Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal, severely debilitating and rapidly progressing disorder affecting motor neurons in the brain, brainstem, and spinal cord. Unfortunately, there are few effective treatments, thus there remains a critical need to find novel interventions that can mitigate against its effects. Whilst the aetiology of ALS remains unclear, ageing is the major risk factor. Ageing is a slowly progressive process marked by functional decline of an organism over its lifespan. However, it remains unclear how ageing promotes the risk of ALS. At the molecular and cellular level there are specific hallmarks characteristic of normal ageing. These hallmarks are highly inter-related and overlap significantly with each other. Moreover, whilst ageing is a normal process, there are striking similarities at the molecular level between these factors and neurodegeneration in ALS. Nine ageing hallmarks were originally proposed: genomic instability, loss of telomeres, senescence, epigenetic modifications, dysregulated nutrient sensing, loss of proteostasis, mitochondrial dysfunction, stem cell exhaustion, and altered inter-cellular communication. However, these were recently (2023) expanded to include dysregulation of autophagy, inflammation and dysbiosis. Hence, given the latest updates to these hallmarks, and their close association to disease processes in ALS, a new examination of their relationship to pathophysiology is warranted. In this review, we describe possible mechanisms by which normal ageing impacts on neurodegenerative mechanisms implicated in ALS, and new therapeutic interventions that may arise from this.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentlessly fatal and rapidly progressing disorder affecting motor neurons in the brain, brainstem, and spinal cord, resulting in gradual muscle paralysis. With a poor prognosis and severely debilitating symptoms it is important to identify the underlying mechanisms that trigger ALS. The average age of diagnosis of ALS is 55 years, and ageing is its biggest risk factor. Ageing is a slowly progressive, continuous decline in the normal function of an organism over its lifespan. It is also marked by an increased sensitivity to ageing-related diseases and increased risk of death. Importantly, the World Health Organization (WHO) estimates that the proportion of the global population over 60 years will nearly double (12% to 22%) from 2015 and 2050, implying that the incidence of age-related neurodegenerative diseases such as ALS will increase significantly in the coming decades. However, it is important to note that these estimations may be revised in the future due to the unique circumstances and challenges posed by the COVID-19 pandemic. We are currently living in the United Nations Decade of Healthy Ageing (2021–2030), a global collaboration led by the WHO, recognising the importance of ageing to health. It is also essential to note the difference between lifespan (total number of years an individual survives from birth until death) and healthspan (total number of years an individual remains healthy, without chronic disease). Thus, healthy ageing should also consider healthspan as well as lifespan.
It is well-established that changes in the morphology and function of the brain are present during ageing, involving weight and volume decreases, loss of white and grey matter, and the degeneration of neurites and synapses [1]. Whilst the effect of normal ageing on the spinal cord remains poorly studied in comparison, significant alterations have been described, including the loss of alpha motor neurons (α-MNs)[2], reminiscent of ALS. Muscle cells, like motor neurons, also display many of the classic hallmarks of ageing.
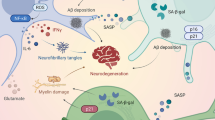
Whilst ageing itself is a normal process, there are striking similarities between neurodegeneration and normal ageing at the molecular and cellular level, because the specific ‘hallmarks’ associated with ageing [3] overlap significantly with pathophysiological mechanisms implicated in ALS (Fig. 1). However, it remains poorly defined how exactly ageing promotes the increased risk of ALS. The molecular and cellular hallmarks of ageing have been recently updated [3]. Hence, a new examination of the relationship between ageing and neurodegeneration in the pathophysiology of ALS is warranted and is the subject of this review.
Molecular hallmarks of ageing in ALS. The molecular hallmarks of ageing denote a collection of inter-connected molecular and cellular features that are widely linked to the ageing process in diverse tissues and organisms. These mechanisms offer a framework for comprehending the intricacies of ageing. Strikingly, the molecular hallmarks of ageing exhibit notable similarities and substantially overlap with the pathophysiological mechanisms described in ALS
Ageing and neurodegeneration
Ageing occurs with time in all organisms, although it progresses at different rates within a species. The differences between individuals are due to variations in genetic makeup, environment, lifestyle, and adaptation [4, 5] and are evident on an organismal, organ, cellular and molecular level. We provide below a brief overview of the major theories of ageing.
Theories of ageing
Whilst the factors that control human lifespan remain unclear, current theories of ageing mainly fall into two broad categories. The ‘Programmed Theory’ proposes that normal ageing follows a biological timetable (similar to that regulating childhood growth) that results in changes in expression of genes involved in cellular maintenance [6]. In contrast, the ‘Damage or Error’ theory suggests that ageing is the result of progressive damage to cells and organs over time [6]. However, currently there is no consensus on the causes of ageing in humans. Moreover, many of the cellular mechanisms implicated in ageing interact extensively and thus may act together to accelerate the underlying molecular processes.
The programmed theory of ageing
The programmed theory of ageing can be further divided into three subtypes. The ‘Programmed Longevity’ theory implies that ageing results from changes in gene expression, leading to age-associated deficits and a subsequent cellular senescent phenotype [7]. Secondly the ‘Endocrine Theory’ proposes that hormones act as biological clocks to control the rate of ageing [8]. Thirdly, the ‘Immunological Theory’ states that the function of the immune system is at its peak during puberty, but it declines thereafter, resulting in an increased susceptibility to inflammation [9].
Damage or error theory of ageing
The ‘Damage or Error’ Theory of ageing can be further divided into five subtypes. First, the ‘Wear and Tear’ theory proposes that cellular components naturally wear out over time from consistent repeated usage [6]. Second, the ‘Rate of Living’ theory states that the lifespan of an organism becomes shorter with higher rates of basal oxygen consumption [10, 11]. Third, the ‘Cross Linking Theory’ [12] proposes that proteins become cross-linked and then aggregate over time [12, 13]. The ‘Free Radical’ theory [14, 15] suggests that superoxide and other free radicals accumulate and damage cellular components (nucleic acids, lipids, sugars, and proteins) during ageing [15]. Whilst antioxidants counteract this to some extent, eventually this becomes ineffective during normal ageing [15]. Finally, the ‘Somatic DNA Damage’ theory proposes that DNA damage occurs continuously in cells. Whilst these lesions are initially repaired, increasing damage over time results in mutations, which impair genome integrity and thus cellular function. Damage to both nuclear and mitochondrial DNA is implicated in this process [16].
Genetics and ageing in ALS
It remains unclear how ageing increases the risk of ALS, but it probably involves a combination of genetic, environmental, and age-related factors [17]. Whilst most (~ 90%) ALS cases arise sporadically, the remaining proportion are familial, and can provide insights into the underlying pathophysiology [17]. Hexanucleotide repeat expansions (GGGGCC) in the first intron of chromosome 9 open reading frame 72 (C9ORF72) gene are the most common genetic cause of both familial (~ 40%) and sporadic ALS (~ 8–10%), as well as the related condition, frontotemporal dementia (FTD), both sporadic (~ 5–10%) and familial forms (~ 25–30%) [18]. FTD primarily affects the frontal and temporal lobes of brain and patients exhibit a combination of cognitive, behavioural, and/or motor symptoms, although these can vary widely [19]. Some individuals with FTD may also develop symptoms of ALS and vice versa, referred to as 'ALS-FTD' [20]. Hence, there is significant genetic and pathological overlap between ALS and FTD. Three main mechanisms are implicated in neurodegeneration induced by hexanucleotide C9ORF72 mutations; production of toxic RNA, non-AUG translation (RAN) to produce dipeptide repeat proteins (DPRs) and haploinsufficiency due to lack of C9ORF72 protein [21].
Mutations in the genes encoding superoxide dismutase 1 (SOD1) and TAR DNA-binding protein 43 (TDP-43) cause another ~ 20% and ~ 4% cases respectively of familial ALS cases [22,23,24]. TDP-43 is an RNA/DNA binding protein normally located primarily in the nucleus. However, the presence of pathological forms of TDP-43, involving its truncation, abnormal aggregation, and mislocalization to the cytoplasm, are the characteristic hallmark of almost all (~ 97%) ALS cases [25]. Fused in Sarcoma (FUS) is another RNA-binding protein with structural and functional similarities to TDP-43 and mutations in FUS also cause ~ 4% cases [26]. Over 30 other genes have been linked to familial ALS, though each account for a smaller proportion of cases. These genes include CCNF, CHCHD10, ATXN2, KIF5A, hnRNPA2/B1, UBQLN2, TBK1. OPTN, PRPH, NEK1, VCP, and PFN1, among others [17, 27, 28].
Whilst ALS involves the degeneration and death of motor neurons, glial cells, which provide important supportive roles to neurons, also contribute to pathophysiology via non-cell autonomous mechanisms. Astrocytes regulate blood flow within the CNS, recycle neurotransmitters, and form the blood brain barrier. Microglia function in phagocytosis, the immune response, neuroinflammation, and immune surveillance and activation. Thus they act as the immune cells of the CNS [29]. Oligodendrocytes myelinate neuronal axons within the CNS to facilitate synaptic transmission and provide metabolic support to neurons, and Schwann cells myelinate neuronal axons in the peripheral nervous system (PNS). The latter cells also perform important roles in maintaining the function of the neuromuscular junction (NMJ) [30].
The clinical manifestations in ALS are driven by loss of voluntary muscle function, facilitated normally by motor neurons at the NMJ [31, 32]. Previously, ALS was considered to affect motor neurons primarily, and the involvement of skeletal muscle was thought to be a secondary consequence. However, the role of muscle in the pathogenesis of ALS is gaining increasing recognition (reviewed recently [33]).
Molecular hallmarks of ageing in ALS
The molecular and cellular hallmarks of ageing are defined by specific criteria [3]; (a) a hallmark should alter in a time-dependent fashion during the ageing process, (b) it should be enhanced by experimental acceleration of ageing, and (c) modulating the hallmark should inhibit, halt or even reverse ageing. Nine ageing hallmarks were originally proposed (in 2013) [3]: genomic instability, loss of telomeres, senescence, epigenetic modifications, dysregulated nutrient sensing, loss of proteostasis, mitochondrial dysfunction, stem cell exhaustion, and altered intercellular communication. However, these hallmarks were recently (2023) [3] expanded to include dysregulation of autophagy, inflammation and dysbiosis. It is important to note however that these twelve age-related hallmarks overlap significantly and are highly inter-related, with much crosstalk between these pathways (Fig. 1). Several are implicated as ‘primary’ hallmarks and drivers of the ageing process [3], including genomic instability, telomere dysfunction, epigenetic dysregulation, and proteostasis dysregulation (Fig. 2). In contrast, the ‘antagonistic’ hallmarks refer to cellular reactions to damage, including nutrient-sensing, mitochondrial dysfunction, and senescence. Finally, the ‘integrative’ hallmarks reflect the lack of ability of the cell to cope with the age-associated damage, involving defects in inter-cellular communication, stem cell exhaustion and dysbiosis. Below, we detail each of the twelve hallmarks, and how these relate to mechanisms of neurodegeneration in ALS.
Primary drivers of ageing and ALS. Ageing and neurodegeneration in ALS are complex processes influenced by a combination of genetic, environmental, and cellular factors. Whilst the precise causes of both ageing and ALS are not fully understood, genomic instability, telomere attrition, epigenetic alterations, proteostasis dysfunction, dysregulated autophagy and mitochondrial dysfunction are thought to be primary drivers
Primary hallmarks of ageing and how they relate to ALS
Genomic instability
Genomic instability refers to the high frequency of mutations within the genome [34]. This can result from both exogenous and endogenous sources, such as environmental agents and DNA replication errors, respectively. The DNA damage response (DDR) refers to the signalling pathways that normally detect and repair DNA damage, and the efficiency of DNA repair decreases during ageing [3]. Genomic instability results from either alterations in the nuclear architecture, damage to nuclear and/or mitochondrial DNA, and defective DNA repair mechanisms [35]. However, whilst genomic instability increases significantly with ageing, direct evidence showing that it modulates ageing specially is lacking.
Nuclear architecture alterations
The architecture of the nucleus maintains multiple aspects of genome stability. This primarily involves the nuclear lamina, a filamentous scaffold mesh underneath the nuclear envelope that tethers proteins and chromatin. Nuclear lamin proteins are its main constituents, and they are strongly associated with ageing and genome stability. Importantly, mutations in the genes encoding several of these proteins cause accelerated ageing disorders such as Hutchinson–Gilford progeria syndrome (HGPS, or progeria) [36, 37], which results from an abnormal truncated form of Lamin A (progerin), that also accumulates normally with age [38]. Dysregulation of Lamin B1 disrupts the shelterin complex and drives telomere instability in human cells [39].
Defects to the nucleus and impairment of nucleocytoplasmic transport are well-described in ALS. Nuclear pore pathology is detected in brains of sporadic ALS, TDP-43 and C9ORF72 patients [40]. Pathological forms of TDP-43 disrupt the nuclear architecture and nuclear pore complexes in ALS [40]. ALS-associated variant FUSR521G interacts with nucleoporins, which form the nuclear pore complex, and disrupts nucleocytoplasmic transport [41]. The C9ORF72 RNA and DPRs also interact with and disrupt various components of the nuclear transport machinery such as nuclear transport receptors, Ran GTPase, nucleoporins and nuclear envelope proteins [42]. However, nuclear morphology is unaltered in C9ORF72 ALS/FTD [43]. Loss of the nucleoporin NUP50 has been implicated as a risk factor for ALS [44]. Mutations in loss of never-in-mitosis A (NIMA)-related kinase-1 (NEK1) in induced pluripotent stem cell (iPSC) derived motor neurons also disrupt the nuclear architecture and import of proteins [45].
Damage to nuclear DNA
Cells are highly prone to DNA damage and insults arise at a rate of tens of thousands per day per cell [34]. Somatic mutations normally accumulate over time and the rate of formation is inversely correlated with lifespan [3]. During normal ageing, the efficiency of DNA repair mechanisms declines, resulting in the accumulation of DNA damage [35]. Furthermore, mutations in several DNA repair proteins cause several human progeroid disorders, directly linking DNA repair deficiencies to ageing. Double-stranded DNA breaks (DSBs) are the most toxic type of damage, which in neurons are repaired primarily by the error-prone non-homologous end-joining (NHEJ) mechanism. Neurons are also prone to oxidative DNA damage, which is repaired by base excision repair (BER) [46].
There is now extensive evidence for DNA damage in the pathophysiology of ALS. Several proteins central to ALS, including C9ORF72, FUS, TDP-43, SOD1, NEK1, C21orf2, senataxin, and valosin containing protein 1 (VCP), are known to function in DNA repair [47]. We and others have shown that TDP-43 is recruited to γH2AX foci where it functions in NHEJ [48], interacts with Ku 70 and is implicated in the repair of R loops [49, 50]. FUS interacts with histone deacetylase 1 (HDAC1) to repair DSBs [51] and it also functions in BER by mediating PARP1-dependent recruitment of XRCC1/DNA Ligase IIIα (LigIII). C21orf72 interacts with NEK1 and is thought to be involved in DSB repair [52,53,54]. VCP and senataxin are also involved in the maintenance of genomic integrity by facilitating transcription, DNA replication and the DDR [55, 56].
DNA damage is also induced by pathological forms of the same proteins in ALS [57]. ALS-mutant TDP-43 displays impaired activity in NHEJ, which disrupts R-loop homeostasis and induces TDP-43 pathology [48, 58]. TDP-43 pathology is associated with genome instability, encompassing splicing changes, somatic mutations, and gene fusions [59]. Loss of TDP-43 in the nucleus correlates with increased accumulation of DSBs [60, 61]. Similarly, ALS-associated mutant FUSR521C induces DNA damage and RNA splicing defects [62]. Loss of nuclear FUS impairs DNA nick ligation by inhibiting recruitment of XRCC1/LigIII [63], inducing aggregate formation and neurodegeneration [64]. In addition, ALS-associated variants of other proteins implicated in ALS also induce DNA damage. DNA repair genes are activated in response to DNA damage caused by SOD1G93A mutations in iPSC-derived motor neurons [65]. Hexanucleotide mutations in C9ORF72 induce DNA damage in neuronal cells, and motor neurons of ALS patients [66]. This has been associated with deficiencies in DSB and R loop repair and H2A ubiquitylation [67]. The C9ORF72 DPRs poly-glycine arginine (poly-GA) and poly-proline-arginine (poly-PA) induce DSBs, and phosphorylation of ataxia telangiectasia mutated (pATM) [68]. There is also evidence linking DNA repair defects to motor neuron loss. Ercc1Δ/− mice lacking DNA repair mechanisms nucleotide excision repair (NER), inter-strand crosslink repair, NHEJ and homologous recombination (HR) display aberrant motor neuron loss, microglia and astrocyte activation, Golgi apparatus dysfunction, genotoxic stress and NMJ pathology [69]. However, neither TDP-43 nor FUS pathology were detected in motor neurons in these mice, indicating that loss of Ercc1 alone is enough to induce ALS-related pathology [69, 70]. Together these data imply there is a strong correlation between ALS and DNA damage, raising the possibility that normal ageing increases genomic instability and thus the risk of neurodegeneration. However, this has not been shown directly.
