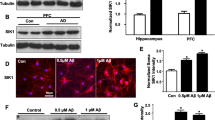
Abstract
Ca2+ is a critical mediator of neurotransmitter release, synaptic plasticity, and gene expression, but also excitotoxicity. Ca2+ signaling and homeostasis are coordinated by an intricate network of channels, pumps, and calcium-binding proteins, which must be rapidly regulated at all expression levels. Τhe role of neuronal miRNAs in regulating ryanodine receptors (RyRs) and inositol 1,4,5-triphosphate receptors (IP3Rs) was investigated to understand the underlying mechanisms that modulate ER Ca2+ release. RyRs and IP3Rs are critical in mounting and propagating cytosolic Ca2+ signals by functionally linking the ER Ca2+ content, while excessive ER Ca2+ release via these receptors is central to the pathophysiology of a wide range of neurological diseases. Herein, two brain-restricted microRNAs, miR-124-3p and miR-153-3p, were found to bind to RyR1-3 and IP3R3 3′UTRs, and suppress their expression at both the mRNA and protein level. Ca2+ imaging studies revealed that overexpression of these miRNAs reduced ER Ca2+ release upon RyR/IP3R activation, but had no effect on [Ca2+]i under resting conditions. Interestingly, treatments that cause excessive ER Ca2+ release decreased expression of these miRNAs and increased expression of their target ER Ca2+ channels, indicating interdependence of miRNAs, RyRs, and IP3Rs in Ca2+ homeostasis. Furthermore, by maintaining the ER Ca2+ content, miR-124 and miR-153 reduced cytosolic Ca2+ overload and preserved protein-folding capacity by attenuating PERK signaling. Overall, this study shows that miR-124-3p and miR-153-3p fine-tune ER Ca2+ homeostasis and alleviate ER stress responses.









Similar content being viewed by others
Availability of data and materials
Data are contained within the article or supplementary material.
Abbreviations
- miRNAs:
-
MicroRNAs
- UTR:
-
Untranslated region
- ER:
-
Endoplasmic reticulum
- CNS:
-
Central nervous system
- Ca2+ :
-
Calcium
- RyRs:
-
Ryanodine receptors
- IP3Rs:
-
Inositol 1,4,5-triphosphate receptors
- CICR:
-
Ca2+-induced Ca2+ release
- UPR:
-
Unfolded protein response
- Tm:
-
Tunicamycin
- CAFF:
-
Caffeine
- CCh:
-
Carbachol
- Tg:
-
Thapsigargin
- TuD:
-
Tough Decoy
- PERK:
-
Protein kinase RNA (PKR)-like endoplasmic reticulum kinase
- IRE1α:
-
Inositol-requiring enzyme 1 alpha
- ATF6:
-
Activating transcription factor 6
- GRP78/Bip:
-
Glucose-regulated protein 78/binding-immunoglobulin protein
- eIF2α:
-
Eukaryotic initiation factor-alpha
- ATF4:
-
Activating transcription factor 4
- CHOP/DDIT3:
-
CCAAT/enhancer-binding protein (C/EBP) homologous protein/DNA damage-inducible transcript 3
- XBP1u/s:
-
Unspliced/spliced X-box-binding protein 1
References
Doxakis E (2013) Principles of miRNA-target regulation in metazoan models. Int J Mol Sci 14(8):16280–16302. https://doi.org/10.3390/ijms140816280 (Epub 2013/08/24. PubMed PMID: 23965954)
Yoo AS, Staahl BT, Chen L, Crabtree GR (2009) MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460(7255):642–646. https://doi.org/10.1038/nature08139 (Epub 2009/06/30. PubMed PMID: 19561591; PubMed Central PMCID: PMC2921580)
Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y et al (2011) MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476(7359):228–231. https://doi.org/10.1038/nature10323 (Epub 2011/07/15. PubMed PMID: 21753754)
Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J et al (2005) Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433(7027):769–773. https://doi.org/10.1038/nature03315 (Epub 2005/02/03. PubMed PMID: 15685193)
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27(3):435–448. https://doi.org/10.1016/j.molcel.2007.07.015 (PubMed PMID: 17679093; PubMed Central PMCID: PMC3139456)
Mokabber H, Najafzadeh N, Mohammadzadeh VM (2019) miR-124 promotes neural differentiation in mouse bulge stem cells by repressing Ptbp1 and Sox9. J Cell Physiol 234(6):8941–8950. https://doi.org/10.1002/jcp.27563 (Epub 2018/11/13. PubMed PMID: 30417370)
Xue Q, Yu C, Wang Y, Liu L, Zhang K, Fang C et al (2016) miR-9 and miR-124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci Rep 6:26781. https://doi.org/10.1038/srep26781 (Epub 2016/05/26. PubMed PMID: 27221778; PubMed Central PMCID: PMCPMC4879704)
Han D, Dong X, Zheng D, Nao J (2019) MiR-124 and the underlying therapeutic promise of neurodegenerative disorders. Front Pharmacol 10:1555. https://doi.org/10.3389/fphar.2019.01555 (Epub 2020/02/06. PubMed PMID: 32009959; PubMed Central PMCID: PMCPMC6978711)
Mishima T, Mizuguchi Y, Kawahigashi Y, Takizawa T, Takizawa T (2007) RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res 1131(1):37–43. https://doi.org/10.1016/j.brainres.2006.11.035 (Epub 2006/12/22. PubMed PMID: 17182009)
Sun Y, Luo ZM, Guo XM, Su DF, Liu X (2015) An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci 9:193. https://doi.org/10.3389/fncel.2015.00193 (Epub 2015/06/05. PubMed PMID: 26041995; PubMed Central PMCID: PMCPMC4438253)
Fischbach SJ, Carew TJ (2009) MicroRNAs in memory processing. Neuron 63(6):714–716. https://doi.org/10.1016/j.neuron.2009.09.007 (Epub 2009/09/26. PubMed PMID: 19778498)
Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ et al (2009) Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63(6):803–817. https://doi.org/10.1016/j.neuron.2009.05.029 (Epub 2009/09/26. PubMed PMID: 19778509; PubMed Central PMCID: PMC2875683)
Soreq H, Wolf Y (2011) NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol Med 17(10):548–555. https://doi.org/10.1016/j.molmed.2011.06.009 (Epub 2011/08/05. PubMed PMID: 21813326)
Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA et al (2012) The miR-124 regulates the expression of BACE1/beta-secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209(1):94–105. https://doi.org/10.1016/j.toxlet.2011.11.032 (Epub 2011/12/20. PubMed PMID: 22178568)
