and 100% specificity.
PVI ROC analysis (Fig. 4) revealed AUC 0.842 (SE 0.124, 95% CI 0.692 to 0.938, Z statistic 2.749), and the sensitivity was 60.53% and the specificity was 100%.
Pairwise comparison of ROC analysis of PPV and PVI
Analysis of the ROC after fluid administration showed a non-significant predictive value between the two variables (P = 0.4605); the difference between the two areas was 0.0921 (SE 0125 and 95% CI − 0.152 to 0.337, Z statistic: 0.738) (Fig. 5).
Discussion
From our study, we found that PVI can predict fluid responsiveness in patients who are candidates for liver donation but is not as effective as PPV. Living organ donation usually puts the whole surgical and anesthetic team under stress to avoid the incidence of any complications such as bleeding during the dissection phase, blood transfusion complications, and fluid overload or deficiency. In our practice, fluid management and intravascular volume status directly affect the hepatic dissection by affecting the sinusoidal pressure and IVC pressure through the right side pressures of the heart. On the other hand, adequate intravascular volume is mandatory and crucial to prevent hypoperfusion and hypovolemia which may lead to elevated lactate levels and renal impairment (Cannesson et al., 2008a). During the past year, we were trying to be less invasive with the donors by stopping the insertion of internal jugular vein (central venous line) rendering the low CVP technique during hepatectomy not applicable in these patients; the other invasive tool in our anesthetic management is the placement of arterial line for continuous monitoring of blood pressure, blood gases, and lactate sampling in addition to dynamic measurements of fluid response by PPV (Poeze et al., 2005). Dynamic assessment of fluid status and response during surgery are usually invasive, and several studies proved their effectiveness in the stability of hemodynamics, hospital stay duration, and financial benefits (Biais et al., 2008). One of the new non-invasive methods to assess volume response is the PVI with several studies proved its dynamic parameters (Salzwedel et al., 2013), which took our attention to the possibility of implementation of PVI readings for dynamic fluid response in donors based on the previous study done in cardiac surgery by Canneson and his colleagues confirming the predictability of cardiovascular response to fluids administration (Cannesson et al., 2008b).
Most of the literature studies assessed the stroke volume variation (SVV) or stroke volume index (SVI) to differentiate between the responders and non-responders (Vos et al., 2013; Zimmermann et al., 2010); in our study, we used the CO as a method to differentiate between the fluids responders and non-responders supported by many work done in this field through the non-invasive LIDCO monitor for goal directed-fluid therapy (Ripollésa et al., 2016; Grocott et al., 2013). We used this monitor to implement the idea of non-invasive measurements in this procedure and try to validate it.
In our surgical procedure (donor hepatectomy), we are in deep need to replace all invasive monitors with non-invasive methods, especially for fluid responsiveness; among our 38 responders to bolus fluids, fingertip PVI showed a significant decrease (P < 0.001) causing a significant rise in the CO (P < 0.001) and accurately can reflect the cardio-pulmonary changes under ventilation; these results were supported by other studies (Hood & Wilson, 2011; Haas et al., 2012; Cai et al., 2010), but these studies were done in other surgical procedures, such as colorectal, cardiac, and general surgeries.
PVI has been suggested by a met-analysis as an accurate and reliable fluid response predictor (Yin & Ho, 2012); Zimmerman et al. identified a PVI value of more than 9.5 was noticed as a predicable parameter of fluid response with 93% sensitivity and 100% specificity (AUC = 0.97) (Zimmermann et al., 2010).
Our results did not match the previous results. A possible explanation for the difference is surgical stability in the previous procedures; also, their readings were taken after anesthetic induction, and our patients of hepatic resection usually have longer times of abdominal exposure and subjected to periods of hypothermia despite aggressive warming methods available, which was reported in several studies that perfusion index (PI) and PVI is usually affected by several factors such as hypothermia, low CO, and peripheral vascular disease (Haas et al., 2012; Cai et al., 2010).
In our study, we had no patients who received vasoactive drugs and peripheral vascular disease, despite that ROC analysis of the PVI response to fluids showed a sensitivity of 60.53% and specificity of 100% ( AUC = 0.842).
Hood & Wilson, 2011 had similar results as our study by recording the PVI in two different situations after induction with 86% sensitivity and 100% specificity (AUC = 0.96); the parameters were decreased to 65% and 67% (AUC = 0.71) during the surgical dynamic changes.