Damage to mitochondrial DNA
Mitochondrial DNA (mtDNA) is particularly vulnerable to age-associated somatic mutations because of its proximity to oxidative phosphorylation sites and lack of protection by histones [71]. It accumulates oxidative damage in an age-dependent manner [71]. Furthermore, whilst mtDNA repair mechanisms are not as well-studied as those of nuclear DNA, they appear to be less efficient [71].
Mutations in mDNA and increased oxidative stress are implicated in both ageing and the development of ALS [72, 73]. Both wildtype TDP-43 and mutant TDP-43Q331K localise to mitochondria and trigger the release of mtDNA through the mitochondrial permeability transition pore [74]. The mtDNA accumulation then activates the cGAS/STING pathway, inducing neuroinflammation and neurodegeneration [74]. Cytoplasmic mtDNA is also present in spinal cords of ALS patients and iPSC-derived motor neurons [74]. Therefore, together these studies imply that damage to mtDNA is present in ALS, although this is not well-characterised.
Telomere attrition
Telomeres are non-coding repetitive DNA sequences (TTAGGG)n found at the distal ends of chromosomes that protect the integrity of the genome during replication. During normal ageing, the length of telomeres decreases, and rodents with short or long telomeres display inhibition or extension of lifespan, respectively [3]. Telomere shortening is thus one of the major features of ageing that is implicated in many age-related diseases [75]. Telomerase reverse transcriptase (TERT) prevents telomere shortening by maintaining telomere length [75], and whilst telomere shortening induces genomic instability and DNA damage, it is recognised as a separate hallmark of ageing [3].
Dysregulation in the length of telomeres has also been described in ALS. Knockout of telomerase leads to telomere shortening and an accelerated ALS phenotype in the SOD1G93A mice model [76]. In addition, age-dependent telomere shortening was detected in iPSC motor neurons from C9ORF72 patients [77]. However, a recent whole genome sequencing study concluded that longer telomeres are a risk factor for ALS and worsen prognosis, including in the brain [78]. Similarly, longer telomere length is associated with FTD [79]. Hence, it is possible that maintaining a balanced telomere length is essential in ALS and that alterations in telomere length, both lengthening and shortening, are both relevant to neurodegeneration. In contrast, genome wide association studies found no association between telomere length and ALS in leukocytes, implying that telomere length is cell-type specific [80]. Thus, these contrasting findings imply that more studies are required to characterise telomere length and activity in ALS.
Epigenetic alterations
Epigenetics refers to heritable changes in the regulation of gene expression independent of the DNA sequence. Multiple epigenetic modifications are known to alter during ageing [81], including DNA methylation, histone acetylation, chromatin remodelling and regulation of non-coding RNAs [81]. These alterations affect DNA replication and repair, gene transcription and silencing, cell division, and maintenance of telomere length [81]. DNA methylation on cytosine is one the most studied epigenetic modifications.
Chromatin, containing both genomic DNA and histones, regulates accessibility of the transcription machinery and thus gene expression. During ageing, chromatin alterations occur, including structural remodelling and changes in chromatin architecture, loss of histones and histone post-translational modifications. Histone acetylation is regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs) [82, 83]. Decreased global histone acetylation results in dysregulated metabolic gene expression and metabolic homeostasis [82]. Hyper-or hypo-acetylation of histones is regulated by HAT/HDAC homeostasis, and imbalance in this process induces defects in the integrated stress response and DNA repair mechanisms [84]. HDAC inhibitors have been implicated as a therapeutic strategy to prevent ageing [85].
There is increasing evidence for a role for epigenetic modifications in the pathogenesis of ALS, particularly in relation to the C9ORF72 repeat expansion [86]. Increased methylation of a CpG island near the GGGGCC repeat in the C9ORF72 promoter decreases C9ORF72 protein expression [87]. Furthermore, age-accelerated DNA methylation in the CpG-island 5′ is associated with more severe disease phenotype, early onset, and short disease duration in C9ORF72 patients [88]. Histones H3 and H4 undergo hyper-methylation of the promoter CpG-island [89] in ALS and FTD patients [90,91,92,93]. Hypermethylation also inhibits the formation of RNA foci and DPR aggregation in ALS [94]. Less nuclear 5-methyl cytosine (5mC) and 5hmC methylation was detected in lower motor neurons displaying TDP-43 pathology compared to those lacking pathology [95]. In addition, iPSC-derived motor neurons from ALS-associated FUS variants express more DNA methyltransferases and display more methylation in the FUS promoter region [96]. Studies in SOD1G93A mice also identified aberrant DNA and RNA methylation (increased or decreased) in spinal cords and skeletal muscles compared to control mice [97].
Epigenetic alterations to chromatin are also described in ALS. A chromatin remodelling complex, neuronal Brahma-related gene 1 (Brg1)-associated factor complex (nBAF), which in neurons regulates differentiation, dendritic expansion, and synaptic function, was lacking in cultured motor neurons expressing ALS-associated FUSR521G or TDP-43G348C [98]. Wildtype TDP-43 expression also disrupts chromatin dynamics due to impaired functioning of the chromatin remodelling enzyme CHD2 in Drosphilia [99]. HAT/HDAC homeostasis is altered in the brain and spinal cord of FUS-ALS patients [84]. HDAC inhibition using ACY-738 restores global histone acetylation, improves survival, and reduces metabolic abnormalities in a mouse model overexpressing wildtype FUS [100]. HDAC inhibitors have been examined extensively in ALS models (SOD1G93A mice, FUS and C9ORF72 mice models (detailed further in the "Therapeutic interventions for ageing and ageing-related diseases" section) [85, 101].
Loss of proteostasis
Protein homeostasis, or 'proteostasis', refers to the dynamic network of processes that regulate the protein synthesis, folding, trafficking and degradation machinery [102]. Proteostasis depends on the proper functioning of molecular chaperones, autophagy, the ubiquitin proteasome system (UPS), and endoplasmic reticulum (ER)-associated degradation (ERAD). Loss of proteostasis occurs if these protein quality control mechanisms fail and this can result in the accumulation of misfolded or aggregated proteins [103]. During normal ageing, the efficiency of proteostasis declines, and the consequent accumulation of damaged and aggregated misfolded proteins is a key hallmark of ageing and neurodegeneration. Lipofuscin aggregates—granules composed of misfolded proteins and lipids as a by-product of lysosomal digestion—also accumulate in motor neurons during normal ageing [104,105,106]. Proteins can also become post-translationally modified during ageing by oxidative damage from reactive oxygen species (ROS) or sugars, and the later modification results in the formation of advanced glycation end products (AGEs)[107]. The rate of protein translation decreases with age, and slowed translation elongation induces protein misfolding and ageing [108]. Proteostasis collapse refers to the breakdown or failure of the cellular machinery responsible for maintaining protein homeostasis and it is implicated as an important driver of cellular ageing in humans [103, 109].
The expression of protein chaperones such as the heat shock proteins (HSPs) decreases with ageing [110], implying that protein folding becomes impaired with increasing age. Administering recombinant human HSP70 to mice delays senescence, enhances proteasome activity and cognitive functions, reduces brain lipofuscin levels, and extends lifespan [111]. Feeding young fruit flies with AGEs and lipofuscin inhibits the UPS, which accelerates ageing and reduces lifespan [112]. Similarly, another chaperone, the oxidoreductase protein disulphide isomerase (PDI) is protective against cellular ageing in several models including replicative senescent human mesenchymal stem cells (RS hMSCs), HGPS hMSCs, Werner syndrome (WS) hMSCs and human primary hMSCs [113]. In addition, stabilising dysfunctional proteostasis using the chemical chaperone 4‐phenyl butyrate (PBA) improves cognitive behaviour and inhibits ageing [114].
The pathological ALS hallmark of misfolded protein aggregates strongly implicates proteostasis defects in pathophysiology [102]. Dysregulation of most proteostasis and protein quality control mechanisms are also well-described in ALS, including defects in autophagy, the UPS, ER-Golgi transport and ERAD [102]. Numerous molecular chaperones are also dysregulated in ALS including PDI proteins and HSPs [102]. PDI proteins have also been linked to ALS as a protective mechanism and as a genetic risk factor [115,116,117,118].
The formation of stress granules (SGs) is increasingly recognised in the maintenance of proteostasis [119]. SGs are cytoplasmic membrane-less organelles (also known as biomolecular condensates) composed of protein and RNA [120,121,122,123]. Functionally, they are implicated in the storage of biomolecules and as mRNA triage locations to regulate translation and the stability of mRNA [124, 125]. The formation of SGs is regulated by liquid phase separation (LLPS), the process by which proteins and nucleic acids in solution separate into liquid droplets (similar to droplets of oil forming in water) [126]. SGs assemble and disassemble in response to exogenous or environmental conditions, thus promoting survival during cellular stress [127]. LLPS is driven by proteins with intrinsically disordered domains, which includes misfolded proteins associated with ALS, including TDP-43 and FUS [128]. Recent studies have shown that SGs sequester misfolded proteins, preventing them from building up in the nucleus or cytoplasm, thus maintaining proteostasis [119]. However abnormal SGs disrupt proteostasis and during normal ageing defects in regulating the normal assembly/disassembly and dynamics of SGs is related to loss of proteostasis [33].
SGs are present in the pathological aggregates in ALS. Moreover, they are implicated in the formation of misfolded protein inclusions via nucleation of these aggregates [129]. TDP-43 localises in SGs in the presence of ER stress, oxidative stress, mitochondrial stress, osmotic stress, and inhibition of the proteasome [49, 130,131,132,133,134]. ALS-associated variant SOD1G93A colocalizes with SGs, unlike wildtype SOD1 [135, 136]. Co-localization of TDP-43 aggregates and SG markers has been detected in ALS patient tissues [120, 130, 137, 138], although cellular studies could not detect co-localization between mutant TDP-43 A315T, M337V and SGs under stress conditions [139, 140]. Similarly, colocalization between ALS-mutant FUS R495X and SGs has been reported in cell lines, primary neurons, human tissues [137, 141,142,143,144,145].
Dysregulated macroautophagy
Autophagy is a catabolic process responsible for the degradation and recycling of cellular components. Macroautophagy is the major type of autophagy, which involves the formation of double-membraned vesicles, or autophagosomes. Dysregulation of macroautophagy is well-described in ageing and was recently categorised as a separate hallmark from proteostasis, because organelles and non-protein cellular components are also subject to macroautophagy [3]. Expression of autophagy-related genes, including ATG5, ATG7, and OPTN, are known to decline with age [146, 147]. This results in the accumulation of protein aggregates and dysfunctional organelles during ageing [146]. In addition, stimulation or activation of autophagy increases healthspan and lifespan in humans and model organisms [146]. Autophagy is also reduced in muscle samples obtained from elderly patients [148]. Knocking out autophagy-related gene 7 (ATG7) in mice leads to increased muscle atrophy, muscular inflammation, abnormal structure, and reduced lifespan [148].
Dysregulated macroautophagy is implicated in neurodegeneration in ALS [149] and ALS-associated variants in C9ORF72, SOD1, TARDBP, TBK1, FUS, FIG4, OPTN, UBLN2, SQSTM1, CHMP2B, ALS2 dysregulate macroautophagy [150]. When autophagy is inhibited genetically or pharmacologically, ageing is accelerated and motor neuron toxicity is enhanced in ALS [146, 151]. Autophagy also plays a vital role in clearing protein aggregates associated with neurodegeneration in ALS [152]. Increased activation of autophagy proteins is detected in SOD1G93A transgenic mice [153]. Similarly, progesterone is neuroprotective through activation of autophagy in SOD1G93A mice [154]. C9ORF72 itself interacts with Rab1a and Unc-51-like kinase 1 (ULK1) complex to initiate autophagy via the formation of autophagosomes [155] and loss of C9ORF72 impairs autophagy [156, 157]. C9ORF72 DPRs co-localise with including p62-positive inclusions, suggesting that DPRs are targeted for clearance by the UPS and/or autophagy [158]. TDP-43A315T mutation activates ER stress and induces autophagy to clearance misfolded protein aggregates [159].
Antagonistic hallmarks of ageing in ALS
Cellular senescence
Senescence is implicated as an important characteristic and driver of the ageing process. Many studies have shown that senescence regulates age-associated phenotypes and is present in age-related diseases [160, 161]. Senescent cells were previously considered to be harmful because their elimination extends the lifespan of mice [162]. However, more recent studies in liver have reported that senescent cells positively impact on healthy ageing and lifespan and may have important functional roles in ageing [163]. In cycling cells, senescence is characterised by a state of eternal cell cycle arrest although they remain metabolically active [164]. DNA damage in the nucleus (mainly in the form of DSBs) and telomere shortening are key features of senescence [165,166,167,168,169]. The senescence-associated DDR involves ATR, ATM, and p53, which induces activation of cyclin-dependent kinase inhibitors p16, p21, and p27 and hyperphosphorylation of retinoblastoma protein (Rb), which results in withdrawal from the cell cycle [170]. Senescence is induced following diverse endogenous and exogenous stimulii, including oxidative stress, neuroinflammation, oncogenic activation, inactivation of tumour suppressor genes and mitochondrial dysfunction [168, 171]. During senescence, cells undergo several phenotypic modifications, including profound chromatin and secretome changes, and tumour-suppressor activation [172]. The senescence-associated secretory phenotype (SASP) is a prominent feature of senescence that induces inflammation via accumulation of pro-inflammatory cytokines, chemokines and growth factors [173]. Consequently, senescent cells can induce significant alterations in the cellular microenvironment through SASP, which can worsen inflammation [174]. Microglia in the white matter are thought to be the primary cell type undergoing senescence in the CNS during ageing [175].
As neurons are post-mitotic, they do not undergo classic ‘replicative’ senescence, so this mechanism was originally thought to be restricted to dividing cells. However, neurons express senescence markers, SASP is present in the ageing brain, and recent findings have revealed that neurons undergo a similar process as senescence in response to stress (‘stress-induced premature senescence’) [170, 176,177,178,179]. Hence, senescence in normal ageing neurons may compromise viability and increase their susceptibility to additional insults [180]. However, our understanding of senescence in neurons remains limited [181].
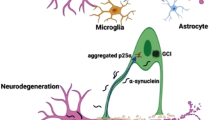
Senescence has been described in ALS, although this has been detected predominately in glial cells. In lumbar spinal cords of symptomatic SOD1G93A rats, microglia with characteristics of senescence were detected [174]. Senescence markers, including loss of nuclear lamin B1 expression and significantly increased p16INK4a, p53, matrix metalloproteinase-1 (MMP-1) were present compared to non-transgenic or asymptomatic transgenic rats [174]. Interestingly, other cell types in the degenerating lumbar spinal cord, including ChAT-positive motor neurons and GFAP-expressing astrocytes, also exhibited nuclear p16INK4a staining. Similarly, in astrocytes generated from iPSCs of individuals with sporadic ALS and ALS-C9ORF72 patients, there was a significant rise in expression of senescence markers [182]. The brains of ALS patients also display elevated numbers of senescent astrocytes [183].
Satellite cells are skeletal muscle adult stem cells that reside between muscles fibres and basement membranes and self-replicate and/or differentiate to new form new muscle fibres following injury [184]. Senescence has been reported in these cells in geriatric mice, resulting in halted muscle fibre regeneration [185]. B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) knockdown results in senescence-like phenotypes in young satellite cells [185]. Protein arginine methyltransferase 7 (PRMT7) [186] is associated with muscle regeneration capacity and its expression decreases in an age-dependant manner [187]. Decreased skeletal muscle mass, impaired satellite cell regeneration and premature senescence, were detected in PRMT7 knockout mice [186]. Together these findings lend support to the idea that senescence plays a role in the development of ALS, although this is not well-characterised [182].
Mitochondrial dysfunction
Mitochondria are multi-functional organelles that have long been associated with ageing. They provide the primary sources of cellular energy, and also regulate innate immunity, inflammation and apoptosis [188]. During ageing, mitochondrial functions become impaired by defects in membrane potential, decreased respiratory capacity, increased free radical production, reduced turnover, and dynamics, as well as accumulation of mutations in mtDNA [73, 188].