Doeppner TR, Doehring M, Bretschneider E, Zechariah A, Kaltwasser B, Muller B et al (2013) MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathol 126(2):251–265. https://doi.org/10.1007/s00401-013-1142-5 (PubMed PMID: 23754622)
Wang H, Ye Y, Zhu Z, Mo L, Lin C, Wang Q et al (2016) MiR-124 regulates apoptosis and autophagy process in MPTP model of Parkinson’s disease by targeting to bim. Brain Pathol 26(2):167–176. https://doi.org/10.1111/bpa.12267 (Epub 2015/05/16. PubMed PMID: 25976060)
Yip PK, Bowes AL, Hall JCE, Burguillos MA, Ip THR, Baskerville T et al (2019) Docosahexaenoic acid reduces microglia phagocytic activity via miR-124 and induces neuroprotection in rodent models of spinal cord contusion injury. Hum Mol Genet 28(14):2427–2448. https://doi.org/10.1093/hmg/ddz073 (PubMed PMID: 30972415)
Paschou M, Maier L, Papazafiri P, Selescu T, Dedos SG, Babes A et al (2020) Neuronal microRNAs modulate TREK two-pore domain K(+) channel expression and current density. RNA Biol 17(5):651–662. https://doi.org/10.1080/15476286.2020.1722450 (PubMed PMID: 31994436; PubMed Central PMCID: PMC7237132)
Gong X, Wang H, Ye Y, Shu Y, Deng Y, He X et al (2016) miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am J Transl Res 8(5):2127–2137 (Epub 2016/06/28. PubMed PMID: 27347320; PubMed Central PMCID: PMCPMC4891425)
Dong RF, Zhang B, Tai LW, Liu HM, Shi FK, Liu NN (2018) The Neuroprotective role of MiR-124-3p in a 6-hydroxydopamine-induced cell model of Parkinson’s disease via the regulation of ANAX5. J Cell Biochem 119(1):269–277. https://doi.org/10.1002/jcb.26170 (Epub 2017/05/26. PubMed PMID: 28543594)
Geng L, Liu W, Chen Y (2017) miR-124-3p attenuates MPP(+)-induced neuronal injury by targeting STAT3 in SH-SY5Y cells. Exp Biol Med (Maywood) 242(18):1757–1764. https://doi.org/10.1177/1535370217734492 (Epub 2017/09/30. PubMed PMID: 28958159; PubMed Central PMCID: PMCPMC5714150)
Du X, Huo X, Yang Y, Hu Z, Botchway BOA, Jiang Y et al (2017) miR-124 downregulates BACE 1 and alters autophagy in APP/PS1 transgenic mice. Toxicol Lett 280:195–205. https://doi.org/10.1016/j.toxlet.2017.08.082 (Epub 2017/09/05. PubMed PMID: 28867212)
Slota JA, Booth SA (2019) MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA 5(2):35. https://doi.org/10.3390/ncrna5020035 (PubMed PMID: 31022830)
Ghafouri-Fard S, Shoorei H, Bahroudi Z, Abak A, Majidpoor J, Taheri M (2021) An update on the role of miR-124 in the pathogenesis of human disorders. Biomed Pharmacother 135:111198. https://doi.org/10.1016/j.biopha.2020.111198 (Epub 2021/01/08. PubMed PMID: 33412388)
Fowler A, Thomson D, Giles K, Maleki S, Mreich E, Wheeler H et al (2011) miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. Eur J Cancer 47(6):953–963. https://doi.org/10.1016/j.ejca.2010.11.026 (PubMed PMID: 21196113)
Silber J, Lim DA, Petritsch C, Persson AI, Maunakea AK, Yu M et al (2008) miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 6:14. https://doi.org/10.1186/1741-7015-6-14 (Epub 2008/06/26. PubMed PMID: 18577219; PubMed Central PMCID: PMCPMC2443372)
Sun Y, Li Q, Gui H, Xu DP, Yang YL, Su DF et al (2013) MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res 23(11):1270–1283. https://doi.org/10.1038/cr.2013.116 (Epub 2013/08/28. PubMed PMID: 23979021; PubMed Central PMCID: PMCPMC3817544)
Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6(3):337–350. https://doi.org/10.1111/j.1474-9726.2007.00275.x (Epub 2007/03/03. PubMed PMID: 17328689)
Doxakis E (2010) Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem 285(17):12726–12734. https://doi.org/10.1074/jbc.M109.086827 (Epub 2010/01/29. PubMed PMID: 20106983; PubMed Central PMCID: PMCPMC2857101)
Qiao J, Zhao J, Chang S, Sun Q, Liu N, Dong J et al (2020) MicroRNA-153 improves the neurogenesis of neural stem cells and enhances the cognitive ability of aged mice through the notch signaling pathway. Cell Death Differ 27(2):808–825. https://doi.org/10.1038/s41418-019-0388-4 (PubMed PMID: 31296962; PubMed Central PMCID: PMC7206122)
Stappert L, Borghese L, Roese-Koerner B, Weinhold S, Koch P, Terstegge S et al (2013) MicroRNA-based promotion of human neuronal differentiation and subtype specification. PLoS ONE 8(3):e59011. https://doi.org/10.1371/journal.pone.0059011 (PubMed PMID: 23527072; PubMed Central PMCID: PMC3601127)
Xu C, Wang C, Meng Q, Gu Y, Wang Q, Xu W et al (2019) miR153 promotes neural differentiation in the mouse hippocampal HT22 cell line and increases the expression of neuronspecific enolase. Mol Med Rep 20(2):1725–1735. https://doi.org/10.3892/mmr.2019.10421 (Epub 2019/07/02. PubMed PMID: 31257504; PubMed Central PMCID: PMCPMC6625396)
Liang C, Zhu H, Xu Y, Huang L, Ma C, Deng W et al (2012) MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res 1455:103–113. https://doi.org/10.1016/j.brainres.2011.10.051 (PubMed PMID: 22510281)
Long JM, Ray B, Lahiri DK (2012) MicroRNA-153 physiologically inhibits expression of amyloid-beta precursor protein in cultured human fetal brain cells and is dysregulated in a subset of Alzheimer disease patients. J Biol Chem 287(37):31298–31310. https://doi.org/10.1074/jbc.M112.366336 (Epub 2012/06/27. PubMed PMID: 22733824; PubMed Central PMCID: PMCPMC3438960)
Wei C, Thatcher EJ, Olena AF, Cha DJ, Perdigoto AL, Marshall AF et al (2013) miR-153 regulates SNAP-25, synaptic transmission, and neuronal development. PLoS ONE 8(2):e57080. https://doi.org/10.1371/journal.pone.0057080 (PubMed PMID: 23451149; PubMed Central PMCID: PMC3581580)
Banks SA, Pierce ML, Soukup GA (2020) Sensational microRNAs: neurosensory roles of the microRNA-183 family. Mol Neurobiol 57(1):358–371. https://doi.org/10.1007/s12035-019-01717-3 (Epub 2019/07/31. PubMed PMID: 31359323)
Dambal S, Shah M, Mihelich B, Nonn L (2015) The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res 43(15):7173–7188. https://doi.org/10.1093/nar/gkv703 (PubMed PMID: 26170234; PubMed Central PMCID: PMC4551935)
Schluter T, Berger C, Rosengauer E, Fieth P, Krohs C, Ushakov K et al (2018) miR-96 is required for normal development of the auditory hindbrain. Hum Mol Genet 27(5):860–874. https://doi.org/10.1093/hmg/ddy007 (PubMed PMID: 29325119)