Another support to our study was from the previous work; they included ASA physical status of I and II with relatively low-risk candidates similar to our study.
PVI and PPV: are they on the opposite side of the same fence?
We included candidates of living donor right lobe hepatectomy due to the presence of major shift of fluids, taking into consideration the hemodynamic disturbances during right lobe hepatectomy (IVC clamping, hepatic manipulation) and average surgery duration of 6 h. Changing all the invasive measurements of fluid response into non-invasive techniques is very challenging and promotes more patient safety, provided that there is good credibility in the readings.
In our work, we did not measure the trustworthiness of the Masimo oximeter; it is supposed to work well in low perfusion index, we used it as a monitor for fluid response. The validity of PPV as a predictor of fluids is proven in a meta-analysis (Haas et al., 2012) with a specificity of 89% and sensitivity of 88%.
The present study results revealed the validity of both PVI using Masimo oximeter and PPV using Drager Infinity Kappa monitor to predict fluid response in patients undergoing right lobe hepatectomy for organ donation; on the other hand, the correlation between the two variables PVI and PPV as shown in Fig. 5 revealed lower sensitivity (60.5%) of PVI justifying to a poor correlation between PPV and PVI, excluding the idea of replacing PPV by the PVI.
Our study strengthens by the simultaneous measurement of the readings of PPV and PVI during periods of hypovolemia followed by bolus fluids administration.
Conclusions
We concluded from this study that PVI is an unreliable indicator for fluid response in low-risk donors undergoing right lobe hepatectomy compared to PPV. We need further studies with unbiased PVI monitors in order to implement a non-invasive and safe method for fluids responsiveness.
Limitations
Using Masimo Rainbow set is affected by mild hypothermia which could not be avoided due to wide exposure of surgical incision and duration of surgery. Future models of Masimo oximeter can assist in the accurate reliable measurements without interference.
Availability of data and materials
The datasets used or analyzed during our study are available from the corresponding author upon request.
Abbreviations
- PVI:
-
Pleth Variability Index
- PPV:
-
Pulse pressure variation
- CO:
-
Cardiac output
- HCV:
-
Hepatitis C virus
- SVV:
-
Stroke volume variation
- SVI:
-
Stroke volume index
- CVP:
-
Central venous pressure
- IJV:
-
Internal jugular vein
- IVC:
-
Inferior vena cava
- CVL:
-
Central venous line
- PI:
-
Perfusion index
- ROC:
-
Receiver operating characteristics
- HR:
-
Heart rate
References
Beavers KL, Sandler RS, Fair JH, Johnson MW, Shrestha R (2001) The living donor experience: donor health assessment and outcomes after living donor liver transplantation. Liver Transpl 7(11):943–947. https://doi.org/10.1053/jlts.2001.28443
Biais M, Nouette-Gaulain K, Cottenceau V, Revel P, Sztark F (2008) Uncalibrated pulse contour-derived stroke volume variation predicts fluid responsiveness in mechanically ventilated patients undergoing liver transplantation. Br J Anaesth. 101(6):761–768. https://doi.org/10.1093/bja/aen277
Broelsch CE, Malago M, Testa G, Valentin Gamazo C (2000) Living donor liver transplantation in adults: outcome in Europe. Liver Transpl 6(6 Suppl. 2):S64–S65
Cai QF, Mi WD, Yuan WX (2010) The ability of Pleth Variability Index to predict fluid responsiveness in mechanically ventilated patients under general anaesthesia. Zhonghua Wai Ke Za Zhi 48:1628–1632
Cannesson M, Attof Y, Rosamel P, Desebbe O, Joseph P, Metton O, Bastien O, Lehot JJ (2007) Respiratory variations in pulse oximetery plethysmographic waveform amplitude to predict fluid responsiveness in the operating room. Anesthesiology 106(6):1105–1111. https://doi.org/10.1097/01.anes.0000267593.72744.20
Cannesson M, Delannoy B, Morand A, Attof Y, Bastien O, Lehot JJ (2008a) Does the Pleth Variability Index indicate the respiratory induced variation in the plethysmogram and arterial pressure waveforms. Anesth Analg 106(4):1189–1194. https://doi.org/10.1213/ane.0b013e318167ab1f