Mitochondrial dysfunction is widely described in ALS [73, 188, 189]. Deficiencies in mitochondrial respiratory chain complex 1 are present in motor neurons obtained from lumbar spinal cord sections from sporadic ALS patients [106, 190]. Decreased mitochondrial membrane potential is present in C9ORF72 and mutant TDP-43M337V human iPSC-derived motor neurons [191] and fibroblasts [192]. C9ORF72 haploinsufficiency impairs mitochondrial bioenergetics and function, and expression of electron transport chain complexes [193, 194]. Overexpression of C9ORF72 DPRs (particularly poly-GR) induces mitochondrial DNA damage, disrupts mitochondrial membrane potential, and increases ROS production [195]. Poly-GR binds to mitochondrial ATP synthase Atp5a1, inducing defects in mitochondrial structure and morphology [196]. Abnormal accumulation of mitochondria is present in spinal cord motor neurons of mutant TDP-43A315T and SOD1G93A transgenic mice [197] and mitochondria dysfunction and transport abnormalities are present in cells expressing ALS mutant TDP-43Q331K, M337V [198,199,200] and mutant SOD1G93A, G85R [201]. Mutations in UBQLN2P497S [202] and FUSR514G also induce mitochondrial abnormalities [203]. ALS-linked oxidised SOD1 triggers mitochondrial dysfunction and cellular senescence, which further accelerates ageing, providing a more direct link between oxidative stress, ALS and ageing[204]. Together, these findings suggest that mitochondrial dysfunction is closely associated with the major ALS pathological proteins.
Dysregulated nutrient sensing
During ageing, there is a decline in key metabolic signaling pathways relevant to ageing and neurodegeneration [210], involving the adrenergic, dopamine, insulin/insulin-like growth factor 1 (IGF1), AMP-activated protein kinase (AMPK), sirtuin (SIRT) and mTOR pathways. IGF-1 is a primary mediator of the action of growth hormone (GH) that modulates carbohydrate metabolism via insulin. Ageing results in reduced IGF-1 and GH levels [205], including in the brain [206]. AMPK is a sensor of cellular energy status, and its activation restores energy balance. Moreover, reduced AMPK activity is implicated in ageing [207]. mTOR, a serine-threonine protein kinase, is a negative regulator of ageing that promotes SASP [208]. In yeast, worms, and flies, blocking mTORC1 prolongs lifespan [209].
Nicotinamide adenine dinucleotide (NAD +) is a coenzyme central to energy metabolism and an essential cofactor in cellular redox reactions and SIRT activities [211]. It influences DNA repair, chromatin remodelling, and senescence, and reduced NAD + levels are detected during ageing [212, 213]. SIRTs are a family of seven proteins that regulate cell/tissue survival and metabolism, and they possess many functions associated with ageing. This includes DNA repair and genome stability, senescence, and mitochondrial function, and they inhibit oxidative stress, inflammation, and apoptosis [214, 215]. SIRT-1, 2, 3 and 6 also increase lifespan in species ranging from fruit flies to mammals [216,217,218]. Expression of SIRT1 decreases during ageing, hence elevating expression of SIRTs may protect against age-related events [219].
Changes in metabolic pathways are associated with the heterogeneity and diverse clinical characteristics of ALS. mTOR inhibition in mutant SOD1G93A transgenic mice hastens disease progression and increases motor neuron degeneration [220]. However, mTOR inhibition is protective in a transgenic mouse model involving neuron-specific wildtype TDP-43 overexpression [221]. IGF-1 overexpression in primary motor neurons is protective against glutamate-induced toxicity in ALS [222]. AMPK activation has been detected in motor neurons of ALS patients as well as in the spinal cord of SOD1G93A mice [223]. Dysregulation of SIRT has been described in ALS [224,225,226] and SIRT1 sensitive lysine-136 acetylation drives LLPS and pathological aggregation of TDP-43 [227, 228]. SIRT-1 activation has been examined therapeutically using resveratrol, which initially displayed promising effects by improving motor impairment and extending lifespan in SOD1G93A mice [229]. However, it failed in clinical trials [230].
Integrative hallmarks and ALS
Impaired intercellular communication
Cells can communicate with each other by either direct physical interactions or by intermediates such as extracellular vesicles (EVs) that act as inter-cellular messengers. During normal ageing, there is a gradual decline in the quality of communication between cells, which impacts on several processes relevant to ALS. These are discussed in the sections below.
Senescence and intercellular communication
Senescent cells are metabolically active and can communicate with, and influence the behaviour of, neighbouring cells through paracrine signalling [231]. Senescence is also an important part of inter-cellular communication and ageing [232] via SASP [233]. As well as the secretion of pro-inflammatory molecules, senescent cells also communicate with other cells via membrane-bound intercellular bridges or ‘tunnelling nanotubes’, that facilitate direct physical connections between cells [234]. The role of senescence in ageing and ALS is described in the 'Integrative hallmarks and ALS' section.
Neuroinflammation and inter-cellular communication between glia and neurons
Within the CNS, neurons, astrocytes, microglia, and oligodendrocytes must normally communicate with each other and the surrounding environment to maintain homeostasis. Motor neuron health and viability relies on efficient communication with glial cells and skeletal muscles [235, 236].
ALS is a non-cell-autonomous disease, and extrinsic inter-cellular communication amongst motor neurons, microglia, oligodendrocytes, and astrocytes is implicated in pathophysiology. This occurs through alterations in trophic factor support to motor neurons, signalling factors that impact on glial cell receptors and changes in direct cell-to-cell interactions [236]. Intrathecal administration of CSF from ALS patients in mice reduces the expression of trophic factors BDNF, fibroblast growth factor 2 (FGF2), and IGF-1 [237]. Pro-inflammatory cytokines and apoptosis-triggering TNF-α and Fas ligand (FASL) produced by activated microglia and astrocytes induce damage to motor neurons [238, 239]. Mouse cortical neurons treated with iPSC-derived astrocytes from C9ORF72 patients show increased oxidative stress and neurotoxicity [182]. Degenerating and morphologically altered oligodendrocytes are dramatically increased in mutant SOD1G93A mice and are surrounded by clustered activated microglia [240]. Astrocytes derived from post-mortem familial ALS (SOD 1A4V) and sporadic ALS patient brains are toxic to motor neurons, but this is alleviated by reducing SOD1 expression in astrocytes [241]. In mutant SOD1G93A mice model, senescent astrocytes display less support to motor neurons. Furthermore, IL-6 levels increase in astrocytes of SOD1G93A rodent models which recruits immune cells to clear the senescent cells [242, 243].
Extracellular vesicles and intercellular communication
EVs are tiny membrane-bound structures, typically ranging in size from 50 to 1000 nm [244]. They contain both protein and nucleic acid and they are released by various cell types in both physiological and pathological conditions [244]. Extracellular RNAs (exRNAs) are important mediators of cell-to-cell communication that are secreted as either EVs or in a complex with RNA binding proteins (RBPs)[245]. Senescence-associated EVs are implicated in the DDR and SASP [246, 247]. The levels of EVs alter during senescence and ageing, although it is controversial whether they increase or decrease [248,249,250].
ExRNAs and EVs are also implicated in ALS pathogenesis. Some exRNAs, including mRNA, microRNA and circular RNA, are present in exosomes and as they are dysregulated in ALS they have been proposed as potential biomarkers [174, 175] [251] (RNA dysregulation in ALS is reviewed in more detail in the 'Defects in RNA dysfunction section). Transgenic SOD1G93A mice release astrocyte-derived EVs containing mutant SOD1G93A that transfer to spinal neurons and selectively trigger death [252]. Microvesicles isolated from ALS patients contain higher levels of pathological proteins (SOD1, TDP-43, FUS) compared to controls, unlike exosomes, despite the mean size for both EV types being larger in ALS than controls [253].
Misfolded proteins are known to transmit between cells in ALS and other neurodegenerative diseases, particularly SOD1 [254, 255]. Several studies have described ‘prion-like’ characteristics of misfolded SOD1, including its capacity to transfer between cells and cause the misfolding of wildtype SOD1 within cells [256] and in vivo [257]. The transmission of toxic aggregates via EVs is not well-understood [244]. Misfolded SOD1, whether wildtype or ALS-associated variants A4V, G93A, G127X, are secreted as EVs in NSC-34 and HEK cells [254]. Astrocytes and neurons constitute the primary sources of EVs in vivo containing misfolded SOD1 in spinal cords of both SOD1G93A transgenic mice and SOD1-ALS patients [258]. Similarly, ‘prion- like’ behaviour for TDP-43 has been described in mice [259, 260]. Further studies have demonstrated both exosome-dependent and independent mechanisms are involved in TDP-43 inter-cellular transmission [261]. Similarly, C9ORF72 DPRs, poly-GA, poly-GP, poly-GR, and poly-PA transmit from cell-to-cell by exosome dependent and independent mechanisms [262].
Neuroinflammation and ageing
Inflammation increases significantly during normal ageing, both systemically and in the nervous system (neuroinflammation). Senescent cells also contribute to the persistent inflammatory environment via SASP [162], and their accumulation leads to sustained inflammation [263]. Inflammasomes, multimeric protein complexes that activate inflammatory caspase 1, are integral components of the innate immune system that become activated during the ageing process [264]. This includes the nucleotide oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome [265].
Neuroinflammation plays a significant role in ageing of the CNS and associated pathological conditions [266]. During ageing, activated microglia and astrocytes display altered morphologies and produce pro-inflammatory cytokines, leading to neuroinflammation [267,268,269]. When activated, astrocytes can display either neurotoxic, pro-inflammatory (A1) or neuroprotective, anti-inflammatory (A2) phenotypes. Similarly, microglia display both inflammatory and anti-inflammatory states, M1 and M2, respectively. RNA sequencing of brain-derived astrocytes throughout the lifespan of mice [270, 271] revealed up-regulation of A1-phenotype genes associated with neuroinflammation [271], linking astrocytes to cognitive impairment during ageing. Mice are protected from age-related reactive astrogliosis in the absence of microglial proinflammatory cytokines, suggesting that microglia are responsible for initiating the neuroinflammation that occurs with ageing [271]. However, astrocytes exert detrimental effects on microglia during ageing, impairing their phagocytic capabilities, resulting in a prolonged pro-inflammatory state [272].
Neuroinflammation is well-described in both human ALS and animal models [273]. Infiltration of peripheral lymphocytes, natural killer (NK) cells and macrophages, along with activation of astrocytes and microglia and the excessive production of inflammatory cytokines, is present in both humans and mice [274]. Interestingly, transcriptomic analysis of spinal cords of SOD1G93A mice revealed a significant overlap (90% shared transcripts) between gene expression patterns associated with normal ageing and ALS, particularly inflammation and immune system activation [219]. The NLRP3 inflammasome, along with expression of caspase-1, IL-1β, IL-18, and NFκB, is increased in the SOD1G93A transgenic rat [275]. In astrocytes of the spinal cord from SOD1G93A mice [276] and sporadic ALS patients, elevated levels of NLRP3, apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC), IL18, and active caspase 1, are present [277]. Microglia in mutant and wild-type SOD1G93A and TDP-43Q331K ALS mice express NLPR3, consistent with elevated expression of inflammasome components in vivo [265]. TDP-43 binds to CD14 receptors in microglia, macrophages, and monocytes, activating NFκB and stimulating the NLRP3 inflammasome [278]. In SOD1G93A rats, progression of paralysis was linked to neuroinflammation and motor neuron toxicity via microglia [174]. There is evidence for both neuroprotective and neurotoxic effects of astrocytes and microglia in ALS (reviewed in Clarke et al. 2020) [279, 280].
Stem cell exhaustion
Stem cells have self-renewal and multi-differentiation capabilities and thus regenerate tissue growth during ageing. Neural stem cells (NSCs) are responsible for producing neurons during prenatal development and maintaining the nervous system throughout adult life [281, 282]. However, during ageing, the functionality and regenerative capacity of NSCs deteriorates. This exhaustion of stem cells can be induced experimentally by upregulation of DNA damage, altered DNA repair mechanisms, decreased regenerative ability, epigenetic alterations, increased genomic instability, altered protein homeostasis, dysfunctional mitochondria, and senescence [290]. Several studies have identified possible ways to improve stem cell function during ageing, such as by increasing the levels of transcription factor FOXO4, HSP70 [290], or alternatively by exposing young blood to aged animals through heterochronic parabiosis [288].
A meta-analysis of eleven studies demonstrated that isolating and transplanting NSCs from the CNS into the spinal cord of transgenic mutant SOD1G93A mice slowed disease progression [281]. This was related to improvement of neurotrophic factor production, reduced neuroinflammation, and preservation of neuromuscular function [281]. Regenerating and renewing aged stem cells may be beneficial therapeutically in neurodegenerative diseases including ALS, although this has not been well-studied.
Dysbiosis
The gut microbiome is now recognised to play a critical role in health and well-being [3] including ageing [4, 5], and it is shaped by genetics, age, stress, illness, medication, diet, and the environment. However, the microbiome is dysregulated in many pathological conditions, which is known as 'dysbiosis’ [3]. Most gut microorganisms are bacteria, and they are implicated in metabolism, defence against pathogens, development of the immune system, and synthesis of vitamins, short-chain fatty acids and other metabolites [3]. Importantly, the microbiome interacts with the CNS via the gut-brain axis, the bidirectional network linking the enteric nervous system to the CNS [2].
The gut microbiome is established during childhood. Whilst it displays significant diversity among individuals, [3] during normal ageing, changes in the composition of gut microbiota and reduced species diversity are associated with frailty, cognitive function, depressive symptoms, and inflammatory processes [3]. Furthermore, mouse models of progeria and progeria patients with HGPS or Nestor-Guillermo progeria syndrome (NGPS) display dysbiosis, characterised by loss and gain of specific species [36]. Transplantation of faecal microbiota between wildtype mice and progeria mice confirm the existence of a strong link to healthspan/lifespan [37, 38] and in the maintenance of brain health and immunity during ageing [11]. Similarly, administration of gut microbiota metabolites improves age-related pathologies in mice [2, 3]. Collectively, these findings suggest that ageing is closely associated with dysbiosis.
Dysbiosis is also linked to neurodegeneration in ALS. Dysregulation of the gut microbiome correlates with disease severity in both mutant SOD1G93A transgenic mice and human patients [283]. Sodium butyrate is a bacterial metabolite produced in the gut by Butyrivibrio fibrisolvens, and reduced levels of this organism were detected in the SOD1G93A mouse [284]. Increased intestinal permeability to toxins was also detected [284] and treatment of SOD1G93A mice with butyrate also delayed weight loss and improved survival [285], implying that interventions aimed at restoring the gut microbiome may extend lifespan and healthspan in ALS. Alterations in gut microbiota have also been detected in C9ORF72-mutant mice [286], and C9ORF72 itself was found to inhibit systemic and neural inflammatory responses induced by gut bacteria [286]. Together these studies imply that the gut microbiome contributes both to ageing and the pathogenesis of ALS.
Defects in RNA functions
Defects in RNA metabolism are not included as a hallmark of ageing [3], but it has been proposed they should be designated as one, given increasing evidence highlighting their importance to ageing [287]. Given that ageing cells lose their ability to maintain RNA metabolism [288], and dysfunctional RNA metabolism is strongly implicated in the pathophysiology of ALS [289], here we consider this as an ageing hallmark that is discussed in relation to ALS.
The RNA milieu within a cell consists of coding messenger RNAs (mRNAs) and non-coding RNAs (ncRNAs), both of which interact with RNA-binding proteins (RBPs) within ribonucleoprotein complexes (RNPs). RBPs play important roles in RNA metabolism, including alternative splicing of pre-mRNA, transport, and stability, which are fine-tuned by modulation of their own expression and that of other RBPs [290]. They are also involved in modulation of SG dynamics by interaction with cytoplasmic RNAs and other RBPs. Dysregulation of RBPs also induces metabolic dysfunction, ageing, and senescence [291].
The transcriptome of the ageing cell results in global changes in gene expression with down-regulation of genes related to oxidative respiration, protein translation and growth signalling, and up-regulation of genes related to innate immunity, inflammation and DNA damage [292,293,294,295,296]. The multiple layers of processing that determine gene expression, including mRNA modification such as splicing, capping and polyadenylation, RNA export, localization, turnover, and translation, are affected by ageing and ALS [297]. In multiple species, including humans and mice, ageing results in shorter RNA transcripts in nearly 80% of tissues, disrupting the balance of long and short RNA transcripts [297].
The signalling pathways that control alternative splicing are some of the most dysregulated processes in normal ageing [292, 298] and senescence [287, 299]. Higher rates of alternative splicing, including back-splicing and circular RNA formation, reduced transcript quality, and mismatches with genome sequences are also detected during ageing [296]. Furthermore, during natural ageing, cryptic splice sites become revealed. These are sequences within introns that incorporate into the transcript during splicing, resulting in a premature stop codon and loss of function of the associated protein [300].
The ageing transcriptome may further be influenced by altered RNA polymerase II (Pol II) activity [296, 301]. The speed of RNA polymerase II elongation within introns increases with age across multiple cellular and animal models and human samples [296]. In contrast, stalling of Pol II at DNA damage sites increases with age which results in transcriptional stress and shorter transcripts [301]. Cells have stringent RNA quality control systems to prevent these detrimental processes. Aberrantly spliced mRNA with premature stop codons are degraded by 'nonsense mediated decay (NMD)' in order to prevent translation into deleterious non-functional proteins [302]. This process is however dysregulated with ageing and it particularly affects post-mitotic neurons that are more dependent on strong RNA quality control and efficient NMD processes [302].