Xiang L, Chen XJ, Wu KC, Zhang CJ, Zhou GH, Lv JN et al (2017) miR-183/96 plays a pivotal regulatory role in mouse photoreceptor maturation and maintenance. Proc Natl Acad Sci USA 114(24):6376–6381. https://doi.org/10.1073/pnas.1618757114 (PubMed PMID: 28559309; PubMed Central PMCID: PMC5474811)
Chen HP, Zhou W, Kang LM, Yan H, Zhang L, Xu BH et al (2014) Intrathecal miR-96 inhibits Nav1.3 expression and alleviates neuropathic pain in rat following chronic construction injury. Neurochem Res 39(1):76–83. https://doi.org/10.1007/s11064-013-1192-z (PubMed PMID: 24234845)
Dong Y, Han LL, Xu ZX (2018) Suppressed microRNA-96 inhibits iNOS expression and dopaminergic neuron apoptosis through inactivating the MAPK signaling pathway by targeting CACNG5 in mice with Parkinson’s disease. Mol Med 24(1):61. https://doi.org/10.1186/s10020-018-0059-9 (PubMed PMID: 30486773; PubMed Central PMCID: PMC6263543)
Kinoshita C, Aoyama K, Matsumura N, Kikuchi-Utsumi K, Watabe M, Nakaki T (2014) Rhythmic oscillations of the microRNA miR-96-5p play a neuroprotective role by indirectly regulating glutathione levels. Nat Commun 5:3823. https://doi.org/10.1038/ncomms4823 (PubMed PMID: 24804999; PubMed Central PMCID: PMC4024755)
Kye MJ, Niederst ED, Wertz MH, Goncalves Ido C, Akten B, Dover KZ et al (2014) SMN regulates axonal local translation via miR-183/mTOR pathway. Hum Mol Genet 23(23):6318–6331. https://doi.org/10.1093/hmg/ddu350 (PubMed PMID: 25055867; PubMed Central PMCID: PMC4271102)
Ubhi K, Rockenstein E, Kragh C, Inglis C, Spencer B, Michael S et al (2014) Widespread microRNA dysregulation in multiple system atrophy—disease-related alteration in miR-96. Eur J Neurosci 39(6):1026–1041. https://doi.org/10.1111/ejn.12444 (PubMed PMID: 24304186; PubMed Central PMCID: PMC4052839)
Berridge MJ (1998) Neuronal calcium signaling. Neuron 21(1):13–26. https://doi.org/10.1016/s0896-6273(00)80510-3 (Epub 1998/08/11. PubMed PMID: 9697848)
Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3:61. https://doi.org/10.3389/fphar.2012.00061 (PubMed PMID: 22518105; PubMed Central PMCID: PMC3325487)
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21. https://doi.org/10.1038/35036035 (Epub 2001/06/20. PubMed PMID: 11413485)
Carreras-Sureda A, Pihan P, Hetz C (2018) Calcium signaling at the endoplasmic reticulum: fine-tuning stress responses. Cell Calcium 70:24–31. https://doi.org/10.1016/j.ceca.2017.08.004 (Epub 2017/10/22. PubMed PMID: 29054537)
Chen S, Novick P, Ferro-Novick S (2013) ER structure and function. Curr Opin Cell Biol 25(4):428–433. https://doi.org/10.1016/j.ceb.2013.02.006 (Epub 2013/03/13. PubMed PMID: 23478217; PubMed Central PMCID: PMCPMC5614462)
Berridge MJ (2002) The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32(5–6):235–249. https://doi.org/10.1016/s0143416002001823 (Epub 2003/01/25. PubMed PMID: 12543086)
Primeau JO, Armanious GP, Fisher ME, Young HS (2018) The SarcoEndoplasmic reticulum calcium ATPase. Subcell Biochem 87:229–258. https://doi.org/10.1007/978-981-10-7757-9_8 (Epub 2018/02/22. PubMed PMID: 29464562)
Fill M (2003) Mechanisms that turn-off intracellular calcium release channels. Front Biosci 8:d46-54. https://doi.org/10.2741/924 (Epub 2002/11/29. PubMed PMID: 12456314)
Hakamata Y, Nakai J, Takeshima H, Imoto K (1992) Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312(2–3):229–235. https://doi.org/10.1016/0014-5793(92)80941-9 (PubMed PMID: 1330694)
Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K (1994) Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci 14(8):4794–4805 (PubMed PMID: 8046450; PubMed Central PMCID: PMC6577160)
Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V (1995) The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128(5):893–904. https://doi.org/10.1083/jcb.128.5.893 (PubMed PMID: 7876312; PubMed Central PMCID: PMC2120385)
Imagawa T, Smith JS, Coronado R, Campbell KP (1987) Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem 262(34):16636–16643 (PubMed PMID: 2445748)
Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N et al (1989) Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339(6224):439–445. https://doi.org/10.1038/339439a0 (PubMed PMID: 2725677)
Marks AR, Tempst P, Hwang KS, Taubman MB, Inui M, Chadwick C et al (1989) Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci USA 86(22):8683–8687. https://doi.org/10.1073/pnas.86.22.8683 (PubMed PMID: 2813419; PubMed Central PMCID: PMC298352)
Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA et al (1990) Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 265(4):2244–2256 (PubMed PMID: 2298749)
Otsu K, Willard HF, Khanna VK, Zorzato F, Green NM, MacLennan DH (1990) Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J Biol Chem 265(23):13472–13483 (PubMed PMID: 2380170)
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2(11):a003996. https://doi.org/10.1101/cshperspect.a003996 (Epub 2010/10/22. PubMed PMID: 20961976; PubMed Central PMCID: PMCPMC2964179)
Verkhratsky A, Shmigol A (1996) Calcium-induced calcium release in neurones. Cell Calcium 19(1):1–14. https://doi.org/10.1016/s0143-4160(96)90009-3 (Epub 1996/01/01. PubMed PMID: 8653752)
Meissner G (2017) The structural basis of ryanodine receptor ion channel function. J Gen Physiol 149(12):1065–1089. https://doi.org/10.1085/jgp.201711878 (Epub 2017/11/11. PubMed PMID: 29122978; PubMed Central PMCID: PMCPMC5715910)
Bootman MD, Berridge MJ, Roderick HL (2002) Calcium signalling: more messengers, more channels, more complexity. Curr Biol 12(16):R563–R565. https://doi.org/10.1016/s0960-9822(02)01055-2 (Epub 2002/08/27. PubMed PMID: 12194839)
Murayama T, Ogawa Y (2004) RyR1 exhibits lower gain of CICR activity than RyR3 in the SR: evidence for selective stabilization of RyR1 channel. Am J Physiol Cell Physiol 287(1):C36-45. https://doi.org/10.1152/ajpcell.00395.2003 (Epub 2004/02/27. PubMed PMID: 14985235)