Cannesson M, Deseble O, Rosamel P, Delannoy B, Robin J, Bastien O et al (2008b) Plethysmographic variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 101(2):200–206. https://doi.org/10.1093/bja/aen133
Chhibber A, Dziak J, Kolano J, Norton JR, Lustik S (2007) Anesthesiacare for adult live donor hepatectomy: our experiences with 100cases. Liver Transpl 13:537–542 PMID: 17394151. https://doi.org/10.1002/lt.21074
Feissel M, Teboul JL, Merlani P, Badie J, Faller JP, Bendjelid K (2007) Plethysmographic dynamic indices predict fluid responsiveness in septic ventilated patients. Intensive Care Med 33(6):993–999. https://doi.org/10.1007/s00134-007-0602-6
Grocott MP, Dushianthan A, Hamiltom MA et al (2013) Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane systematic review. Br J Anaesth 111(4):535–548. https://doi.org/10.1093/bja/aet155
Haas S, Trepte C, Hinteregger M, Fahje R, Sill B, Herich L, Reuter DA (2012) Prediction of volume responsiveness using Pleth Variability Index in patients undergoing cardiac surgery after cardiopulmonary bypass. J Anesth. 26(5):696–701. https://doi.org/10.1007/s00540-012-1410-x
Hood JA, Wilson RJ (2011) Pleth Variability Index to predict fluid responsiveness in colorectal surgery. Anesth Analg. 113(5):1058–1063. https://doi.org/10.1213/ANE.0b013e31822c10cd
Marik PE, Cavallazzi R, Vasu T, Hirani A (2009) Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 37(9):2642–2647. https://doi.org/10.1097/CCM.0b013e3181a590da
Poeze M, Greve JWM, Ramsay G (2005) Meta-analysis of haemodynamic optimization relationship to methodological quantity. Crit Care. 9(6):771–779. https://doi.org/10.1186/cc3902
Ripollésa J, Espinosa A, Martínez-Hurtado M et al (2016) Intraoperative goal directed hemodynamic therapy in non-cardiac surgery: a systematic review and meta analysis. J Clin Anesth 28:105–115. https://doi.org/10.1016/j.jclinane.2015.08.004
Salzwedel C, Puig J, Carstens A, Bein B, Molnar Z, Kiss K, Hussain A, Belda J, Kirov MY, Sakka SG, Reuter DA (2013) Perioperative goal-directed hemodynamic therapy based on radial arterial pulse pressure variation and continuous cardiac index trending reduces postoperative complications after major abdominal surgery: a multi-center, prospective, randomized study. Crit Care. 17(5):R191. https://doi.org/10.1186/cc12885
Vos JJ, Kalmar AF, Struys MM, Wietasch JK, Hendriks HG, Scheeren TW (2013) Comparison of arterial pressure and plethysmographic waveform-based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth. 110(6):940–946. https://doi.org/10.1093/bja/aes508
Yin JY, Ho KM (2012) Use of plethysmographic variability index derived from the Massimo pulse oximeter to predict fluid or preload responsiveness: a systematic review and meta-analysis. Anaesthesia 67(7):777–783. https://doi.org/10.1111/j.1365-2044.2012.07117.x
Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, Wiesenack C (2010) Accuracy of stroke volume variation compared with Pleth Variability Index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol. 27(6):555–561. https://doi.org/10.1097/EJA.0b013e328335fbd1
Acknowledgments
Not applicable
Funding
This study did not receive any funding source or grant from any funding agency.
Author information
Authors and Affiliations
Contributions
AA designed the study, collected data, revised literature, measured vital data, and wrote manuscript. KA performed analysis, revised literature, collected data. MK revised manuscript, critically reviewed and collected data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval of research ethical committee of Ain Shams University, Faculty of Medicine obtained with number FMASU R 53/2018 and registered at clinicaltrials.gov under number NCT03921125. Written informed consent was obtained from all patients after clear explanation of the study.
Consent for publication
Not applicable
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hilal Abdou, A.M., Abdou, K.M. & Kamal, M.M. The accuracy of Pleth Variability Index for directed fluid optimization in donors in living donor liver transplantation. Ain-Shams J Anesthesiol 14, 2 (2022). https://doi.org/10.1186/s42077-021-00199-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42077-021-00199-7