Chemical modifications to RNA regulate RNA metabolism and are known to contribute to at least eight of the classical hallmarks of ageing, including cellular senescence, epigenetic changes, immune and stem cell dysfunction, concomitant metabolic dysregulation and loss of proteostasis [303]. These RNA modifications include methylation and A-to-I editing. Decreased m6A modifications are present in aged human PBMCs [304], impairing synaptic protein synthesis and synaptic functions relevant to ageing and neurodegeneration [305], suggesting that m6A RNA methylation contributes to cognitive decline in ageing [305]. A-to-I editing also declines during ageing specifically in the human brain [306]. Similarly, mice lacking mRNA editing apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC1) in microglia show acceleration of age-related neurodegeneration and motor deficiencies [307].
The expression of various ncRNAs is altered with ageing and influences its hallmarks. An array of ncRNAs, including long ncRNAs (lncRNAs), microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), small nucleolar RNA (snoRNA), small nuclear RNA (snRNA), ribosomal RNA (rRNA), small Cajal body specific RNA (scaRNA), transfer RNA (tRNA) and tRNA derived fragments (tRFs), are differentially expressed in ageing tissues [308]. Of these, miRNAs were the most modified as a result of ageing [308]. Age-related changes in global gene expression also correlate with the corresponding miRNA expression [309], which is not surprising given that miRNAs regulate mRNA expression. LncRNAs regulate histone methyltransferases and other chromatin modifying enzymes and thus they epigenetically modify gene expression [310]. They may also protect cells from senescence, because the lncRNA senescence-associated noncoding RNA (SAN) was increased in aged adipose-derived stem cells (ASCs)[311] and another lncRNA, NEAT1 suppressed cellular senescence in hepatocellular carcinoma [312]. NEAT1 plays a crucial role in forming a flexible environment within cells, increasing LLPS and condensation of RBPs and nucleic acids [313]. CircRNAs, a more recently described class of mostly ncRNAs, are gaining recognition as potent regulators of gene expression via their interaction with miRNAs [314], and are emerging players in ageing [315] and age-related diseases [316], including ALS [317, 318].
There is now strong evidence that dysfunctional RNA metabolism is present in ALS. Interestingly, degenerating neurons in ALS show similar RNA metabolic defects (RNA processing, modifications and transport) to ageing neurons [298]. Some of the major proteins dysregulated in ALS are RBPs, including TDP43, FUS, TAF15 and hnRNPA1 [28, 291]. TDP43 and FUS mislocalise and aggregate in the cytoplasm in sporadic ALS [319], which reduces their expression in the nucleus [288], resulting in loss of essential functions, including splicing and regulation of transcription. Loss of nuclear TDP43 also leads to the emergence of cryptic splice sites, which are now increasingly recognised as contributors to ALS. This includes a cryptic splice site in the first intron of the stathmin-2 gene (STMN2), leading to loss of protein and inability to repair axons following motor neuron injury [320].
RNA modifications and quality control of RNA such as m6A methylation, A-to-I editing, NMD and RNA surveillance are also dysregulated in ALS [321,322,323]. The C9ORF72 expansion repeats sequester RNA export factors [324, 325] and inhibit NMD [326]. In contrast, NMD hyperactivation was detected in fibroblasts derived from ALS-associated FUS patients [327], which reduces protein biosynthesis and contributes to motor neuron death in ALS [328]. TDP-43 interacts with NEAT1 resulting in its condensation into nuclear bodies in response to stress and pathological features of ALS, such as phosphorylation and its mis-localisation [329].
These studies therefore provide significant evidence linking dysregulated RNA metabolism to ageing and ALS. However it is important to recognise that these events are intricately interconnected [330]. For example, loss of the RBP HuR causes a reduction in methylation (C106) of the lncRNA component of telomerase (TERC), impairing telomerase function and resulting in telomere attrition and accelerated ageing [331] (Table 1).
Ageing of motor neurons and non-neuronal cells in ALS
The hallmarks described above detail ageing-related events at the molecular and cellular level. Below, we also briefly discuss below how ageing specifically affects the cell types relevant to ALS; motor neurons, glia, and skeletal muscle cells.
Ageing of motor neurons and glial cells in ALS
Motor neurons
It is unclear whether motor neurons are lost during normal ageing because conflicting findings have been obtained. Some studies have concluded that the size and number of motor neurons remains constant [367], whereas others report that there is a progressive motor neuron loss during physiological ageing [105, 368], similar to ALS, leaving the remaining aged motor neurons under stress [369].
It is clear that during normal ageing there are alterations in the properties of spinal α-motor neurons and a decline in neurotransmitter function [370]. This impairs their membrane and electrical properties, rendering them more susceptible to degeneration. Voluntary movements require efficient intra-neuronal excitatory (glutamatergic and cholinergic) and inhibitory (GABAergic and glycinergic) signalling [105]. Decreased cholinergic and glutamatergic synaptic inputs terminating on motor neurons are present in the ventral horn of old rhesus monkeys and mice [105]. In mice, membrane depolarization and increased expression of voltage-gated sodium channel isoform Nav1.8 l are present in aged motor neuron axons [371]. In addition, as neurons age, they lose their excitatory synaptic connections across the cell body and dendritic branches. Consequently, older motor neurons possess a diminished balance of excitatory to inhibitory synapses, which could impair their ability to initiate motor movements [305]. The expression of matrix metalloproteinase 1 (dMMP1) rises during ageing, which leads to motor functional impairments that worsen with ageing Drosophila motor neurons [372].
Alterations in synaptic transmission and the excitability of motor neurons are one of the first events in ALS. Hyperexcitability of both upper and lower motor neurons is frequently observed in SOD1G93A mouse models, ALS-iPSC derived motor neurons, and ALS patients [373,374,375]. Excitotoxicity, referring to excessive activation of glutamate receptors and subsequent neuronal injury or death, is also commonly described in disease models [376]. During disease progression, however, motor neurons become hypo-excitable, although this could be a compensatory process [376]. In ALS patients, electrophysiological studies have identified abnormalities in sodium and potassium currents, implying that membrane depolarization and age-related changes in membrane excitability are present in median motor axons [377]. Increased expression of Drosophila dMMP1 in motor neurons contributes to the decline in motor function observed during ageing [372]. TDP-43 overexpression in neurons accelerates neuronal death by triggering dMMP1 expression, suggesting potential connections between ageing and ALS [372]. With ageing, motor neurons may become less efficient in transmitting signals to muscles, leading to slower response times and decreased motor control [378].
It remains unclear why motor neurons are selectively targeted in ALS. Neurons themselves display features that may render them more susceptible to the effects of ageing. Neurons rely on error-prone NHEJ for DSB repair [379], and being post-mitotic, they are unable to dilute the effect of DNA repair errors by cell division, unlike other cell types. Thus, they may be particularly vulnerable to DNA damage, as well as senescence [181, 379]. Post-mitotic neurons also may be more susceptible to the accumulation of misfolded proteins than other cell types, where the effect of protein aggregation can also be reduced by cell division. Hence, they are likely to be more susceptible to proteostasis dysfunction.
However, motor neurons possess distinctive characteristics compared to other neurons, which may render them uniquely vulnerable to neurodegeneration in ALS [1]. Motor neurons are large cells, with very long axons (up to 1m in an adult human), which may render them more prone to injury [380]. Also, they need to transmit signals over long distances and sustain constant firing and communication with muscles. Thus, the electrical activity of motor neurons may also contribute to their susceptibility in ALS, because this necessitates a significant amount of energy [380]. The constant firing of action potentials and the high metabolic rates required to maintain electrochemical gradients across neuronal membranes also increases oxidative stress [381]. Motor neurons are also highly susceptible to glutamate excitotoxicity compared to other neurons [382].
Within motor neurons, susceptibility to neurodegeneration in ALS is not uniform. Specific populations of motor neurons, including those in the oculomotor and Onuf's nuclei, remain relatively spared and do not degenerate until later stages of disease in humans [383] and mouse models [384,385,386,387]. Similarly, oculomotor neurons are not usually affected during ageing. In contrast, spinal motor neurons are targeted in both ALS and ageing, implying that ageing increases the susceptibility of spinal motor neurons to degeneration [388, 389]. Several studies have identified different gene expression profiles between oculomotor and spinal motor neurons. Microarray and laser capture microdissection of motor neurons isolated from oculomotor/trochlear nuclei, the hypoglossal nucleus, and the lateral column of the cervical spinal cord in humans and SOD1G93A rats have revealed unique expression patterns in pathways associated with the ageing hallmarks, including loss of proteostasis, mitochondrial impairment and dysregulated autophagy [390, 391].
Differential susceptibility among motor neuron subtypes, even within the same motor unit, is also observed in ALS. Fast-fatigable (FF) motor neurons degenerate early in disease course, and the fatigue-resistant (FR) types subsequently follow later. In contrast, the slow (S) MNs are resistant to degeneration and are retained, even up to late in disease course. The reasons for the selective vulnerability of motor neuron subtypes remain unclear. However, both FF and FR subtypes are affected first during normal ageing whereas the slow subtypes are affected later, implying that ageing increases the susceptibility of FF and FR motor neurons in ALS [392,393,394]. The motor neuron subtypes also display noticeable differences in their properties. FF motor neurons possess somas with large-diameter whereas S motor neurons contain much smaller soma [385]. Also, FF motor neurons are much less excitable than the FR subtype which in turn are less excitable than the S subtype, also linking motor neuron susceptibility to excitability. Different gene expression profiles are also evident between these subgroups [385].
The unique combination of features in motor neurons, including their long axons, high energy demands, increased DNA damage and error-prone DNA repair mechanisms, neuronal senescence, susceptibility to excitotoxicity, and heterogeneity in susceptibility among subtypes, may therefore collectively render them more vulnerable to the effects of ageing [395]. Further exploration of these aspects is crucial for understanding the mechanisms underlying age-related motor neuron degeneration. Characterising these events may pave the way for targeted interventions to promote motor neuronal health during the normal ageing process.
Glial cells
Age-related changes and the presence of the ageing hallmarks are also detected in glial cells, as detailed below. The role of neuroinflammation induced by reactive astrocytes and microglia is discussed in the 'Neuroinflammation and ageing' section.
Astrocytes
Ageing is associated with morphological changes to astrocytes, characterized by atrophy and shrinking of processes such as branches and leaflets, and a decline in their function [396]. More specifically during ageing there is a decrease in synaptic connections and plasticity [397]. Astrocyte marker glial fibrillary acidic protein (GFAP) is highly increased in the aged brain, representing activation and gliosis during neurodegeneration [398]. The blood brain barrier is also maintained by astrocytes which also becomes compromised and leaky during ageing [398]. Dysregulation of astrocyte function, leading to sustained release of pro-inflammatory molecules such as IL-8, IL-1β, IL-6, IL-18, TNF-α, is implicated in ageing and age-related neurogenerative diseases [399].
Many studies have implicated astrocytes in ALS pathogenesis. A meta-analysis of studies involving human iPSC-derived astrocytes with variations in SOD1, C9ORF72, and FUS, with those using mouse astrocyte models expressing the SOD1G93A mutation or TDP-43 deletion, or Tmem259 (membralin) deletion, revealed a consistent pattern of gene expression changes amongst these models [400], involving upregulation of genes associated with extracellular matrix dynamics, ER stress responses, and the immune system [400]. Reactive astrocytes induce neurofilament and SOD1 aggregation by disrupting autophagy via the action of TGF-β1, leading to motor neuron degeneration [401]. This occurs through the disruption of autophagy, primarily mediated by TGF-β1 [401]. Increased oxidative stress, reduced survival of motor neurons and neurotoxicity were observed in human iPSC-derived astrocytes from C9ORF72 ALS patients [182]. Similarly, iPSC-derived astrocytes from FUS R521H, P525L ALS patients impair motor neuron-neurite outgrowth, and formation and functionality of the NMJ formation [402].
Microglia
Microglia also undergo morphological changes with age [29]. In aged rats, microglia are characterized by shortened and less intricately branched structures containing nerve fibres and myelin sheets [403]. In the brains of aged individuals, a subset of microglia demonstrated elevated expression of activation markers, including MHC classII, CD45, and CD4. This expression pattern suggested that aged microglia exhibited increased inclusions, indicative of heightened phagocytosis activity [404]. Aged microglia cells also display altered mTOR signaling and increased oxidative stress [29].
There is debate whether microglial activation is present in ALS patients—transitioning from a ramified or stellate shape (inactive state) to an ameboid form (active state)—and whether there is increased proliferation and/or upregulation of inflammatory pathways in post-mortem ALS tissue [279, 405]. Evidence from mouse models including SOD1G93A, A4V, TDP-43WT, M337V, A315T, FUSWT and C9ORF72 knockouts revealed microglia activation and morphological changes compared to controls [279, 406,407,408]. Reactive microglia are neuroprotective in a TDP-43 mice model [409]. Monocyte-derived microglia-like cells display pathological hallmarks of ALS, including cytoplasmic aggregation and phosphorylation of TDP-43, DNA damage, and cell-specific impairment of phagocytosis associated with disease progression [410].
Oligodendrocytes
Whilst oligodendrocytes normally myelinate axons in the CNS [411], during ageing, myelin regeneration becomes slowed [411]. Ageing oligodendrocytes also display reduced expression of myelin-associated genes MOG, PLP, and CNP [412], and HMGCS1, which is associated with cholesterol synthesis [412]. Conversely, ageing oligodendrocytes display increased expression of genes related to ribosome biogenesis, RPl6, RPS29, and RPl23A, and upregulation of immune-related genes such as C4B and Il33 [412].
Selective removal of mutant SOD1 selectively from oligodendrocytes substantially delays disease onset and prolonged survival in SOD1G93A mice [413]. Oligodendrocytes supply energy to axons through glucose and lactose shuttling and blockage in these pathways is implicated in motor neuron degeneration in ALS [414]. Oligodendrocytes also induce motor neuron death via human SOD1-dependent mechanisms in ALS [415]. Morphological changes and the presence of TDP-43 inclusions were detected in oligodendrocytes in human sporadic ALS spinal cords [416].
Schwann cells
Schwann cells are peripheral myelin generating cells that have a remarkable capacity to regenerate and remyelinate but during ageing these abilities decline [417, 418]. The ageing process in Schwann cells is also linked to abnormalities in myelination in mice [418]. Moreover, older mice show a significant decrease in the number of myelinated nerve fibres [419].
The role of Schwann cells in ALS pathology is poorly understood. However, they induce peripheral nerve inflammation through expression of CSF1, IL-34, and SCF factors in ALS patients [420, 421] and transgenic SOD1G93A rodents [421, 422]. Reducing the levels of SOD1G37R from Schwann cells accelerated disease progression in mice [384]. Terminal and pre-terminal Schwann cells are lost in transgenic SOD1G93A mice, which impairs reinnervation following muscle denervation [422].
Ageing of skeletal muscle/NMJs
Physiological ageing can significantly affect skeletal muscles [423], both structurally and functionally. This can result in progressive loss of muscle mass or ‘sarcopenia’, leading to muscle weakness and motor control [423, 424]. The neuromuscular junction (NMJ) is a specialized tripartite chemical synapse that entails highly coordinated communication between the presynaptic motor neuron, postsynaptic skeletal muscle, and terminal Schwann cells. Muscle denervation is a major contributor to sarcopenia [425] and alterations in the NMJ during ageing play a pivotal role in this process [425,426,427]. In muscles, age-related decline in mitochondrial function [428] of 25–30% between the ages 30 and 70 years occurs [429]. Similarly, reduced oxygen consumption rates and elevated ROS production in muscles are present during normal ageing in mice [430].
Satellite cells display many classic hallmarks of ageing, and their capability to regenerate becomes compromised during normal ageing [185]. These are also similar to features detected in ALS, including increased DNA damage, mitochondrial dysfunction, loss of proteostasis, oxidative stress, and autophagy defects [351, 431]. Elderly satellite cells display disrupted antioxidant activity and increased membrane fluidity compared to those of young individuals [432], indicating age-dependent imbalance in the antioxidant system during ageing. Age related dysfunction of satellite cells and decreased regenerative capacity are also present in ALS [433].
Impaired NMJs are well-described in ALS although there is controversy whether this involves ‘dying forward’ (originating in the motor cortex before descending to the NMJ) or "dying back" (beginning at the NMJ and then propagating to the motor neuron cell body) mechanisms. Impairment in NMJs is detected in ALS patient tissues and disease models [434]. Transgenic SOD1G93A mice [435] and mutant FUSR521C mouse models exhibit changes in the NMJ early in disease course [387, 436]. Similarly, transgenic SOD1G93A zebrafish display early defects in motor neuron outgrowth, axonal branching and dysregulated NMJ with reduced postsynaptic volume [437, 438]. ALS transgenic mice displaying TDP-43 pathology that lack the nuclear localization signal, also exhibit substantial muscle denervation [439]. Similarly, inducible expression of the C9ORF72 hexanucleotide repeat expansion in mice result in structural abnormalities in NMJs. In addition, early inhibition of C9ORF72 expression leads to structural NMJ abnormalities and rapid muscle dystrophy [440]. It has been proposed that as NMJ impairment in ALS leads to motor neuron dysfunction [441], and degeneration of motor neurons results in further NMJ dysfunction, this creates a viscous cycle, resulting in the progressive loss of muscle control and strength [441].