McGrew SG, Wolleben C, Siegl P, Inui M, Fleischer S (1989) Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry 28(4):1686–1691. https://doi.org/10.1021/bi00430a039 (PubMed PMID: 2541762)
Bezprozvanny I, Watras J, Ehrlich BE (1991) Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351(6329):751–754. https://doi.org/10.1038/351751a0 (PubMed PMID: 1648178)
Dulhunty AF, Pouliquin P (2003) What we don’t know about the structure of ryanodine receptor calcium release channels. Clin Exp Pharmacol Physiol 30(10):713–723. https://doi.org/10.1046/j.1440-1681.2003.03904.x (Epub 2003/10/01. PubMed PMID: 14516409)
Xiao B, Masumiya H, Jiang D, Wang R, Sei Y, Zhang L et al (2002) Isoform-dependent formation of heteromeric Ca2+ release channels (ryanodine receptors). J Biol Chem 277(44):41778–41785. https://doi.org/10.1074/jbc.M208210200 (PubMed PMID: 12213830)
Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda N, Mikoshiba K (1989) Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 342(6245):32–38. https://doi.org/10.1038/342032a0 (PubMed PMID: 2554142)
Maeda N, Niinobe M, Mikoshiba K (1990) A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J 9(1):61–67 (PubMed PMID: 2153079; PubMed Central PMCID: PMC551630)
Blondel O, Takeda J, Janssen H, Seino S, Bell GI (1993) Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem 268(15):11356–11363 (PubMed PMID: 8388391)
Yamamoto-Hino M, Sugiyama T, Hikichi K, Mattei MG, Hasegawa K, Sekine S et al (1994) Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Recept Channels 2(1):9–22 (Epub 1994/01/01. PubMed PMID: 8081734)
Michell RH (1982) Inositol lipid metabolism in dividing and differentiating cells. Cell Calcium 3(4–5):429–440. https://doi.org/10.1016/0143-4160(82)90028-8 (Epub 1982/10/01. PubMed PMID: 6760977)
Berridge MJ (2016) The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96(4):1261–1296. https://doi.org/10.1152/physrev.00006.2016 (Epub 2016/08/12. PubMed PMID: 27512009)
Prole DL, Taylor CW (2016) Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol 594(11):2849–2866. https://doi.org/10.1113/JP271139 (Epub 2016/02/03. PubMed PMID: 26830355; PubMed Central PMCID: PMCPMC4887697)
Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K (2007) Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem 282(17):12755–12764. https://doi.org/10.1074/jbc.M609833200 (PubMed PMID: 17327232)
Nerou EP, Riley AM, Potter BV, Taylor CW (2001) Selective recognition of inositol phosphates by subtypes of the inositol trisphosphate receptor. Biochem J 355(Pt 1):59–69. https://doi.org/10.1042/0264-6021:3550059 (PubMed PMID: 11256949; PubMed Central PMCID: PMC1221712)
Baker MR, Fan G, Seryshev AB, Agosto MA, Baker ML, Serysheva II (2021) Cryo-EM structure of type 1 IP3R channel in a lipid bilayer. Commun Biol 4(1):625. https://doi.org/10.1038/s42003-021-02156-4
Betzenhauser MJ, Fike JL, Wagner LE 2nd, Yule DI (2009) Protein kinase A increases type-2 inositol 1,4,5-trisphosphate receptor activity by phosphorylation of serine 937. J Biol Chem 284(37):25116–25125. https://doi.org/10.1074/jbc.M109.010132 (PubMed PMID: 19608738; PubMed Central PMCID: PMC2757215)
Soulsby MD, Wojcikiewicz RJ (2007) Calcium mobilization via type III inositol 1,4,5-trisphosphate receptors is not altered by PKA-mediated phosphorylation of serines 916, 934, and 1832. Cell Calcium 42(3):261–270. https://doi.org/10.1016/j.ceca.2006.12.002 (PubMed PMID: 17257671; PubMed Central PMCID: PMC1975771)
Bimboese P, Gibson CJ, Schmidt S, Xiang W, Ehrlich BE (2011) Isoform-specific regulation of the inositol 1,4,5-trisphosphate receptor by O-linked glycosylation. J Biol Chem 286(18):15688–15697. https://doi.org/10.1074/jbc.M110.206482 (PubMed PMID: 21383013; PubMed Central PMCID: PMC3091177)
Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE (2007) Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation. J Neurosci 27(50):13813–13821. https://doi.org/10.1523/JNEUROSCI.2069-07.2007 (PubMed PMID: 18077693; PubMed Central PMCID: PMC6673603)
Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262(5134):744–747. https://doi.org/10.1126/science.8235595 (Epub 1993/10/29. PubMed PMID: 8235595)
De Stefani D, Rizzuto R, Pozzan T (2016) Enjoy the trip: calcium in mitochondria back and forth. Annu Rev Biochem 85:161–192. https://doi.org/10.1146/annurev-biochem-060614-034216 (PubMed PMID: 27145841)
La Rovere RM, Roest G, Bultynck G, Parys JB (2016) Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60(2):74–87. https://doi.org/10.1016/j.ceca.2016.04.005 (Epub 2016/05/10. PubMed PMID: 27157108)
Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM et al (2005) The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem 280(49):40892–40900. https://doi.org/10.1074/jbc.M506623200 (PubMed PMID: 16192275)
Mangla A, Guerra MT, Nathanson MH (2020) Type 3 inositol 1,4,5-trisphosphate receptor: A calcium channel for all seasons. Cell Calcium 85:102132. https://doi.org/10.1016/j.ceca.2019.102132 (PubMed PMID: 31790953; PubMed Central PMCID: PMC6917836)
Mignery GA, Sudhof TC, Takei K, De Camilli P (1989) Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature 342(6246):192–195. https://doi.org/10.1038/342192a0 (PubMed PMID: 2554146)
Seo MD, Enomoto M, Ishiyama N, Stathopulos PB, Ikura M (2015) Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim Biophys Acta 1853(9):1980–1991. https://doi.org/10.1016/j.bbamcr.2014.11.023 (Epub 2014/12/03. PubMed PMID: 25461839)
Santulli G, Nakashima R, Yuan Q, Marks AR (2017) Intracellular calcium release channels: an update. J Physiol 595(10):3041–3051. https://doi.org/10.1113/jp272781 (Epub 2017/03/18. PubMed PMID: 28303572; PubMed Central PMCID: PMCPMC5430224)
Berridge MJ (2006) Calcium microdomains: organization and function. Cell Calcium 40(5–6):405–412. https://doi.org/10.1016/j.ceca.2006.09.002 (Epub 2006/10/13. PubMed PMID: 17030366)
McGeown JG (2004) Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two? Cell Calcium 35(6):613–619. https://doi.org/10.1016/j.ceca.2004.01.016 (Epub 2004/04/28. PubMed PMID: 15110151)
