Abstract
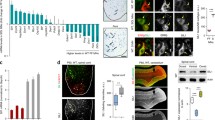
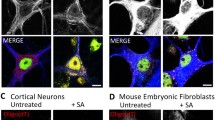
TDP-43 has been implicated in the pathogenesis of amyotrophic lateral sclerosis and other neurodegenerative diseases. Here we demonstrate, using neuronal and spinal cord organotypic culture models, that chronic excitotoxicity, oxidative stress, proteasome dysfunction and endoplasmic reticulum stress mechanistically induce mislocalization, phosphorylation and aggregation of TDP-43. This is compatible with a lack of function of this protein in the nucleus, specially in motor neurons. The relationship between cell stress and pathological changes of TDP-43 also includes a dysfunction in the survival pathway mediated by mitogen-activated protein kinase/extracellular signal-regulated kinases (ERK1/2). Thus, under stress conditions, neurons and other spinal cord cells showed cytosolic aggregates containing ERK1/2. Moreover, aggregates of abnormal phosphorylated ERK1/2 were also found in the spinal cord in amyotrophic lateral sclerosis (ALS), specifically in motor neurons with abnormal immunoreactive aggregates of phosphorylated TDP-43. These results demonstrate that cellular stressors are key factors in neurodegeneration associated with TDP-43 and disclose the identity of ERK1/2 as novel players in the pathogenesis of ALS.







Similar content being viewed by others
References
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445
Arai K, Lee SR, van Leyen K et al (2004) Involvement of ERK MAP kinase in endoplasmic reticulum stress in SH-SY5Y human neuroblastoma cells. J Neurochem 89:232–239
Arai T, Hasegawa M, Akiyama H et al (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Atkin JD, Farg MA, Walker AK et al (2008) Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis 30:400–407
Ayala YM, Misteli T, Baralle FE (2008) TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proc Natl Acad Sci USA 105:3785–3789
Ballif BA, Blenis J (2001) Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ 12:397–408
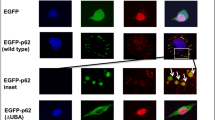
Brady OA, Meng P, Zheng Y et al (2011) Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem 116:248–259
Buratti E, Brindisi A, Giombi M et al (2005) TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem 280:37572–37584
Buratti E, Baralle FE (2001) Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem 276:36337–36343
Bush KT, Goldberg AL, Nigam SK (1997) Proteasome inhibition leads to a heat-shock response, induction of endoplasmic reticulum chaperones, and thermotolerance. J Biol Chem 272:9086–9092
Czubryt MP, Austria JA, Pierce GN (2000) Hydrogen peroxide inhibition of nuclear protein import is mediated by the mitogen-activated protein kinase, ERK2. J Cell Biol 148:7–16
Dewey CM, Cenik B, Sephton CF et al (2011) TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol Cell Biol 31:1098–1108
Dormann D, Capell A, Carlson AM et al (2009) Proteolytic processing of TAR DNA binding protein-43 by caspases produces C-terminal fragments with disease defining properties independent of progranulin. J Neurochem 110:1082–1094
Ferrante RJ, Browne SE, Shinobu LA et al (1997) Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem 69:2064–2074
Foulds P, McAuley E, Gibbons L et al (2008) TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol 116:141–146
Freibaum BD, Chitta RK, High AA et al (2010) Global analysis of TDP-43 interacting proteins reveals strong association with RNA splicing and translation machinery. J Proteome Res 9:1104–1120
Gendron TF, Josephs KA, Petrucelli L (2010) Review: transactive response DNA-binding protein 43 (TDP-43): mechanisms of neurodegeneration. Neuropathol Appl Neurobiol 36:97–112
Giordana MT, Piccinini M, Grifoni S et al (2010) TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol 20:351–360
Granado-Serrano AB, Martin MA, Haegeman G et al (2010) Epicatechin induces NF-kappaB, activator protein-1 (AP-1) and nuclear transcription factor erythroid 2p45-related factor-2 (Nrf2) via phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) and extracellular regulated kinase (ERK) signalling in HepG2 cells. Br J Nutr 103:168–179
Henkel JS, Engelhardt JI, Siklos L et al (2004) Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol 55:221–235
Higashi S, Iseki E, Yamamoto R et al (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–294
Hirano A, Nakano I, Kurland LT et al (1984) Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 43:471–480
Ilieva EV, Ayala V, Jove M et al (2007) Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain 130:3111–3123