Therapeutic interventions for ageing and ageing-related diseases
Whilst normal ageing is an inevitable consequence for all individuals, despite the increasing aged population, few therapeutic approaches have been developed for ageing itself. In contrast, there has been intense efforts to develop treatments for age-related diseases, including ALS [442]. There has been considerable progress in this area and there are now seven FDA-approved drugs for ALS. However, these treatments have limited efficacy or are restricted to specific groups of patients. Thus it is important to continue to develop new approaches.
During normal ageing there is a progressive decrease in functionality and in ALS this becomes accelerated. Thus, therapeutic approaches should aim to promote healthspan as well as lifespan. In humans, lifestyle interventions such as calorie restriction and exercise have been examined as approaches to prevent/delay ageing. Furthermore, numerous therapeutic studies have been performed in animal models, demonstrating that delayed ageing and prolonging longevity and healthspan are possible [442]. In particular, treatments that inhibit mTOR are showing promise [209]. In cells, senescence phenotypes such as size and granularity, β-galactosidase staining and fibroblastic spindle morphology are reversed by mTOR inhibition [443]. Rapamycin, an mTOR inhibitor, extends the lifespan of yeast, nematodes, fruit flies and mice [444]. Rapamycin is safe and well-tolerated in a randomised, double-blinded, placebo controlled ALS clinical trial, but further trials are necessary to understand the clinical and biological effects of the drug in ALS [445].
As the levels of NAD+ decline with normal ageing, NAD+ precursors that increase the levels of NAD+ have been examined therapeutically, including nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN), NAD+ biosynthetic enzymes, and NAD+ degradation inhibitors [446]. NMN (CD38 inhibitor) and NR (PARP inhibitor) administration in transgenic SOD1G93A mice delay senescence, improve stem cell renewal, and enhance lifespan [447]. However, deletion of CD38 had no effect in survival in two hSOD1 ALS mouse models [448, 449]. These compounds are in various stages of human clinical trials for ALS [335, 449].
Compounds that enhance SIRT activity may also be protective against ageing given that this function is decreased with age [450]. These include polyphenols such as resveratrol, which activates SIRT1 NAD+-dependent histone deacetylase activity [451]. Administration of dietary resveratrol extends lifespan of several organisms including yeast, nematode and fruit flies [452] and mice models of ageing [453]. Resveratrol also enhances mitochondrial biogenesis and improves oxidative capacity in ageing mice models [454]. Resveratrol treatment also prevented motor neuron loss, relieved muscle atrophy, and improved mitochondrial function in muscles [455] and extended survival in SOD1G93A mice [229]. However, another study reported that in SOD1G93A mice it did not improve motor function or increase survival when given as a single dose, in contrast to these other studies [456].
Metformin, used in Europe as traditional medicine since the early nineteenth century, is also implicated as an anti-ageing treatment, because it suppresses cellular senescence phenotypes [457] and has been described as a possible protective factor against neurodegeneration [458, 459]. Metformin treatment of iPSCs generated from HGPS patients decreases progerin expression and reduces abnormalities in nuclear architecture, suggesting it has therapeutic potential to reduce ageing [460]. Lonafarnib is a farnesyltransferase inhibitor that reduces the effects of progerin on nuclear morphology in HGPS and is the only FDA approved treatment for HGPS [461]. FOXO4 peptide treatment and immunotherapy reverses senescence-associated loss of tissue by restoring the apoptotic role of p53 [462]. A small molecule telomerase activator, TA-65, inhibited telomere shortening and enhanced immune function [463], increased healthspan and rescued DNA damage defects in old adult mice [464]. Similarly, supplementation of TA-65 led to an improvement of healthspan indicators in several studies [465]. A recent RNA-seq analyses identified IGF1 and mitochondrial translation as longevity signatures common to 41 mammalian species and identified compounds that extended lifespan in mice [466]. However, none of these approaches have yet been tested in ALS (Table 2).
DNMTs, HDACs and HATs have also been examined in ageing. HDACs include the ‘classical’ Zn2+-dependent deacetylases (class I, II, and IV) and SIRT deacetylases (class III) that are also implicated in longevity [473]. Inactivating HDAC homologs in C. elegans and Drosophila improved lifespan, and delayed age-related physical decline [336]. HDAC inhibitors trichostatin A, scriptaid, tubastatin A, valproic acid, entinostat, sodium phenylbutyrate improved lifespan, delayed disease onset and improved motor function in SOD1G93A mice [101, 472]. HDAC inhibitors may therefore have potential in age-related diseases such as ALS [474]. However, whilst HDAC inhibitors sodium phenylbutyrate and valproic acid are safe and tolerable in human ALS phase II clinical trials, there was no difference in survival compared to placebo [101].Therapeutic strategies involving preventing protein aggregation and restoring proteostasis have been widely studied in ALS. Molecular chaperones such as HSPs [475] and PDI family members are neuroprotective against ALS mutant SOD1 and TDP-43-induced pathologies, including in vivo [115, 338, 476]. Compounds targeting neuroinflammation have largely been ineffective in ALS clinical trials, although a phase II study involving tyrosine kinase inhibitor masitinib with riluzole showed promising results in ALS patients [477].
Conclusion
The universal phenomenon of ageing is the largest risk factor for age-related neurodegenerative diseases, including ALS [335, 478]. Hence, therapeutic strategies that target ageing mechanisms may be beneficial in these conditions. However, to date, ageing has not been explored specifically as a drug development target in relation to ALS. Furthermore, the molecular links between ageing and neurodegeneration remain poorly understood. Normal ageing is a complex, multi-layered phenomenon that is difficult to separate from many age-associated diseases. Furthermore, the recognised molecular and cellular hallmarks of ageing overlap significantly and are strongly related to each other (Fig. 1). Moreover, there are numerous similarities between ageing and the pathophysiology of ALS. However, whilst most neurodegenerative mechanisms implicated in ALS are related to the key hallmarks of ageing, more studies are required to confirm a direct connection between dysfunction in these events and ageing in ALS. The pathophysiology underlying ALS involves a complex interaction between ageing, genetic, epigenetic, and environmental factors. As pathogenesis in ALS is thought to be multistep process requiring six steps [479], ageing may therefore be an important accelerator of neurodegeneration in ALS.
Given the complexity of ALS, it is important to employ a multifaceted approach to target age-associated molecular mechanisms. Maintenance of the integrity of both the genome and proteome is of central importance in this process and increasingly described links between these pathways are described [479, 480]. Similarly, restoring proteostasis and improving resilience to cellular stress will be essential to withstand the insults associated with ALS. The increasing recognition of skeletal muscle in the etiology of ALS must also be considered, particularly as many features of normal ageing in muscle are also described in ALS. However, understanding the primary mechanisms that drive ageing at molecular and cellular level will facilitate in unravelling the mysteries of why the risk of ALS increases with age. Normal ageing is an inevitable consequence for all individuals but it will be important to consider age-related molecular events when considering novel therapeutic approaches for ALS in the future to design more effective strategies to halt neurodegeneration [442].
Availability of data and materials
Not applicable.
References
Blinkouskaya Y et al (2021) Brain aging mechanisms with mechanical manifestations. Mech Ageing Dev 200:111575
Piekarz KM et al (2020) Molecular changes associated with spinal cord aging. GeroScience 42(2):765–784
López-Otín C et al (2023) Hallmarks of aging: an expanding universe. Cell 186(2):243–278
Carmona JJ, Michan S (2016) Biology of healthy aging and longevity. Rev Invest Clin 68(1):7–16
Khan SS, Singer BD, Vaughan DE (2017) Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16(4):624–633
Jin K (2010) Modern biological theories of aging. Aging Dis 1(2):72–74
Davidovic M et al (2010) Old age as a privilege of the “selfish ones.” Aging Dis 1(2):139–146
van Heemst D (2010) Insulin, IGF-1 and longevity. Aging Dis 1(2):147–157
Fulop T et al (2014) On the immunological theory of aging. Interdiscip Top Gerontol 39:163–176
Brys K, Vanfleteren JR, Braeckman BP (2007) Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp Gerontol 42(9):845–851
Hulbert AJ et al (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87(4):1175–1213
Bjorksten J (1968) The crosslinkage theory of aging. J Am Geriatr Soc 16(4):408–427
Bjorksten J, Tenhu H (1990) The crosslinking theory of aging–added evidence. Exp Gerontol 25(2):91–95
Gerschman R et al (1954) Oxygen poisoning and x-irradiation: a mechanism in common. Science 119(3097):623–626
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11(3):298–300
Freitas AA, de Magalhães JP (2011) A review and appraisal of the DNA damage theory of ageing. Mutat Res 728(1–2):12–22
Taylor JP, Brown RH Jr, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539(7628):197–206
Renton AE et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72(2):257–268
Jellinger KA (2023) The spectrum of cognitive dysfunction in amyotrophic lateral sclerosis: an update. Int J Mol Sci 24(19):14647
Cividini C et al (2021) Amyotrophic lateral sclerosis-frontotemporal dementia: shared and divergent neural correlates across the clinical spectrum. Neurology 98(4):e402–e415
Sattler R et al (2023) Roadmap for C9ORF72 in frontotemporal dementia and amyotrophic lateral sclerosis: report on the C9ORF72 FTD/ALS summit. Neurol Ther 12(6):1821–1843
Rosen DR et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362(6415):59–62
Kabashi E et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40(5):572–574
Sreedharan J et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319(5870):1668–1672
Neumann M et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314(5796):130–133
Vance C et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323(5918):1208–1211
Mead RJ et al (2023) Amyotrophic lateral sclerosis: a neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov 22(3):185–212
Akçimen F et al (2023) Amyotrophic lateral sclerosis: translating genetic discoveries into therapies. Nat Rev Genet 24(9):642–658
Antignano I et al (2023) Aging microglia. Cell Mol Life Sci 80(5):126
Snyder-Warwick AK et al (2018) Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell 17(4):e12776
Verma A (2021) Clinical manifestation and management of amyotrophic lateral sclerosis. In: Araki T (ED) Amyotrophic Lateral Sclerosis. Exon Publications Copyright: The Authors: Brisbane (AU)
Hobson EV, McDermott CJ (2016) Supportive and symptomatic management of amyotrophic lateral sclerosis. Nat Rev Neurol 12(9):526–538
Cao X, Jin X, Liu B (2020) The involvement of stress granules in aging and aging-associated diseases. Aging Cell 19(4):e13136
Jackson SP, Bartek J (2009) The DNA-damage response in human biology and disease. Nature 461(7267):1071–1078
Yousefzadeh M et al (2021) DNA damage-how and why we age? Elife 10:e62852
De Sandre-Giovannoli A et al (2003) Lamin A truncation in Hutchinson–Gilford progeria. Science 300(5628):2055
Eriksson M et al (2003) Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423(6937):293–298
Olive M et al (2010) Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol 30(11):2301–2309
Pennarun G et al (2021) Increase in lamin B1 promotes telomere instability by disrupting the shelterin complex in human cells. Nucleic Acids Res 49(17):9886–9905
Chou C-C et al (2018) TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci 21(2):228–239
Lin YC et al (2021) Interactions between ALS-linked FUS and nucleoporins are associated with defects in the nucleocytoplasmic transport pathway. Nat Neurosci 24(8):1077–1088
Chandra S, Lusk CP (2022) Emerging connections between nuclear pore complex homeostasis and ALS. Int J Mol Sci 23(3):1329
Coyne AN, Rothstein JD (2021) Nuclear lamina invaginations are not a pathological feature of C9orf72 ALS/FTD. Acta Neuropathol Commun 9(1):45
Megat S et al (2021) Loss of nucleoporin Nup50 is a risk factor for amyotrophic lateral sclerosis. MedRxiv. https://doi.org/10.1101/2021.08.23.21262299
Mann JR et al (2023) Loss of function of the ALS-associated NEK1 kinase disrupts microtubule homeostasis and nuclear import. Sci Adv 9(33):eadi5548
Narciso L et al (2016) The response to oxidative DNA damage in neurons: mechanisms and disease. Neural Plast 2016:3619274
Konopka A, Atkin JD (2022) DNA damage, defective dna repair, and neurodegeneration in amyotrophic lateral sclerosis. Front Aging Neurosci 14:786420
Konopka A et al (2020) Impaired NHEJ repair in amyotrophic lateral sclerosis is associated with TDP-43 mutations. Mol Neurodegener 15(1):51
Freibaum BD et al (2010) Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res 9(2):1104–1120
Hill SJ et al (2016) Two familial ALS proteins function in prevention/repair of transcription-associated DNA damage. Proc Natl Acad Sci U S A 113(48):E7701-e7709
Wang WY et al (2013) Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat Neurosci 16(10):1383–1391
Pelegrini AL et al (2010) Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest. Mutagenesis 25(5):447–454
Lai CK et al (2011) Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell 22(7):1104–1119
Fang X et al (2015) The NEK1 interactor, C21ORF2, is required for efficient DNA damage repair. Acta Biochim Biophys Sin (Shanghai) 47(10):834–841
Zhu C et al (2020) Phospho-Ser(784)-VCP is required for DNA damage response and is associated with poor prognosis of chemotherapy-treated breast cancer. Cell Rep 31(10):107745
Rawal CC et al (2020) Senataxin ortholog Sen1 limits DNA:RNA hybrid accumulation at DNA double-strand breaks to control end resection and repair fidelity. Cell Rep 31(5):107603
Shadfar S et al (2023) Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl Neurodegener 12(1):18
Giannini M et al (2020) TDP-43 mutations link Amyotrophic Lateral Sclerosis with R-loop homeostasis and R loop-mediated DNA damage. PLoS Genet 16(12):e1009260
Ziff OJ et al (2023) Integrated transcriptome landscape of ALS identifies genome instability linked to TDP-43 pathology. Nat Commun 14(1):2176
Mitra J et al (2019) Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proc Natl Acad Sci U S A 116(10):4696–4705
Kawaguchi T et al (2020) Changes to the TDP-43 and FUS interactomes induced by DNA damage. J Proteome Res 19(1):360–370
Qiu H et al (2014) ALS-associated mutation FUS-R521C causes DNA damage and RNA splicing defects. J Clin Invest 124(3):981–999
Wang H et al (2018) Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat Commun 9(1):3683
Naumann M et al (2018) Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat Commun 9(1):335
Barbosa LF et al (2010) Increased SOD1 association with chromatin, DNA damage, p53 activation, and apoptosis in a cellular model of SOD1-linked ALS. Biochim Biophys Acta 1802(5):462–471
Farg MA et al (2017) The DNA damage response (DDR) is induced by the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Hum Mol Genet 26(15):2882–2896
Walker C et al (2017) C9orf72 expansion disrupts ATM-mediated chromosomal break repair. Nat Neurosci 20(9):1225–1235
Nihei Y et al (2020) Poly-glycine-alanine exacerbates C9orf72 repeat expansion-mediated DNA damage via sequestration of phosphorylated ATM and loss of nuclear hnRNPA3. Acta Neuropathol 139(1):99–118
de Waard MC et al (2010) Age-related motor neuron degeneration in DNA repair-deficient Ercc1 mice. Acta Neuropathol 120(4):461–475
Shadfar S, Brocardo M, Atkin JD (2022) The complex mechanisms by which neurons die following DNA damage in neurodegenerative diseases. Int J Mol Sci 23(5):2484
Druzhyna NM, Wilson GL, LeDoux SP (2008) Mitochondrial DNA repair in aging and disease. Mech Ageing Dev 129(7–8):383–390
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Kudryavtseva AV et al (2016) Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 7(29):44879–44905
Yu CH et al (2020) TDP-43 Triggers Mitochondrial DNA release via mPTP to Activate cGAS/STING in ALS. Cell 183(3):636-649.e18
Yu X et al (2023) Telomerase reverse transcriptase and neurodegenerative diseases. Front Immunol 14:1165632
Linkus B et al (2016) Telomere shortening leads to earlier age of onset in ALS mice. Aging (Albany NY) 8(2):382–393
Robinson H et al (2022) Telomere attrition in induced pluripotent stem cell-derived neurons from ALS/FTD-related C9ORF72 repeat expansion carriers. Front Cell Dev Biol 10:874323
Al Khleifat A et al (2022) Telomere length analysis in amyotrophic lateral sclerosis using large-scale whole genome sequence data. Front Cell Neurosci 16:1050596