Niggli E (1999) Localized intracellular calcium signaling in muscle: calcium sparks and calcium quarks. Annu Rev Physiol 61:311–335. https://doi.org/10.1146/annurev.physiol.61.1.311 (Epub 1999/04/01. PubMed PMID: 10099691)
Manita S, Ross WN (2009) Synaptic activation and membrane potential changes modulate the frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons. J Neurosci 29(24):7833–7845. https://doi.org/10.1523/JNEUROSCI.0573-09.2009 (Epub 2009/06/19. PubMed PMID: 19535595; PubMed Central PMCID: PMCPMC2756180)
Miyazaki K, Ross WN (2013) Ca2+ sparks and puffs are generated and interact in rat hippocampal CA1 pyramidal neuron dendrites. J Neurosci 33(45):17777–17788. https://doi.org/10.1523/JNEUROSCI.2735-13.2013 (Epub 2013/11/08. PubMed PMID: 24198368; PubMed Central PMCID: PMCPMC3818551)
McDaid J, Mustaly-Kalimi S, Stutzmann GE (2020) Ca(2+) dyshomeostasis disrupts neuronal and synaptic function in Alzheimer’s disease. Cells. https://doi.org/10.3390/cells9122655 (Epub 2020/12/17. PubMed PMID: 33321866; PubMed Central PMCID: PMCPMC7763805)
Grosskreutz J, Van Den Bosch L, Keller BU (2010) Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 47(2):165–174. https://doi.org/10.1016/j.ceca.2009.12.002 (Epub 2010/02/02. PubMed PMID: 20116097)
Naia L, Ferreira IL, Ferreiro E, Rego AC (2017) Mitochondrial Ca(2+) handling in Huntington’s and Alzheimer’s diseases—role of ER-mitochondria crosstalk. Biochem Biophys Res Commun 483(4):1069–1077. https://doi.org/10.1016/j.bbrc.2016.07.122 (Epub 2016/08/04. PubMed PMID: 27485547)
Orem BC, Pelisch N, Williams J, Nally JM, Stirling DP (2017) Intracellular calcium release through IP(3)R or RyR contributes to secondary axonal degeneration. Neurobiol Dis 106:235–243. https://doi.org/10.1016/j.nbd.2017.07.011 (Epub 2017/07/16. PubMed PMID: 28709993)
Zhao H, Tong G, Liu J, Wang J, Zhang H, Bai J et al (2019) IP3R and RyR channels are involved in traffic-related PM(2.5)-induced disorders of calcium homeostasis. Toxicol Ind Health 35(5):339–348. https://doi.org/10.1177/0748233719843763 (Epub 2019/04/27. PubMed PMID: 31023176)
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21(8):421–438. https://doi.org/10.1038/s41580-020-0250-z (Epub 2020/05/28. PubMed PMID: 32457508)
Ghemrawi R, Khair M (2020) Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. Int J Mol Sci. https://doi.org/10.3390/ijms21176127 (Epub 2020/08/29. PubMed PMID: 32854418; PubMed Central PMCID: PMCPMC7503386)
Stefani IC, Wright D, Polizzi KM, Kontoravdi C (2012) The role of ER stress-induced apoptosis in neurodegeneration. Curr Alzheimer Res 9(3):373–387. https://doi.org/10.2174/156720512800107618 (Epub 2012/02/04. PubMed PMID: 22299619)
Hetz C, Saxena S (2017) ER stress and the unfolded protein response in neurodegeneration. Nat Rev Neurol 13(8):477–491. https://doi.org/10.1038/nrneurol.2017.99 (Epub 2017/07/22. PubMed PMID: 28731040)
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086. https://doi.org/10.1126/science.1209038 (Epub 2011/11/26. PubMed PMID: 22116877)
Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27(1):91–105. https://doi.org/10.1016/j.molcel.2007.06.017 (Epub 2007/07/07. PubMed PMID: 17612493)
Agarwal V, Bell GW, Nam JW, Bartel DP (2015) Predicting effective microRNA target sites in mammalian mRNAs. Elife. https://doi.org/10.7554/eLife.05005 (Epub 2015/08/13. PubMed PMID: 26267216; PubMed Central PMCID: PMCPMC4532895)
Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z et al (2004) A combined computational-experimental approach predicts human microRNA targets. Genes Dev 18(10):1165–1178. https://doi.org/10.1101/gad.1184704 (Epub 2004/05/08. PubMed PMID: 15131085; PubMed Central PMCID: PMC415641)
Paraskevopoulou MD, Georgakilas G, Kostoulas N, Vlachos IS, Vergoulis T, Reczko M et al (2013) DIANA-microT web server v5.0: service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41(Web Server issue):W169–W173. https://doi.org/10.1093/nar/gkt393(PubMed PMID: 23680784; PubMed Central PMCID: PMC3692048)
Wong N, Wang X (2015) miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res 43(Database issue):D146–D152. https://doi.org/10.1093/nar/gku1104(PubMed PMID: 25378301; PubMed Central PMCID: PMC4383922)
Fehlmann T, Ludwig N, Backes C, Meese E, Keller A (2016) Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol 13(11):1084–1088. https://doi.org/10.1080/15476286.2016.1234658 (PubMed PMID: 27687236; PubMed Central PMCID: PMC5100342)
Guo Z, Maki M, Ding R, Yang Y, Zhang B, Xiong L (2014) Genome-wide survey of tissue-specific microRNA and transcription factor regulatory networks in 12 tissues. Sci Rep 4:5150. https://doi.org/10.1038/srep05150 (Epub 2014/06/04. PubMed PMID: 24889152; PubMed Central PMCID: PMCPMC5381490)
Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C et al (2016) Distribution of miRNA expression across human tissues. Nucleic Acids Res 44(8):3865–3877. https://doi.org/10.1093/nar/gkw116 (Epub 2016/02/28. PubMed PMID: 26921406; PubMed Central PMCID: PMCPMC4856985)
Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR (2008) Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J 414(3):441–452. https://doi.org/10.1042/BJ20080489 (Epub 2008/06/04. PubMed PMID: 18518861; PubMed Central PMCID: PMCPMC2747660)
McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C et al (1991) The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron 7(1):17–25. https://doi.org/10.1016/0896-6273(91)90070-g (Epub 1991/07/01. PubMed PMID: 1648939)
Guerreiro S, Marien M, Michel PP (2011) Methylxanthines and ryanodine receptor channels. Handb Exp Pharmacol 200:135–150. https://doi.org/10.1007/978-3-642-13443-2_5 (Epub 2010/09/23. PubMed PMID: 20859795)
Fisher SK, Snider RM (1987) Differential receptor occupancy requirements for muscarinic cholinergic stimulation of inositol lipid hydrolysis in brain and in neuroblastomas. Mol Pharmacol 32(1):81–90 (Epub 1987/07/01. PubMed PMID: 3600615)