Jung T, Engels M, Kaiser B et al (2006) Intracellular distribution of oxidized proteins and proteasome in HT22 cells during oxidative stress. Free Radic Biol Med 40:1303–1312
Kabashi E, Valdmanis PN, Dion P et al (2008) TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 40:572–574
Kametani F, Nonaka T, Suzuki T et al (2009) Identification of casein kinase-1 phosphorylation sites on TDP-43. Biochem Biophys Res Commun 382:405–409
Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802:396–405
Kinoshita Y, Ito H, Hirano A et al (2009) Nuclear contour irregularity and abnormal transporter protein distribution in anterior horn cells in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 68:1184–1192
Kuo PH, Doudeva LG, Wang YT et al (2009) Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res 37:1799–1808
Liu-Yesucevitz L, Bilgutay A, Zhang YJ et al (2010) Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One 5:e13250
Maris C, Dominguez C, Allain FH (2005) The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J 272:2118–2131
McDonald KK, Aulas A, Destroismaisons L et al (2011) TAR DNA-binding protein 43 (TDP-43) regulates stress granule dynamics via differential regulation of G3BP and TIA-1. Hum Mol Genet 20:1400–1410
Menzies FM, Ince PG, Shaw PJ (2002) Mitochondrial involvement in amyotrophic lateral sclerosis. Neurochem Int 40:543–551
Nakashima-Yasuda H, Uryu K, Robinson J et al (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114:221–229
Neumann M, Sampathu DM, Kwong LK et al (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Nishimura AL, Zupunski V, Troakes C et al (2010) Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain 133:1763–1771
Nonaka T, Arai T, Buratti E et al (2009) Phosphorylated and ubiquitinated TDP-43 pathological inclusions in ALS and FTLD-U are recapitulated in SH-SY5Y cells. FEBS Lett 583:394–400
Ou SH, Wu F, Harrich D et al (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69:3584–3596
Rothstein JD, Jin L, Dykes-Hoberg M et al (1993) Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci USA 90:6591–6595
Rothstein JD, Tsai G, Kuncl RW et al (1990) Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol 28:18–25
Sasaki S, Takeda T, Shibata N et al (2010) Alterations in subcellular localization of TDP-43 immunoreactivity in the anterior horns in sporadic amyotrophic lateral sclerosis. Neurosci Lett 478:72–76
Schwab C, Arai T, Hasegawa M et al (2008) Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol 67:1159–1165
Sephton CF, Cenik C, Kucukural A et al (2011) Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J Biol Chem 286:1204–1215
Spencer JP, Rice-Evans C, Williams RJ (2003) Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem 278:34783–34793
Tatom JB, Wang DB, Dayton RD et al (2009) Mimicking aspects of frontotemporal lobar degeneration and Lou Gehrig’s disease in rats via TDP-43 overexpression. Mol Ther 17:607–613
Wils H, Kleinberger G, Janssens J et al (2010) TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA 107:3858–3863
Wood JD, Beaujeux TP, Shaw PJ (2003) Protein aggregation in motor neurone disorders. Neuropathol Appl Neurobiol 29:529–545
Yamashita M, Nonaka T, Arai T et al (2009) Methylene blue and dimebon inhibit aggregation of TDP-43 in cellular models. FEBS Lett 583:2419–2424
Zhang YJ, Xu YF, Cook C et al (2009) Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA 106:7607–7612
Acknowledgments
We are indebted to tissue donors and their families. This work was supported by grants from Spanish Ministry of Education and Science [grant numbers BFU 2009-11879/BFI, AGL2006-12433, BFU 2009-06427/E]; the Generalitat of Catalunya [grant number 2009SGR-735]; the Spanish Ministry of Health [grant number PI08-1843 to M.P.O., BESAD-P and PI08-0582 to I.F.]; the ALS Catalan Foundation [to I.F.]; and “La Caixa” Foundation. Supported also by the COST B-35 Action. V.C has been supported by a predoctoral fellowship from Govern Balear, Conselleria d’ Economia Hisenda i Innovació and D.C. by a predoctoral fellowship from the Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Ayala and A. B. Granado-Serrano contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayala, V., Granado-Serrano, A.B., Cacabelos, D. et al. Cell stress induces TDP-43 pathological changes associated with ERK1/2 dysfunction: implications in ALS. Acta Neuropathol 122, 259–270 (2011). https://doi.org/10.1007/s00401-011-0850-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-011-0850-y