Kim EJ et al (2021) Increased telomere length in patients with frontotemporal dementia syndrome. J Neurol Sci 428:117565
Gao Y et al (2020) Mendelian randomization implies no direct causal association between leukocyte telomere length and amyotrophic lateral sclerosis. Sci Rep 10(1):12184
Gonzalo S (2010) Epigenetic alterations in aging. J Appl Physiol (1985) 109(2):586–597
Eberharter A, Becker PB (2002) Histone acetylation: a switch between repressive and permissive chromatin. EMBO Rep 3(3):224–229
Lazo-Gómez R et al (2013) Histone deacetylases and their role in motor neuron degeneration. Front Cell Neurosci 7:243
Tejido C, Pakravan D, Bosch LVD (2021) Potential therapeutic role of HDAC inhibitors in FUS-ALS. Front Mol Neurosci 14:686995
Pasyukova EG, Vaiserman AM (2017) HDAC inhibitors: a new promising drug class in anti-aging research. Mech Ageing Dev 166:6–15
Xi Z et al (2015) The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathol 129(5):715–727
Gijselinck I et al (2016) The C9orf72 repeat size correlates with onset age of disease, DNA methylation and transcriptional downregulation of the promoter. Mol Psychiatry 21(8):1112–1124
Zhang M et al (2017) DNA methylation age-acceleration is associated with disease duration and age at onset in C9orf72 patients. Acta Neuropathol 134(2):271–279
Belzil VV et al (2014) Characterization of DNA hypermethylation in the cerebellum of c9FTD/ALS patients. Brain Res 1584:15–21
Xi Z et al (2014) Hypermethylation of the CpG-island near the C9orf72 G4C2-repeat expansion in FTLD patients. Hum Mol Genet 23(21):5630–5637
Xi Z et al (2013) Hypermethylation of the CpG island near the G4C2 repeat in ALS with a C9orf72 expansion. Am J Hum Genet 92(6):981–989
Morahan JM et al (2009) A genome-wide analysis of brain DNA methylation identifies new candidate genes for sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10(5–6):418–429
Figueroa-Romero C et al (2012) Identification of epigenetically altered genes in sporadic amyotrophic lateral sclerosis. PLoS ONE 7(12):e52672
Liu EY et al (2014) C9orf72 hypermethylation protects against repeat expansion-associated pathology in ALS/FTD. Acta Neuropathol 128(4):525–541
Appleby-Mallinder C et al (2021) TDP43 proteinopathy is associated with aberrant DNA methylation in human amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol 47(1):61–72
Hartung T et al (2021) Methylation and expression of mutant FUS in motor neurons differentiated from induced pluripotent stem cells from ALS patients. Front Cell Dev Biol 9:774751
Martin LJ et al (2022) Aberrant DNA and RNA methylation occur in spinal cord and skeletal muscle of human SOD1 mouse models of ALS and in human ALS: targeting DNA methylation is therapeutic. Cells 11(21):3448
Tibshirani M et al (2017) Dysregulation of chromatin remodelling complexes in amyotrophic lateral sclerosis. Hum Mol Genet 26(21):4142–4152
Berson A et al (2017) TDP-43 promotes neurodegeneration by impairing chromatin remodeling. Curr Biol 27(23):3579-3590.e6
Rossaert E et al (2019) Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol Commun 7(1):107
Klingl YE, Pakravan D, Van Den Bosch L (2021) Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis. Br J Pharmacol 178(6):1353–1372
Shahheydari H et al (2017) Protein quality control and the amyotrophic lateral sclerosis/frontotemporal dementia continuum. Front Mol Neurosci 10:119
Santra M, Dill KA, de Graff AMR (2019) Proteostasis collapse is a driver of cell aging and death. Proc Natl Acad Sci 116(44):22173–22178
Moreno-García A et al (2018) An overview of the role of lipofuscin in age-related neurodegeneration. Front Neurosci 12:464
Maxwell N et al (2018) α-Motor neurons are spared from aging while their synaptic inputs degenerate in monkeys and mice. Aging Cell 17(2):12726
Rygiel KA, Grady JP, Turnbull DM (2014) Respiratory chain deficiency in aged spinal motor neurons. Neurobiol Aging 35(10):2230–2238
Nowotny K et al (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5(1):194–222
Gerashchenko MV et al (2020) Translation elongation rate varies among organs and decreases with age. Nucleic Acids Res 49(2):e9–e9
Watanabe Y, Taguchi K, Tanaka M (2023) Roles of stress response in autophagy processes and aging-related diseases. Int J Mol Sci 24(18):13804
Koga H, Kaushik S, Cuervo AM (2011) Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev 10(2):205–215
Bobkova NV et al (2015) Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci 112(52):16006–16011
Tsakiri EN et al (2013) Diet-derived advanced glycation end products or lipofuscin disrupts proteostasis and reduces life span in Drosophila melanogaster. Free Radical Biol Med 65:1155–1163
Cheng F et al (2023) Reducing oxidative protein folding alleviates senescence by minimizing ER-to-nucleus H(2) O(2) release. EMBO Rep 24(8):e56439
Hafycz JM, Strus E, Naidoo N (2022) Reducing ER stress with chaperone therapy reverses sleep fragmentation and cognitive decline in aged mice. Aging Cell 21(6):e13598
Parakh S et al (2020) The redox activity of protein disulfide isomerase inhibits ALS phenotypes in cellular and Zebrafish models. iScience 23(5):101097
Parakh S et al (2021) Protein disulphide isomerase (PDI) is protective against amyotrophic lateral sclerosis (ALS)-related mutant Fused in Sarcoma (FUS) in in vitro models. Sci Rep 11(1):17557
Kwok CT et al (2013) Association studies indicate that protein disulfide isomerase is a risk factor in amyotrophic lateral sclerosis. Free Radical Biol Med 58:81–86
Woehlbier U et al (2016) ALS-linked protein disulfide isomerase variants cause motor dysfunction. EMBO J 35(8):845–865
Xu S et al (2023) Cytosolic stress granules relieve the ubiquitin-proteasome system in the nuclear compartment. EMBO J 42(3):e111802
Aulas A, Vande Velde C (2015) Alterations in stress granule dynamics driven by TDP-43 and FUS a link to pathological inclusions in ALS? Front Cell Neurosci 9:423
Kedersha NL et al (1999) RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 147(7):1431–1442
Anderson P, Kedersha N (2008) Stress granules: the Tao of RNA triage. Trends Biochem Sci 33(3):141–150
Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26(9):668–679
Kedersha N et al (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol 151(6):1257–1268
Kedersha N et al (2002) Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell 13(1):195–210
Li Z, Liu X, Liu M (2022) Stress granule homeostasis, aberrant phase transition, and amyotrophic lateral sclerosis. ACS Chem Neurosci 13(16):2356–2370
Moujaber O et al (2017) Dissecting the molecular mechanisms that impair stress granule formation in aging cells. Biochim Biophys Acta Mol Cell Res 1864(3):475–486
Carey JL, Guo L (2022) Liquid-liquid phase separation of TDP-43 and FUS in physiology and pathology of neurodegenerative diseases. Front Mol Biosci 9:826719
Moreno-Gonzalez I, Soto C (2011) Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol 22(5):482–487
Liu-Yesucevitz L et al (2010) Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS ONE 5(10):e13250
Dewey CM et al (2011) TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol 31(5):1098–1108
McDonald KK et al (2011) TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet 20(7):1400–1410
Colombrita C et al (2009) TDP-43 is recruited to stress granules in conditions of oxidative insult. J Neurochem 111(4):1051–1061
Parker SJ et al (2012) Endogenous TDP-43 localized to stress granules can subsequently form protein aggregates. Neurochem Int 60(4):415–424
Mateju D et al (2017) An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J 36(12):1669–1687
Gal J et al (2016) ALS mutant SOD1 interacts with G3BP1 and affects stress granule dynamics. Acta Neuropathol 132(4):563–576
Bentmann E et al (2012) Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J Biol Chem 287(27):23079–23094
Volkening K et al (2009) Tar DNA binding protein of 43 kDa (TDP-43), 14–3–3 proteins and copper/zinc superoxide dismutase (SOD1) interact to modulate NFL mRNA stability. Implications for altered RNA processing in amyotrophic lateral sclerosis (ALS). Brain Res 1305:168–182
Ayala V et al (2011) Cell stress induces TDP-43 pathological changes associated with ERK1/2 dysfunction: implications in ALS. Acta Neuropathol 122(3):259–270
Meyerowitz J et al (2011) C-Jun N-terminal kinase controls TDP-43 accumulation in stress granules induced by oxidative stress. Mol Neurodegener 6:57
Andersson MK et al (2008) The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biol 9:37
Sama RR et al (2013) FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress. J Cell Physiol 228(11):2222–2231
Vance C et al (2013) ALS mutant FUS disrupts nuclear localization and sequesters wild-type FUS within cytoplasmic stress granules. Hum Mol Genet 22(13):2676–2688
Lenzi J et al (2015) ALS mutant FUS proteins are recruited into stress granules in induced pluripotent stem cell-derived motoneurons. Dis Model Mech 8(7):755–766
Dormann D et al (2010) ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. Embo j 29(16):2841–2857
Barbosa MC, Grosso RA, Fader CM (2018) Hallmarks of aging: an autophagic perspective. Front Endocrinol (Lausanne) 9:790
Metaxakis A, Ploumi C, Tavernarakis N (2018) Autophagy in age-associated neurodegeneration. Cells 7(5):37
Carnio S et al (2014) Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep 8(5):1509–1521
Ramesh N, Pandey UB (2017) Autophagy dysregulation in ALS: when protein aggregates get out of hand. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2017.00263
Nassif M, Woehlbier U, Manque PA (2017) The enigmatic role of C9ORF72 in autophagy. Front Neurosci 11:442
Madeo F et al (2015) Essential role for autophagy in life span extension. J Clin Invest 125(1):85–93
Cuervo AM, Dice JF (2000) Age-related decline in chaperone-mediated autophagy. J Biol Chem 275(40):31505–31513
Morimoto N et al (2007) Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res 1167:112–117
Kim J et al (2013) Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 59:80–85
Webster CP et al (2016) The C9orf72 protein interacts with Rab1a and the ULK1 complex to regulate initiation of autophagy. EMBO J 35(15):1656–1676
Sellier C et al (2016) Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J 35(12):1276–1297
Farg MA et al (2014) C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum Mol Genet 23(13):3579–3595
Al-Sarraj S et al (2011) p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol 122(6):691–702
Wang X et al (2015) Activation of ER stress and autophagy induced by TDP-43 A315T as pathogenic mechanism and the corresponding histological changes in skin as potential biomarker for ALS with the mutation. Int J Biol Sci 11(10):1140–1149
Kritsilis M et al (2018) Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci 19(10):2937
Sahu MR et al (2022) Cellular senescence in the aging brain: a promising target for neurodegenerative diseases. Mech Ageing Dev 204:111675
Childs BG et al (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435
Grosse L et al (2020) Defined p16(High) senescent cell types are indispensable for mouse healthspan. Cell Metab 32(1):87-99.e6
Liao Z et al (2021) Cellular senescence: mechanisms and therapeutic potential. Biomedicines 9(12):1769
Ovadya Y et al (2018) Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9(1):5435
d’Adda di Fagagna F et al (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426(6963):194–198
Herbig U et al (2004) Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell 14(4):501–513
Di Micco R et al (2021) Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol 22(2):75–95
Rossiello F et al (2022) Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol 24(2):135–147
Gorgoulis V et al (2019) Cellular senescence: defining a path forward. Cell 179(4):813–827
Martínez-Cué C, Rueda N (2020) Cellular senescence in neurodegenerative diseases. Front Cell Neurosci 14:16
van Deursen JM (2014) The role of senescent cells in ageing. Nature 509(7501):439–446
Limbad C et al (2020) Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS ONE 15(1):e0227887
Trias E et al (2019) Emergence of microglia bearing senescence markers during paralysis progression in a rat model of inherited ALS. Front Aging Neurosci 11:42
Matsudaira T et al (2023) Cellular senescence in white matter microglia is induced during ageing in mice and exacerbates the neuroinflammatory phenotype. Commun Biol. https://doi.org/10.1038/s42003-023-05027-2
von Zglinicki T, Wan T, Miwa S (2021) Senescence in post-mitotic cells: a driver of aging? Antioxid Redox Signal 34(4):308–323
Sah E et al (2021) The cellular senescence stress response in post-mitotic brain cells: cell survival at the expense of tissue degeneration. Life (Basel) 11(3):229
Ishikawa S, Ishikawa F (2020) Proteostasis failure and cellular senescence in long-term cultured postmitotic rat neurons. Aging Cell 19(1):e13071
Ohashi M et al (2018) Loss of MECP2 leads to activation of P53 and neuronal senescence. Stem Cell Rep 10(5):1453–1463
Pandya VA, Patani R (2021) Region-specific vulnerability in neurodegeneration: lessons from normal ageing. Ageing Res Rev 67:101311
Sikora E et al (2021) Cellular senescence in brain aging. Front Aging Neurosci 13:646924
Birger A et al (2019) Human iPSC-derived astrocytes from ALS patients with mutated C9ORF72 show increased oxidative stress and neurotoxicity. EBioMedicine 50:274–289
Turnquist C et al (2016) p53 isoforms regulate astrocyte-mediated neuroprotection and neurodegeneration. Cell Death Differ 23(9):1515–1528
Morgan JE, Partridge TA (2003) Muscle satellite cells. Int J Biochem Cell Biol 35(8):1151–1156
Sousa-Victor P, Perdiguero E, Muñoz-Cánoves P (2014) Geroconversion of aged muscle stem cells under regenerative pressure. Cell Cycle 13(20):3183–3190
Blanc RS et al (2016) PRMT7 preserves satellite cell regenerative capacity. Cell Rep 14(6):1528–1539
Jeong HJ et al (2016) Prmt7 deficiency causes reduced skeletal muscle oxidative metabolism and age-related obesity. Diabetes 65(7):1868–1882
Amorim JA et al (2022) Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol 18(4):243–258
Bustamante-Barrientos FA et al (2023) Mitochondrial dysfunction in neurodegenerative disorders: potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J Transl Med 21(1):613
Sasaki S, Iwata M (2007) Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 66(1):10–16
Dafinca R et al (2020) Impairment of mitochondrial calcium buffering links mutations in C9ORF72 and TARDBP in iPS-derived motor neurons from patients with ALS/FTD. Stem Cell Rep 14(5):892–908
Onesto E et al (2016) Gene-specific mitochondria dysfunctions in human TARDBP and C9ORF72 fibroblasts. Acta Neuropathol Commun 4(1):47
Wang T et al (2021) C9orf72 regulates energy homeostasis by stabilizing mitochondrial complex I assembly. Cell Metab 33(3):531-546.e9
Mehta AR et al (2021) Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol 141(2):257–279
Lopez-Gonzalez R et al (2016) Poly(GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron 92(2):383–391
Choi SY et al (2019) C9ORF72-ALS/FTD-associated poly(GR) binds Atp5a1 and compromises mitochondrial function in vivo. Nat Neurosci 22(6):851–862
Magrané J et al (2014) Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet 23(6):1413–1424
Lu J et al (2012) Mitochondrial dysfunction in human TDP-43 transfected NSC34 cell lines and the protective effect of dimethoxy curcumin. Brain Res Bull 89(5–6):185–190
Wang W et al (2013) The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum Mol Genet 22(23):4706–4719
Wang W et al (2016) The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med 22(8):869–878
Magrané J et al (2009) Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet 18(23):4552–4564
Lin BC et al (2021) ALS/FTD mutations in UBQLN2 are linked to mitochondrial dysfunction through loss-of-function in mitochondrial protein import. Hum Mol Genet 30(13):1230–1246
Ho WY et al (2021) Dysfunction in nonsense-mediated decay, protein homeostasis, mitochondrial function, and brain connectivity in ALS-FUS mice with cognitive deficits. Acta Neuropathol Commun 9(1):9
Guo Y et al (2024) ALS-linked SOD1 mutations impair mitochondrial-derived vesicle formation and accelerate aging. Redox Biol 69:102972
Yang L et al (2018) The role of insulin/IGF-1/PI3K/Akt/GSK3beta signaling in Parkinson’s disease dementia. Front Neurosci 12:73
Ogundele OM et al (2018) A putative mechanism of age-related synaptic dysfunction based on the impact of IGF-1 Receptor Signaling on synaptic CaMKIIalpha phosphorylation. Front Neuroanat 12:35
Toyama EQ et al (2016) Metabolism AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351(6270):275–281
Herranz N et al (2015) mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17(9):1205–1217
Mannick JB, Lamming DW (2023) Targeting the biology of aging with mTOR inhibitors. Nat Aging 3(6):642–660
Mazucanti CH et al (2015) Longevity pathways (mTOR, SIRT, Insulin/IGF-1) as key modulatory targets on aging and neurodegeneration. Curr Top Med Chem 15(21):2116–2138
Jagaraj CJ, Parakh S, Atkin JD (2020) Emerging evidence highlighting the importance of redox dysregulation in the pathogenesis of amyotrophic lateral sclerosis (ALS). Front Cell Neurosci 14:581950
Chen C et al (2020) SIRT1 and aging related signaling pathways. Mech Ageing Dev 187:111215
Fang EF et al (2017) NAD(+) in aging: molecular mechanisms and translational implications. Trends Mol Med 23(10):899–916
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404(1):1–13