Baird JG, Lambert DG, McBain J, Nahorski SR (1989) Muscarinic receptors coupled to phosphoinositide hydrolysis and elevated cytosolic calcium in a human neuroblastoma cell line SK-N-SH. Br J Pharmacol 98(4):1328–1334. https://doi.org/10.1111/j.1476-5381.1989.tb12681.x (Epub 1989/12/01. PubMed PMID: 2558760; PubMed Central PMCID: PMCPMC1854815)
Lambert DG, Nahorski SR (1990) Muscarinic-receptor-mediated changes in intracellular Ca2+ and inositol 1,4,5-trisphosphate mass in a human neuroblastoma cell line, SH-SY5Y. Biochem J 265(2):555–562. https://doi.org/10.1042/bj2650555 (Epub 1990/01/15. PubMed PMID: 2302186; PubMed Central PMCID: PMCPMC1136919)
White C, McGeown JG (2002) Carbachol triggers RyR-dependent Ca(2+) release via activation of IP(3) receptors in isolated rat gastric myocytes. J Physiol 542(Pt 3):725–733. https://doi.org/10.1113/jphysiol.2002.020131 (Epub 2002/08/03. PubMed PMID: 12154174; PubMed Central PMCID: PMCPMC2290441)
Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA 87(7):2466–2470. https://doi.org/10.1073/pnas.87.7.2466 (Epub 1990/04/01. PubMed PMID: 2138778; PubMed Central PMCID: PMCPMC53710)
Treiman M, Caspersen C, Christensen SB (1998) A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases. Trends Pharmacol Sci 19(4):131–135. https://doi.org/10.1016/s0165-6147(98)01184-5 (Epub 1998/06/05. PubMed PMID: 9612087)
Foufelle F, Fromenty B (2016) Role of endoplasmic reticulum stress in drug-induced toxicity. Pharmacol Res Perspect 4(1):e00211. https://doi.org/10.1002/prp2.211 (Epub 2016/03/16. PubMed PMID: 26977301; PubMed Central PMCID: PMCPMC4777263)
Yoo J, Mashalidis EH, Kuk ACY, Yamamoto K, Kaeser B, Ichikawa S et al (2018) GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nat Struct Mol Biol 25(3):217–224. https://doi.org/10.1038/s41594-018-0031-y (Epub 2018/02/21. PubMed PMID: 29459785; PubMed Central PMCID: PMCPMC5840018)
Wang H, Wang X, Ke ZJ, Comer AL, Xu M, Frank JA et al (2015) Tunicamycin-induced unfolded protein response in the developing mouse brain. Toxicol Appl Pharmacol 283(3):157–167. https://doi.org/10.1016/j.taap.2014.12.019 (Epub 2015/01/27. PubMed PMID: 25620058; PubMed Central PMCID: PMCPMC4361256)
Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR et al (2014) Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol 7(6):1214–1222. https://doi.org/10.1161/CIRCEP.114.001973 (Epub 2014/11/13. PubMed PMID: 25389315; PubMed Central PMCID: PMCPMC4270890)
Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu H et al (2011) Functional SNP in the microRNA-367 binding site in the 3’UTR of the calcium channel ryanodine receptor gene 3 (RYR3) affects breast cancer risk and calcification. Proc Natl Acad Sci USA 108(33):13653–13658. https://doi.org/10.1073/pnas.1103360108 (Epub 2011/08/04. PubMed PMID: 21810988; PubMed Central PMCID: PMCPMC3158174)
Wahlquist C, Jeong D, Rojas-Munoz A, Kho C, Lee A, Mitsuyama S et al (2014) Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature 508(7497):531–535. https://doi.org/10.1038/nature13073 (Epub 2014/03/29. PubMed PMID: 24670661; PubMed Central PMCID: PMCPMC4131725)
Gabani M, Liu J, Ait-Aissa K, Koval O, Kim YR, Castaneda D et al (2019) MiR-204 regulates type 1 IP3R to control vascular smooth muscle cell contractility and blood pressure. Cell Calcium 80:18–24. https://doi.org/10.1016/j.ceca.2019.03.006 (Epub 2019/03/30. PubMed PMID: 30925290; PubMed Central PMCID: PMCPMC6767906)
Diener C, Hart M, Alansary D, Poth V, Walch-Ruckheim B, Menegatti J et al (2018) Modulation of intracellular calcium signaling by microRNA-34a-5p. Cell Death Dis 9(10):1008. https://doi.org/10.1038/s41419-018-1050-7 (Epub 2018/09/29. PubMed PMID: 30262862; PubMed Central PMCID: PMCPMC6160487)
Drawnel FM, Wachten D, Molkentin JD, Maillet M, Aronsen JM, Swift F et al (2012) Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J Cell Biol 199(5):783–798. https://doi.org/10.1083/jcb.201111095 (Epub 2012/11/21. PubMed PMID: 23166348; PubMed Central PMCID: PMCPMC3514786)
Ananthanarayanan M, Banales JM, Guerra MT, Spirli C, Munoz-Garrido P, Mitchell-Richards K et al (2015) Post-translational regulation of the type III inositol 1,4,5-trisphosphate receptor by miRNA-506. J Biol Chem 290(1):184–196. https://doi.org/10.1074/jbc.M114.587030 (Epub 2014/11/08. PubMed PMID: 25378392; PubMed Central PMCID: PMCPMC4281721)
Baker KD, Edwards TM, Rickard NS (2013) The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev 37(7):1211–1239. https://doi.org/10.1016/j.neubiorev.2013.04.011 (Epub 2013/05/04. PubMed PMID: 23639769)
Paschou M, Doxakis E (2012) Neurofibromin 1 Is a miRNA Target in Neurons. PLoS ONE 7(10):e46773. https://doi.org/10.1371/journal.pone.0046773 (PubMed PMID: 23056445; PubMed Central PMCID: PMC3462785)
Paschou M, Paraskevopoulou MD, Vlachos IS, Koukouraki P, Hatzigeorgiou AG, Doxakis E (2012) miRNA regulons associated with synaptic function. PLoS ONE 7(10):e46189. https://doi.org/10.1371/journal.pone.0046189 (PubMed PMID: 23071543; PubMed Central PMCID: PMC3468272)
Paschou M, Doxakis E (2012) Neurofibromin 1 is a miRNA target in neurons. PLoS ONE. https://doi.org/10.1371/journal.pone.0046773
Banerjee S, Hasan G (2005) The InsP3 receptor: its role in neuronal physiology and neurodegeneration. BioEssays 27(10):1035–1047. https://doi.org/10.1002/bies.20298 (Epub 2005/09/16. PubMed PMID: 16163728)
Del Prete D, Checler F, Chami M (2014) Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9:21. https://doi.org/10.1186/1750-1326-9-21 (Epub 2014/06/07. PubMed PMID: 24902695; PubMed Central PMCID: PMCPMC4063224)
Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV et al (1998) Calcium dysregulation in neuronal aging and Alzheimer’s disease: history and new directions. Cell Calcium 24(5–6):417–433. https://doi.org/10.1016/s0143-4160(98)90064-1 (Epub 1999/03/26. PubMed PMID: 10091010)
O’Neill C, Cowburn RF, Bonkale WL, Ohm TG, Fastbom J, Carmody M et al (2001) Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer’s disease. Biochem Soc Symp 67:177–194. https://doi.org/10.1042/bss0670177 (Epub 2001/07/13. PubMed PMID: 11447834)
Gant JC, Sama MM, Landfield PW, Thibault O (2006) Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci 26(13):3482–3490. https://doi.org/10.1523/JNEUROSCI.4171-05.2006 (Epub 2006/03/31. PubMed PMID: 16571755; PubMed Central PMCID: PMCPMC6673869)
Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J et al (2012) Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32(34):11820–11834. https://doi.org/10.1523/JNEUROSCI.0875-12.2012 (Epub 2012/08/24. PubMed PMID: 22915123; PubMed Central PMCID: PMCPMC3458216)
Ruiz A, Matute C, Alberdi E (2009) Endoplasmic reticulum Ca(2+) release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium 46(4):273–281. https://doi.org/10.1016/j.ceca.2009.08.005 (Epub 2009/09/15. PubMed PMID: 19747726)
Zhao S, Deng Y, Liu Y, Chen X, Yang G, Mu Y et al (2013) MicroRNA-153 is tumor suppressive in glioblastoma stem cells. Mol Biol Rep 40(4):2789–2798. https://doi.org/10.1007/s11033-012-2278-4 (Epub 2013/02/12. PubMed PMID: 23397238)
Fragkouli A, Doxakis E (2014) miR-7 and miR-153 protect neurons against MPP(+)-induced cell death via upregulation of mTOR pathway. Front Cell Neurosci 8:182. https://doi.org/10.3389/fncel.2014.00182 (Epub 2014/07/30. PubMed PMID: 25071443; PubMed Central PMCID: PMCPMC4080263)
Tsai PC, Bake S, Balaraman S, Rawlings J, Holgate RR, Dubois D et al (2014) MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biology open. 3(8):741–758. https://doi.org/10.1242/bio.20147765 (Epub 2014/07/27. PubMed PMID: 25063196; PubMed Central PMCID: PMCPMC4133727)
Nolan JC, Salvucci M, Carberry S, Barat A, Segura MF, Fenn J et al (2020) A context-dependent role for MiR-124-3p on cell phenotype, viability and chemosensitivity in neuroblastoma in vitro. Front Cell Dev Biol 8:559553. https://doi.org/10.3389/fcell.2020.559553 (Epub 2020/12/18. PubMed PMID: 33330445; PubMed Central PMCID: PMCPMC7714770)
Ferreiro E, Oliveira CR, Pereira C (2004) Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res 76(6):872–880. https://doi.org/10.1002/jnr.20135 (Epub 2004/05/26. PubMed PMID: 15160398)
Popescu BO, Oprica M, Sajin M, Stanciu CL, Bajenaru O, Predescu A et al (2002) Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. J Cell Mol Med 6(4):555–569. https://doi.org/10.1111/j.1582-4934.2002.tb00454.x (Epub 2003/03/04. PubMed PMID: 12611640; PubMed Central PMCID: PMCPMC6741407)
Ruiz A, Matute C, Alberdi E (2010) Intracellular Ca2+ release through ryanodine receptors contributes to AMPA receptor-mediated mitochondrial dysfunction and ER stress in oligodendrocytes. Cell Death Dis 1:e54. https://doi.org/10.1038/cddis.2010.31 (Epub 2011/03/03. PubMed PMID: 21364659; PubMed Central PMCID: PMCPMC3032558)
Panganiban RA, Park H-R, Sun M, Shumyatcher M, Himes BE, Lu Q (2019) Genome-wide CRISPR screen identifies suppressors of endoplasmic reticulum stress-induced apoptosis. Proc Natl Acad Sci 116(27):13384. https://doi.org/10.1073/pnas.1906275116
Ding C, Dou M, Wang Y, Li Y, Wang Y, Zheng J et al (2020) miR-124/IRE-1α affects renal ischemia/reperfusion injury by regulating endoplasmic reticulum stress in renal tubular epithelial cells. Acta Biochim Biophys Sin 52(2):160–167. https://doi.org/10.1093/abbs/gmz150
Park H-R, Sun R, Panganiban RA, Christiani DC, Lu Q (2020) MicroRNA-124 reduces arsenic-induced endoplasmic reticulum stress and neurotoxicity and is linked with neurodevelopment in children. Sci Rep 10(1):5934. https://doi.org/10.1038/s41598-020-62594-8
Liang H, Xiao J, Zhou Z, Wu J, Ge F, Li Z et al (2018) Hypoxia induces miR-153 through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in breast cancer angiogenesis. Oncogene 37(15):1961–1975. https://doi.org/10.1038/s41388-017-0089-8
Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S (2001) Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett 497(1):1–5. https://doi.org/10.1016/s0014-5793(01)02437-1 (Epub 2001/05/30. PubMed PMID: 11376653)
Subramaniam SR, Chesselet MF (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32. https://doi.org/10.1016/j.pneurobio.2013.04.004 (Epub 2013/05/07. PubMed PMID: 23643800; PubMed Central PMCID: PMCPMC3742021)
Karagas NE, Venkatachalam K (2019) Roles for the endoplasmic reticulum in regulation of neuronal calcium homeostasis. Cells. https://doi.org/10.3390/cells8101232 (Epub 2019/10/30. PubMed PMID: 31658749; PubMed Central PMCID: PMCPMC6829861)
Haraguchi T, Ozaki Y, Iba H (2009) Vectors expressing efficient RNA decoys achieve the long-term suppression of specific microRNA activity in mammalian cells. Nucleic Acids Res 37(6):e43. https://doi.org/10.1093/nar/gkp040 (PubMed PMID: 19223327; PubMed Central PMCID: PMC2665227)
Bak RO, Hollensen AK, Primo MN, Sorensen CD, Mikkelsen JG (2013) Potent microRNA suppression by RNA Pol II-transcribed “Tough Decoy” inhibitors. RNA 19(2):280–293. https://doi.org/10.1261/rna.034850.112 (PubMed PMID: 23249752; PubMed Central PMCID: PMC3543086)
Ravanidis S, Bougea A, Papagiannakis N, Maniati M, Koros C, Simitsi AM et al (2020) Circulating brain-enriched MicroRNAs for detection and discrimination of idiopathic and genetic Parkinson’s disease. Mov Disord 35(3):457–467. https://doi.org/10.1002/mds.27928 (PubMed PMID: 31799764)
Gee KR, Brown KA, Chen WN, Bishop-Stewart J, Gray D, Johnson I (2000) Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium 27(2):97–106. https://doi.org/10.1054/ceca.1999.0095 (Epub 2000/04/11. PubMed PMID: 10756976)
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260(6):3440–3450 (Epub 1985/03/25. PubMed PMID: 3838314)
Funding
This work was supported by grants from the Greek General Secretariat for Research and Technology (GSRT) to ED (MIS380201, 12RUS-11-65), SGD (ARISTEIA II 4475), and PP (ARISTEIA I 1507). The funders had no role in the study design, the collection, data analysis, or the manuscript's preparation.