Grabowska W, Sikora E, Bielak-Zmijewska A (2017) Sirtuins, a promising target in slowing down the ageing process. Biogerontology 18(4):447–476
Taylor JR et al (2022) Sirt6 regulates lifespan in Drosophila melanogaster. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2111176119
Burnett C et al (2011) Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477(7365):482–485
Park S, Mori R, Shimokawa I (2013) Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells 35(6):474–480
Herskovits AZ et al (2018) SIRT1 deacetylase in aging-induced neuromuscular degeneration and amyotrophic lateral sclerosis. Aging Cell 17(6):e12839
Granatiero V et al (2021) Modulation of the IGF1R-MTOR pathway attenuates motor neuron toxicity of human ALS SOD1(G93A) astrocytes. Autophagy 17(12):4029–4042
Wang IF et al (2012) Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A 109(37):15024–15029
Vincent AM et al (2004) Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Med 6(2–3):79–85
Perera ND et al (2014) Mutant TDP-43 deregulates AMPK activation by PP2A in ALS models. PLoS ONE 9(4):e95549
Pasinetti GM, Bilski AE, Zhao W (2013) Sirtuins as therapeutic targets of ALS. Cell Res 23(9):1073–1074
Buck E et al (2017) Comparison of Sirtuin 3 levels in ALS and Huntington’s disease-differential effects in human tissue samples vs Transgenic Mouse Models. Front Mol Neurosci 10:156
Tang BL (2017) Could sirtuin activities modify ALS onset and progression? Cell Mol Neurobiol 37(7):1147–1160
Garcia Morato J et al (2022) Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43. Nat Commun 13(1):1223
Morato JG et al (2021) Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43. BioRxiv. https://doi.org/10.1101/2020.05.26.104356v3.full
Mancuso R et al (2014) Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 11(2):419–432
Hendouei F et al (2020) Resveratrol as adjunctive therapy in treatment of irritability in children with autism: a double-blind and placebo-controlled randomized trial. J Clin Pharm Ther 45(2):324–334
Biran A et al (2015) Senescent cells communicate via intercellular protein transfer. Genes Dev 29(8):791–802
Fafian-Labora JA, O’Loghlen A (2020) Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol 30(8):628–639
Dhirachaikulpanich D et al (2022) Intercellular communication analysis of the human retinal pigment epithelial and choroidal cells predicts pathways associated with aging, cellular senescence and age-related macular degeneration. Front Aging Neurosci 14:1016293
Walters HE, Cox LS (2021) Intercellular transfer of mitochondria between senescent cells through cytoskeleton-supported intercellular bridges requires mTOR and CDC42 signalling. Oxid Med Cell Longevity 2021:6697861
Phatnani HP et al (2013) Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci USA 110(8):E756–E765
Brites D, Vaz AR (2014) Microglia centered pathogenesis in ALS: insights in cell interconnectivity. Front Cell Neurosci 8:117
Deepa P et al (2011) Down regulation of trophic factors in neonatal rat spinal cord after administration of cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients. J Neural Transm (Vienna) 118(4):531–538
Gandelman M et al (2010) Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation 7:33
Glass CK et al (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934
Philips T et al (2013) Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain 136(Pt 2):471–482
Haidet-Phillips AM et al (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol 29(9):824-U79
Das MM, Svendsen CN (2015) Astrocytes show reduced support of motor neurons with aging that is accelerated in a rodent model of ALS. Neurobiol Aging 36(2):1130–1139
Das MM et al (2016) Human neural progenitors differentiate into astrocytes and protect motor neurons in aging rats. Exp Neurol 280:41–49
Kim G, Chen X, Yang Y (2022) Pathogenic extracellular vesicle (EV) signaling in amyotrophic lateral sclerosis (ALS). Neurotherapeutics 19(4):1119–1132
Mateescu B et al (2022) Phase 2 of extracellular RNA communication consortium charts next-generation approaches for extracellular RNA research. Iscience 25(8):104653
Takahashi A et al (2017) Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun 8:15287
Dluzen DF, Noren Hooten N, Evans MK (2017) Extracellular RNA in aging. Wiley Interdiscip Rev RNA 8(2):96–98
Eitan E et al (2017) Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci Rep 7(1):1342
Alibhai FJ et al (2020) Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell 19(3):e13103
Jeon OH et al (2019) Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. https://doi.org/10.1172/jci.insight.125019
Hosaka T et al (2019) Extracellular RNAs as biomarkers of sporadic amyotrophic lateral sclerosis and other neurodegenerative diseases. Int J Mol Sci 20(13):3148
Basso M et al (2013) Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem 288(22):15699–15711
Sproviero D et al (2018) Pathological proteins are transported by extracellular vesicles of sporadic amyotrophic lateral sclerosis patients. Front Neurosci 12:487
Grad LI et al (2014) Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 111(9):3620–5
Munch C, O’Brien J, Bertolotti A (2011) Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A 108(9):3548–53
Furukawa Y et al (2013) Intracellular seeded aggregation of mutant Cu, Zn-superoxide dismutase associated with amyotrophic lateral sclerosis. FEBS Lett 587(16):2500–5
Ekhtiari Bidhendi E et al (2018) Mutant superoxide dismutase aggregates from human spinal cord transmit amyotrophic lateral sclerosis. Acta Neuropathol 136(6):939–953
Silverman JM et al (2019) CNS-derived extracellular vesicles from superoxide dismutase 1 (SOD1)(G93A) ALS mice originate from astrocytes and neurons and carry misfolded SOD1. J Biol Chem 294(10):3744–3759
Mishra PS et al (2020) Transmission of ALS pathogenesis by the cerebrospinal fluid. Acta Neuropathol Commun 8(1):65
Porta S et al (2018) Patient-derived frontotemporal lobar degeneration brain extracts induce formation and spreading of TDP-43 pathology in vivo. Nat Commun 9(1):4220
Iguchi Y et al (2016) Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain 139(Pt 12):3187–3201
Westergard T et al (2016) Cell-to-cell transmission of dipeptide repeat proteins linked to C9orf72-ALS/FTD. Cell Rep 17(3):645–652
Aquino-Martinez R et al (2021) Senescent cells exacerbate chronic inflammation and contribute to periodontal disease progression in old mice. J Periodontol 92(10):1483–1495
Brahadeeswaran S, Sivagurunathan N, Calivarathan L (2022) Inflammasome signaling in the aging brain and age-related neurodegenerative diseases. Mol Neurobiol 59(4):2288–2304
Deora V et al (2020) The microglial NLRP3 inflammasome is activated by amyotrophic lateral sclerosis proteins. Glia 68(2):407–421
Gudkov SV et al (2023) An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res Rev 83:101775
Di Benedetto S et al (2017) Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 75:114–128
Rothhammer V et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557(7707):724–728
Adrover JM, Nicolas-Avila JA, Hidalgo A (2016) Aging: a temporal dimension for neutrophils. Trends Immunol 37(5):334–345
Boisvert MM et al (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep 22(1):269–285
Clarke LE et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115(8):E1896–E1905
Lana D et al (2019) Microglial distribution, branching, and clearance activity in aged rat hippocampus are affected by astrocyte meshwork integrity: evidence of a novel cell-cell interglial interaction. FASEB J 33(3):4007–4020
Liu E, Karpf L, Bohl D (2021) Neuroinflammation in amyotrophic lateral sclerosis and frontotemporal dementia and the interest of induced pluripotent stem cells to study immune cells interactions with neurons. Front Mol Neurosci 14:767041
Liu J, Wang F (2017) Role of neuroinflammation in amyotrophic lateral sclerosis: cellular mechanisms and therapeutic implications. Front Immunol 8:1005
Gugliandolo A et al (2018) NLRP3 inflammasome activation in a transgenic amyotrophic lateral sclerosis model. Inflammation 41(1):93–103
Bellezza I et al (2018) Peroxynitrite activates the NLRP3 inflammasome cascade in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Mol Neurobiol 55(3):2350–2361
Johann S et al (2015) NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia 63(12):2260–73
Zhao W et al (2015) TDP-43 activates microglia through NF-kappaB and NLRP3 inflammasome. Exp Neurol 273:24–35
Clarke BE, Patani R (2020) The microglial component of amyotrophic lateral sclerosis. Brain 143(12):3526–3539
Pehar M et al (2017) Role and therapeutic potential of astrocytes in amyotrophic lateral sclerosis. Curr Pharm Des 23(33):5010–5021
Teng YD et al (2012) Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med 4(165):165ra164-165ra164
Carpentier PA, Palmer TD (2009) Immune influence on adult neural stem cell regulation and function. Neuron 64(1):79–92
Blacher E et al (2019) Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572(7770):474–480
Wu S et al (2015) Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep 3(4):e12356
Zhang Y et al (2017) A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1(-/)(-) mice is correlated to increased cellular senescence. Redox Biol 11:30–37
Burberry A et al (2020) C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 582(7810):89–94
Harries LW (2023) Dysregulated RNA processing and metabolism: a new hallmark of ageing and provocation for cellular senescence. FEBS J 290(5):1221–1234
Nussbacher JK et al (2019) Disruption of RNA metabolism in neurological diseases and emerging therapeutic interventions. Neuron 102(2):294–320
Butti Z, Patten SA (2018) RNA dysregulation in amyotrophic lateral sclerosis. Front Genet 9:712
Fu X-D, Ares M (2014) Context-dependent control of alternative splicing by RNA-binding proteins. Nat Rev Genet 15(10):689–701
Varesi A et al (2023) RNA binding proteins in senescence: a potential common linker for age-related diseases? Ageing Res Rev 88:101958
Harries LW et al (2011) Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging Cell 10(5):868–78
Jung S, Arcos Hodar J, del Sol A (2023) Measuring biological age using a functionally interpretable multi-tissue RNA clock. Aging Cell 22(5):13799
Ren X, Kuan PF (2020) RNAAgeCalc: a multi-tissue transcriptional age calculator. PLoS ONE 15(8):e0237006
Souliotis VL et al (2019) DNA damage response and oxidative stress in systemic autoimmunity. Int J Mol Sci 21(1):55
Debès C et al (2023) Ageing-associated changes in transcriptional elongation influence longevity. Nature 616(7958):814–821
Stoeger T et al (2022) Aging is associated with a systemic length-associated transcriptome imbalance. Nature Aging 2(12):1191–1206
Tollervey JR et al (2011) Analysis of alternative splicing associated with aging and neurodegeneration in the human brain. Genome Res 21(10):1572–82
Holly AC et al (2013) Changes in splicing factor expression are associated with advancing age in man. Mech Ageing Dev 134(9):356–66
Ward AJ, Cooper TA (2010) The pathobiology of splicing. J Pathol 220(2):152–63
Gyenis A et al (2023) Genome-wide RNA polymerase stalling shapes the transcriptome during aging. Nat Genet 55(2):268–279
Son HG et al (2017) RNA surveillance via nonsense-mediated mRNA decay is crucial for longevity in daf-2/insulin/IGF-1 mutant C. elegans. Nat Commun 8(1):14749
McMahon M, Forester C, Buffenstein R (2021) Aging through an epitranscriptomic lens. Nature Aging 1(4):335–346
Min K-W et al (2018) Profiling of m6A RNA modifications identified an age-associated regulation of AGO2 mRNA stability. Aging Cell 17(3):e12753
Castro-Hernández R et al (2023) Conserved reduction of m6A RNA modifications during aging and neurodegeneration is linked to changes in synaptic transcripts. Proc Natl Acad Sci 120(9):e2204933120
Nicholas A et al (2010) Age-related gene-specific changes of A-to-I mRNA editing in the human brain. Mech Ageing Dev 131(6):445–7
Cole DC et al (2017) Loss of APOBEC1 RNA-editing function in microglia exacerbates age-related CNS pathophysiology. Proc Natl Acad Sci 114(50):13272–13277
Wagner V et al (2023) Characterizing expression changes in noncoding RNAs during aging and heterochronic parabiosis across mouse tissues. Nat Biotechnol 42:109–118
Somel M et al (2010) MicroRNA, mRNA, and protein expression link development and aging in human and macaque brain. Genome Res 20(9):1207–18
Butler AA et al (2019) Long noncoding RNA NEAT1 mediates neuronal histone methylation and age-related memory impairment. Sci Signal 12(588):9277
Xiong H et al (2023) Knockdown of long noncoding RNA SAN rejuvenates aged adipose-derived stem cells via miR-143-3p/ADD3 axis. Stem Cell Res Ther 14(1):213
Gurkar AU et al (2023) Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging 3(7):776–790
Srinivas T et al (2023) Roles of lncRNAs in brain development and pathogenesis: emerging therapeutic opportunities. Mol Ther 31(6):1550–1561
Memczak S et al (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495(7441):333–8
Niu R, Liu J (2022) Circular RNA involvement in aging and longevity. Curr Genomics 23(5):318–325
Cai H et al (2019) Circular RNA involvement in aging: an emerging player with great potential. Mech Ageing Dev 178:16–24
Errichelli L et al (2017) FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun 8(1):14741
Colantoni A et al (2023) FUS Alters circRNA metabolism in human motor neurons carrying the ALS-Linked P525L mutation. Int J Mol Sci 24(4):3181
Aksoy YA et al (2020) “STRESSED OUT”: The role of FUS and TDP-43 in amyotrophic lateral sclerosis. Int J Biochem Cell Biol 126:105821
Baughn MW et al (2023) Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies. Science 379(6637):1140–1149
Li Y et al (2023) Globally reduced N6-methyladenosine (m6A) in C9ORF72-ALS/FTD dysregulates RNA metabolism and contributes to neurodegeneration. Nat Neurosci 26(8):1328–1338
Hideyama T et al (2012) Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol Dis 45(3):1121–8
Zhang X et al (2020) Small RNA modifications in Alzheimer’s disease. Neurobiol Dis 145:105058
Lee Y-B et al (2013) Hexanucleotide repeats in ALS/FTD Form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep 5(5):1178–1186
Cooper-Knock J et al (2014) Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain 137(7):2040–2051
Xu W et al (2019) Reactivation of nonsense-mediated mRNA decay protects against C9orf72 dipeptide-repeat neurotoxicity. Brain 142(5):1349–1364
Kamelgarn M et al (2018) ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc Natl Acad Sci U S A 115(51):E11904-e11913
Dobrowolny G et al (2021) A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Discov 7(1):4
Wang C et al (2020) Stress induces dynamic, cytotoxicity-antagonizing TDP-43 nuclear bodies via Paraspeckle LncRNA NEAT1-mediated liquid–liquid phase separation. Mol Cell 79(3):443-458.e7
Li P et al (2020) Identification and characterization of N6-methyladenosine CircRNAs and methyltransferases in the lens epithelium cells from age-related cataract. Invest Ophthalmol Vis Sci 61(10):13–13
Tang H et al (2018) HuR regulates telomerase activity through TERC methylation. Nat Commun 9(1):2213
Wang P et al (2019) TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLoS Genet 15(5):e1007947
Konopka A, Atkin JD (2018) The emerging role of DNA damage in the pathogenesis of the C9orf72 repeat expansion in amyotrophic lateral sclerosis. Int J Mol Sci 19(10):3137
Sengupta D, Sengupta K (2022) Lamin A and telomere maintenance in aging: two to Tango. Mut Res Fundam Mol Mech Mutagen 825:111788
Hou Y et al (2019) Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol 15(10):565–581
Yu R et al (2021) Inactivating histone deacetylase HDA promotes longevity by mobilizing trehalose metabolism. Nat Commun 12(1):1981
Parakh S et al (2018) Rab-dependent cellular trafficking and amyotrophic lateral sclerosis. Crit Rev Biochem Mol Biol 53(6):623–651
Parakh S et al (2018) ERp57 is protective against mutant SOD1-induced cellular pathology in amyotrophic lateral sclerosis. Hum Mol Genet 27(8):1311–1331
Haase G, Rabouille C (2015) Golgi fragmentation in ALS motor neurons. new mechanisms targeting microtubules, tethers, and transport vesicles. Front Neurosci 2015(9):448
Soo KY et al (2015) Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta Neuropathol 130(5):679–97
Valenzuela V, Nassif M, Hetz C (2018) Unraveling the role of motoneuron autophagy in ALS. Autophagy 14(4):733–737
Evans CS, Holzbaur ELF (2019) Autophagy and mitophagy in ALS. Neurobiol Dis 122:35–40
Nguyen DKH, Thombre R, Wang J (2018) Autophagy as a common pathway in amyotrophic lateral sclerosis. Neurosci Lett. https://doi.org/10.1016/j.neulet.2018.04.006
Ho WY et al (2019) The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy 15:1–16
Rudnick ND et al (2017) Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proc Natl Acad Sci U S A 114(39):E8294-e8303
Chen T et al (2018) Mutant UBQLN2(P497H) in motor neurons leads to ALS-like phenotypes and defective autophagy in rats. Acta Neuropathol Commun 6(1):122
Chitiprolu M et al (2018) A complex of C9ORF72 and p62 uses arginine methylation to eliminate stress granules by autophagy. Nat Commun 9(1):2794
Madill M et al (2017) Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Mol Brain 10(1):22
Ma Y, Farny NG (2023) Connecting the dots: neuronal senescence, stress granules, and neurodegeneration. Gene 871:147437
Miwa S et al (2022) Mitochondrial dysfunction in cell senescence and aging. J Clin Invest 132(13):158447