Author information
Authors and Affiliations
Contributions
Conceived the study: ED. Designed and optimized protocols: ED, MP, and PP. Prepared plasmid constructs: ED, MP, and CC. Performed tissue culture and molecular biology experiments: MP and CC. Performed Ca2+ imaging experiments: MP, PP, MZ, and CC. Analyzed data: MP, PP, ED, MZ, and SGD. Wrote the manuscript: ED and MP. All authors read, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
18_2022_4398_MOESM1_ESM.tif
Figure S1. miRNA and RyR1-3, IP3R1-3 mRNA expression in human tissues, primary cortical neurons and SK-N-SH cells. A Quantification of endogenous RNA expression levels (log10) of RyR1-3 and IP3R1-3 mRNAs, miR-96, miR-124, miR-153, and miR-182 in different human tissues measured by RT-qPCR using GAPDH and U6 mRNAs as reference controls. Data show the mean ± SD from two technical replicates. B Boxplot of ΔCT values (CT of mRNA or miRNA minus CT of reference gene mRNA) of RyR1-3, IP3R1-3 mRNA, miR-96, miR-124, miR-153, and miR-182 endogenous expression in primary cortical neurons measured by RT-qPCR. GAPDH mRNA was used as an internal reference control. The boxes indicate the interquartile range (IQR), the whiskers show the range of values within 1.5 x IQR, and the horizontal line indicates the median. Dots represent individual ΔCT values obtained from two biological replicates (two technical replicates) per RNA group. C Boxplot of ΔCT values of miR-96, miR-124, miR-153, and miR-182 endogenous expression in SK-N-SH cells measured by RT-qPCR, using GAPDH mRNA as an internal reference control. Dots represent individual ΔCT values obtained from ten biological replicates per miRNA group. ( D–G) Relative miRNA expression levels in SK-N-SH cells following transfection with pri-miR-96, pri-miR-124, pri-miR-153, and pri-miR-182 pcDNA6.2-GW/EmGFP-miR expression vectors. miRNA levels were quantified by RT-qPCR 48 h later, using GAPDH and U6 mRNAs for normalization. Data show the mean ± SD from three biological replicates. Statistical significance was evaluated by two-tailed Student’s t test for paired samples, compared to the control group (*, p<0.05; **, p<0.01; ***, p<0.001). (TIF 2750 KB)
18_2022_4398_MOESM2_ESM.tif
Figure S2. Design of TuD constructs. A schematic of the TuD-124 (A) and TuD-153 (B) RNA structure and sequence. TuD inhibitors form a hairpin-shaped RNA structure that exposes two opposing miRNA-binding sites (depicted in red color). Endogenous miR-124-3p and miR-153-3p (depicted in green color) bind with imperfect complementarity to these sites, preventing them from binding to target genes. (TIF 2174 KB)
18_2022_4398_MOESM3_ESM.tif
Figure S3. miR-124 and miR-153 reduce RyR1-3 and IP3R3 protein levels in C2C12 cells. Representative immunoblot images of pan-RyR (A) or IP3R3 (B) protein levels in C2C12 cells transfected with miR-124 or miR-153 plasmids for 48 h. β-Adaptin and α-Tubulin were used as internal normalization controls, obtained from different gels with identical protein loading. The molecular weights of the proteins, as indicated by the antibody manufacturers, are depicted on the left of each blot image. Pixel intensity analysis revealed that pan-RyR and IP3R3 protein levels were significantly lower upon miR-124 or miR-153 overexpression than miR-SC control. Data show the mean ± SD from four independent biological replicates. Statistical significance was evaluated by one-way ANOVA followed by Dunnett’s multiple comparison test for repeated measurements (pan-RyR) or two-tailed Student’s t test for paired samples (IP3R3), compared to the control group (*, p<0.05; ***, p<0.001). (TIF 1443 KB)
18_2022_4398_MOESM4_ESM.tif
Figure S4. Effects of different concentrations of Ca2+ modulators on Ca2+ mobilization in SK-N-SH cells. Representative dose-dependent curves of Ca2+ amplitude (expressed as ΔF/F0) in response to (A) CAFF (15, 30, 60 mM), (B) CCh (0.5, 1, 2 mM), and (C) Tg (0.5, 1, 2 μM). Untransfected SK-N-SH cells were loaded with Fluo-4, and differences in the fluorescence intensity were monitored over time, before and after stimulation with the reagents, in extracellular Ca2+ free conditions. ΔF/F0 represents changes in the fluorescence intensity over baseline levels over time. Arrows indicate the timing of the addition of reagents. Black curves depict the response to the concentration of each reagent that was chosen for further experiments (CAFF, 30 mM; CCh, 1 mM; Tg, 2 μM). (TIF 770 KB)
18_2022_4398_MOESM5_ESM.tif
Figure S5. Tunicamycin induces ER stress in a dose-dependent manner. A Representative immunoblot images of untransfected SK-N-SH cells treated with increasing concentrations of Tm (0.5, 1, 2, 5, 10 μg/ml) for 24 h. Protein levels of p-eIF2α, ATF4, IRE1α, ATF6, XBP1s, and GRP78 were measured with total eIF2α, β-Adaptin, α-Tubulin, or GAPDH being used as loading controls for normalization. The molecular weights of the proteins, as indicated by the antibody manufacturers, are depicted on the left of each blot image. The red dashed box highlights the results obtained with 2 μg/ml Tm, chosen for further experiments. (TIF 2816 KB)
18_2022_4398_MOESM6_ESM.tif
Figure S6. Uncropped Western blot images used in Fig 4, Fig 8, Fig 9, Fig S3, and Fig S5. Dashed boxes contain the protein of interest and loading controls (β-Adaptin, α-Tubulin, GAPDH) run in the same gel. Positions of the molecular weight markers and their sizes are depicted on the left of each blot image and the molecular weight of each protein, as indicated by the antibody manufacturers, is displayed on the right. (TIF 13000 KB)
Rights and permissions
About this article
Cite this article
Paschou, M., Papazafiri, P., Charalampous, C. et al. Neuronal microRNAs safeguard ER Ca2+ homeostasis and attenuate the unfolded protein response upon stress. Cell. Mol. Life Sci. 79, 373 (2022). https://doi.org/10.1007/s00018-022-04398-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04398-9