Zhao J et al (2022) The impact of mitochondrial dysfunction in amyotrophic lateral sclerosis. Cells 11(13):2049
Delic V et al (2018) Discrete mitochondrial aberrations in the spinal cord of sporadic ALS patients. J Neurosci Res 96(8):1353–1366
Deng J et al (2018) FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models. Proc Natl Acad Sci U S A 115(41):E9678-e9686
Khalil B, Lievens JC (2017) Mitochondrial quality control in amyotrophic lateral sclerosis: towards a common pathway? Neural Regen Res 12(7):1052–1061
Perera ND et al (2018) Rilmenidine promotes MTOR-independent autophagy in the mutant SOD1 mouse model of amyotrophic lateral sclerosis without slowing disease progression. Autophagy 14(3):534–551
Ugolino J et al (2016) Loss of C9orf72 enhances autophagic activity via deregulated mTOR and TFEB signaling. PLoS Genet 12(11):e1006443
Di Giorgio FP et al (2007) Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10(5):608–14
Rodriguez MJ, Mahy N (2016) Neuron–microglia interactions in motor neuron degeneration. The inflammatory hypothesis in amyotrophic lateral sclerosis revisited. Curr Med Chem 23(42):4753–4772
Parisi C et al (2013) Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis 4:e959
Frakes AE et al (2014) Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81(5):1009–1023
Radford RA et al (2015) The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front Cell Neurosci 9:414
Lee HJ et al (2014) Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS ONE 9(5):e97518
Hoye ML et al (2017) MicroRNA profiling reveals marker of motor neuron disease in ALS models. J Neurosci 37(22):5574–5586
Zhou Y et al (2014) FUS-regulated RNA metabolism and DNA damage repair: Implications for amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Rare Dis 2:e29515
Droppelmann CA et al (2014) RNA metabolism in ALS: when normal processes become pathological. Amyotroph Lateral Scler Frontotemporal Degener 15(5–6):321–36
Alami NH et al (2014) Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81(3):536–543
Lee J, Kim HJ (2022) Normal aging induces changes in the brain and neurodegeneration progress: review of the structural, biochemical, metabolic, cellular, and molecular changes. Front Aging Neurosci 14:931536
Tomlinson BE, Irving D (1977) The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34(2):213–9
Jacob JM (1998) Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev 106(1–2):205–16
Maxwell N et al (2018) α-Motor neurons are spared from aging while their synaptic inputs degenerate in monkeys and mice. Aging Cell 17(2):e12726
Moldovan M et al (2016) Aging-associated changes in motor axon voltage-gated Na(+) channel function in mice. Neurobiol Aging 39:128–39
Azpurua J, Mahoney RE, Eaton BA (2018) Transcriptomics of aged Drosophila motor neurons reveals a matrix metalloproteinase that impairs motor function. Aging Cell 17(2):12729
Wainger BJ et al (2014) Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep 7(1):1–11
Menon P, Kiernan MC, Vucic S (2015) Cortical hyperexcitability precedes lower motor neuron dysfunction in ALS. Clin Neurophysiol 126(4):803–9
Tankisi H et al (2021) Early diagnosis of amyotrophic lateral sclerosis by threshold tracking and conventional transcranial magnetic stimulation. Eur J Neurol 28(9):3030–3039
Dong XX, Wang Y, Qin ZH (2009) Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin 30(4):379–87
Bae JS et al (2008) Effects of age on excitability properties in human motor axons. Clin Neurophysiol 119(10):2282–6
Hunter SK, Pereira HM, Keenan KG (2016) The aging neuromuscular system and motor performance. J Appl Physiol (1985) 121(4):982–995
Wang H et al (2021) DNA damage and repair deficiency in ALS/FTD-associated neurodegeneration: from molecular mechanisms to therapeutic implication. Front Mol Neurosci 14:784361
Kanning KC, Kaplan A, Henderson CE (2010) Motor neuron diversity in development and disease. Annu Rev Neurosci 33:409–40
Singh A et al (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24(8):1583
Urushitani M et al (1998) Mechanism of selective motor neuronal death after exposure of spinal cord to glutamate: involvement of glutamate-induced nitric oxide in motor neuron toxicity and nonmotor neuron protection. Ann Neurol 44(5):796–807
Schrøder H, Reske-Nielsen E (1984) Preservation of the nucleus X-pelvic floor motosystem in amyotrophic lateral sclerosis. Clin Neuropathol 3(5):210–216
Lobsiger CS et al (2009) Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci U S A 106(11):4465–70
Pun S et al (2006) Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci 9(3):408–419
Spiller KJ et al (2016) Selective motor neuron resistance and recovery in a new inducible mouse model of TDP-43 proteinopathy. J Neurosci 36(29):7707–7717
Sharma A et al (2016) ALS-associated mutant FUS induces selective motor neuron degeneration through toxic gain of function. Nat Commun 7(1):10465
Hashizume K, Kanda K, Burke RE (1988) Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol 269(3):425–30
Swash M, Fox KP (1972) The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci 16(4):417–32
Hedlund E et al (2010) Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection. Brain 133(Pt 8):2313–30
Comley L et al (2015) Motor neurons with differential vulnerability to degeneration show distinct protein signatures in health and ALS. Neuroscience 291:216–29
Kadhiresan VA, Hassett CA, Faulkner JA (1996) Properties of single motor units in medial gastrocnemius muscles of adult and old rats. J Physiol 493:543–52
Kanda K, Hashizume K (1989) Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61(4):737–46
Foran E, Trotti D (2009) Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal 11(7):1587–602
Arnold WD, Clark BC (2019) Faster, higher, farther: outpacing age-related motor neuron losses. J Physiol 597(19):4867–4868
Palmer AL, Ousman SS (2018) Astrocytes and aging. Front Aging Neurosci 10:337
Popov A et al (2021) Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20(3):e13334
Verkerke M, Hol EM, Middeldorp J (2021) Physiological and pathological ageing of astrocytes in the human brain. Neurochem Res 46(10):2662–2675
Li X et al (2023) Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther 8(1):239
Ziff OJ et al (2022) Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res 32(1):71–84
Tripathi P et al (2017) Reactive astrocytes promote als-like degeneration and intracellular protein aggregation in human motor neurons by disrupting autophagy through TGF-β1. Stem Cell Rep 9(2):667–680
Stoklund Dittlau K et al (2023) FUS-ALS hiPSC-derived astrocytes impair human motor units through both gain-of-toxicity and loss-of-support mechanisms. Mol Neurodegener 18(1):5
Perry VH, Matyszak MK, Fearn S (1993) Altered antigen expression of microglia in the aged rodent CNS. Glia 7(1):60–7
Vaughan DW, Peters A (1974) Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol 3(4):405–29
Tam OH et al (2019) Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep 29(5):1164-1177.e5
Funikov SY et al (2018) FUS(1–359) transgenic mice as a model of ALS: pathophysiological and molecular aspects of the proteinopathy. Neurogenetics 19(3):189–204
Maniatis S et al (2019) Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364(6435):89–93
Zhou Q et al (2020) Active poly-GA vaccination prevents microglia activation and motor deficits in a C9orf72 mouse model. EMBO Mol Med 12(2):e10919
Spiller KJ et al (2018) Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat Neurosci 21(3):329–340
Quek H et al (2022) ALS monocyte-derived microglia-like cells reveal cytoplasmic TDP-43 accumulation, DNA damage, and cell-specific impairment of phagocytosis associated with disease progression. J Neuroinflammation 19(1):58
Rawji KS, Neumann B, Franklin RJM (2023) Glial aging and its impact on central nervous system myelin regeneration. Ann N Y Acad Sci 1519(1):34–45
Ximerakis M et al (2019) Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci 22(10):1696–1708
Kang SH et al (2013) Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci 16(5):571–579
Allaman I, Bélanger M, Magistretti PJ (2011) Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci 34(2):76–87
Ferraiuolo L et al (2016) Oligodendrocytes contribute to motor neuron death in ALS via SOD1-dependent mechanism. Proc Natl Acad Sci 113(42):E6496–E6505
Niebroj-Dobosz I et al (2007) Myelin composition of spinal cord in a model of amyotrophic lateral sclerosis (ALS) in SOD1G93A transgenic rats. Folia Neuropathol 45(4):236–41
Painter MW et al (2014) Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83(2):331–343
Painter MW (2017) Aging Schwann cells: mechanisms, implications, future directions. Curr Opin Neurobiol 47:203–208
Fuertes-Alvarez S, Izeta A (2021) Terminal schwann cell aging: implications for age-associated neuromuscular dysfunction. Aging Dis 12(2):494–514
Bruneteau G et al (2015) Endplate denervation correlates with Nogo-A muscle expression in amyotrophic lateral sclerosis patients. Ann Clin Transl Neurol 2(4):362–72
Trias E et al (2020) Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 68(6):1165–1181
Carrasco DI, Seburn KL, Pinter MJ (2016) Altered terminal Schwann cell morphology precedes denervation in SOD1 mice. Exp Neurol 275(1):172–81
Marzetti E et al (2017) Sarcopenia: an overview. Aging Clin Exp Res 29(1):11–17
Clegg A et al (2013) Frailty in elderly people. Lancet 381(9868):752–62
Rudolf R et al (2014) Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci 6:99
Gonzalez-Freire M et al (2014) The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci 6:208
Manini TM, Hong SL, Clark BC (2013) Aging and muscle: a neuron’s perspective. Curr Opin Clin Nutr Metab Care 16(1):21–6
Seo DY et al (2016) Age-related changes in skeletal muscle mitochondria: the role of exercise. Integr Med Res 5(3):182–186
Power GA, Dalton BH, Rice CL (2013) Human neuromuscular structure and function in old age: a brief review. J Sport Health Sci 2(4):215–226
Valentine JM et al (2020) NFκB regulates muscle development and mitochondrial function. J Gerontol A Biol Sci Med Sci 75(4):647–653
Van Damme P, Robberecht W, Van Den Bosch L (2017) Modelling amyotrophic lateral sclerosis: progress and possibilities. Dis Model Mech 10(5):537–549
Fulle S et al (2005) Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol 40(3):189–97
Scaramozza A et al (2014) Skeletal muscle satellite cells in amyotrophic lateral sclerosis. Ultrastruct Pathol 38(5):295–302
Cappello V, Francolini M (2017) Neuromuscular junction dismantling in amyotrophic lateral sclerosis. Int J Mol Sci 18(10):2092
Wong M, Martin LJ (2010) Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet 19(11):2284–302
Picchiarelli G et al (2019) FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis. Nat Neurosci 22(11):1793–1805
Sakowski SA et al (2012) Neuromuscular effects of G93A-SOD1 expression in zebrafish. Mol Neurodegener 7:44
Ramesh T et al (2010) A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech 3(9–10):652–62
Walker AK et al (2015) Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol 130(5):643–60
Iyer SR, Shah SB, Lovering RM (2021) The neuromuscular junction: roles in aging and neuromuscular disease. Int J Mol Sci 22(15):8058
Verma S et al (2022) Neuromuscular junction dysfunction in amyotrophic lateral sclerosis. Mol Neurobiol 59(3):1502–1527
Partridge L, Fuentealba M, Kennedy BK (2020) The quest to slow ageing through drug discovery. Nat Rev Drug Discov 19(8):513–532
Walters HE, Deneka-Hannemann S, Cox LS (2016) Reversal of phenotypes of cellular senescence by pan-mTOR inhibition. Aging (Albany NY) 8(2):231
Garay RP (2021) Investigational drugs and+ nutrients for human longevity Recent clinical trials registered in ClinicalTrials.gov and clinicaltrialsregister.eu. Expert Opin Investig Drugs 30(7):749–758
Mandrioli J et al (2023) Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis. Nat Commun 14(1):4970
Trammell SA et al (2016) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7:12948
Zhang H et al (2016) NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352(6292):1436–43
Harlan BA et al (2020) Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp Neurol 327:113219
Zhou Q et al (2020) Nicotinamide riboside enhances mitochondrial proteostasis and adult neurogenesis through activation of mitochondrial unfolded protein response signaling in the brain of ALS SOD1(G93A) Mice. Int J Biol Sci 16(2):284–297
Rajman L, Chwalek K, Sinclair DA (2018) Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab 27(3):529–547
Biala A et al (2010) Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press 19(3):196–205
Vaiserman AM, Lushchak OV, Koliada AK (2016) Anti-aging pharmacology: promises and pitfalls. Ageing Res Rev 31:9–35
Baur JA et al (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–42
Lagouge M et al (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127(6):1109–22
Song L et al (2014) Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Biomed Res Int 2014:483501
Markert CD et al (2010) A single-dose resveratrol treatment in a mouse model of amyotrophic lateral sclerosis. J Med Food 13(5):1081–5
Moiseeva O et al (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12(3):489–98
Patrone C, Eriksson O, Lindholm D (2014) Diabetes drugs and neurological disorders: new views and therapeutic possibilities. Lancet Diabetes Endocrinol 2(3):256–62
Ng TP et al (2014) Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 41(1):61–8
Egesipe A-L et al (2016) Metformin decreases progerin expression and alleviates pathological defects of Hutchinson-Gilford progeria syndrome cells. NPJ Aging Mech Dis 2(1):16026
Abutaleb NO et al (2023) Lonafarnib and everolimus reduce pathology in iPSC-derived tissue engineered blood vessel model of Hutchinson-Gilford Progeria Syndrome. Sci Rep 13(1):5032
Krimpenfort P, Berns A (2017) Rejuvenation by therapeutic elimination of senescent cells. Cell 169(1):3–5
Harley CB et al (2013) A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuvenation Res 16(5):386–395
de Jesus BB et al (2011) The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10(4):604–621
Reichert S et al (2014) Experimental increase in telomere length leads to faster feather regeneration. Exp Gerontol 52:36–38
Tyshkovskiy A et al (2023) Distinct longevity mechanisms across and within species and their association with aging. Cell 186(13):2929-2949.e20
Graf M et al (2005) High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: results of a placebo-controlled double-blind study. J Neural Transm (Vienna) 112(5):649–60
Galbussera A et al (2006) Vitamin E intake and quality of life in amyotrophic lateral sclerosis patients: a follow-up case series study. Neurol Sci 27(3):190–3
Fitzgerald KC et al (2013) Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: pooled results from 5 cohort studies. Ann Neurol 73(2):236–45
de la Rubia JE et al (2019) Efficacy and tolerability of EH301 for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled human pilot study. Amyotroph Lateral Scler Frontotemporal Degener 20(1–2):115–122
Yoshino J, Baur JA, Imai SI (2018) NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 27(3):513–528
Ganai SA, Ramadoss M, Mahadevan V (2016) Histone Deacetylase (HDAC) Inhibitors—emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr Neuropharmacol 14(1):55–71
McIntyre RL et al (2019) From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs. EMBO Mol Med 11(9):e9854
Gomez-Sanchez JA et al (2022) Emerging role of HDACs in regeneration and ageing in the peripheral nervous system: repair schwann cells as pivotal targets. Int J Mol Sci 23(6):2996
Kalmar B, Lu C-H, Greensmith L (2014) The role of heat shock proteins in amyotrophic lateral sclerosis: the therapeutic potential of Arimoclomol. Pharmacol Ther 141(1):40–54
Bilches Medinas D et al (2022) Mutation in protein disulfide isomerase A3 causes neurodevelopmental defects by disturbing endoplasmic reticulum proteostasis. Embo j 41(2):e105531
Mora JS et al (2020) Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Frontotemporal Degener 21(1–2):5–14
Azam S et al (2021) The ageing brain: molecular and cellular basis of neurodegeneration. Front Cell Dev Biol 9:683459
Al-Chalabi A et al (2014) Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol 13(11):1108–1113
Gumeni S et al (2017) Proteome stability as a key factor of genome integrity. Int J Mol Sci 18(10):2036
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by an Australian National Health and Medical Research Council (NHMRC) Dementia Teams Research Grant (1095215), Macquarie University Postgraduate Research Scholarship, and Motor Neuron Disease Research Australia (Peter Stearne Familial MND Research Grant and Linda Rynalski Bridge Funding Grant) and Fight MND Foundation.
Author information
Authors and Affiliations
Contributions
CJJ and JDA conceptualized the structure of the manuscript. CJJ and JDA also conceived the article and edited the manuscript throughout for content and style consistency. SSH and SAK designed and created the figures with BioRender. All the authors wrote sections of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no financial or non-financial competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors have agreed and provided consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jagaraj, C.J., Shadfar, S., Kashani, S.A. et al. Molecular hallmarks of ageing in amyotrophic lateral sclerosis. Cell. Mol. Life Sci. 81, 111 (2024). https://doi.org/10.1007/s00018-024-05164-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-024-05164-9