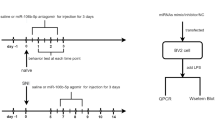
Abstract
MicroRNAs (miRNAs) are short non-coding RNAs that regulate gene expression post-transcriptionally by binding to their cognate target mRNAs. Emerging evidence suggests that miRNAs are critical regulators of neuronal functions. The expression pattern of miRNAs in the peripheral nervous system after peripheral nerve injury suggest that miRNAs may have important and yet unknown roles in the mechanisms of pain. Thus, we examined the role of miR-96 in neuropathic pain using a rat model of the condition chronic constriction sciatic nerve injury (CCI). We found that miR-96 alleviated neuropathic pain. The level of miR-96 was decreased within the ipsilateral dorsal root ganglion (DRG) after peripheral nerve injury but the Nav1.3 level was increased. Specifically, Intrathecal administration of miR-96 suppressed the expression of Nav1.3 induced by CCI. Further examination revealed that miR-96 inhibited the Nav1.3 mRNA expression in the embryonic DRG neurons in vitro. Our findings suggest that miR-96 participate in the regulation of neuropathic pain through inhibiting the expression of Nav1.3 in the DRG of CCI rats.




Similar content being viewed by others
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Ambros V (2006) The functions of animal microRNAs. Nature 431(2004):350–355
Kloosterman WP, Plasterk RH (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450
Marcinowski L, Tanguy M, Krmpotic A, Rädle B, Lisnić VJ, Tuddenham L, Chane-Woon-Ming B, Ruzsics Z, Erhard F, Benkartek C, Babic M, Zimmer R, Trgovcich J, Koszinowski UH, Jonjic S, Pfeffer S, Dölken L (2012) Degradation of cellular mir-27 by a novel, highly abundant viral transcript is important for efficient virus replication in vivo. PLoS Pathog 8:e1002510
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H, Chen G, Wang Z (2007) The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med 13:486–491
Oglesby IK, McElvaney NG, Greene CM (2010) MicroRNAs in inflammatory lung disease—master regulators or target practice? Respir Res 11:148
Ma X, Becker Buscaglia LE, Barker JR, Li Y (2011) MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3:159–166
Eacker SM, Dawson TM, Dawson VL (2009) Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10:837–841
Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH (2010) microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci USA 107:20382–20387
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S (2005) A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci USA 102:16426–16431
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A MicroRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM (2009) Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA 106:13052–13057
Yu B, Zhou S, Wang Y, Ding G, Ding F, Gu X (2011) Profile of microRNAs following rat sciatic nerve injury by deep sequencing: implication for mechanisms of nerve regeneration. PLoS ONE 6:e24612
Liu NK, Wang XF, Lu QB, Xu XM (2009) Altered microRNA expression following traumatic spinal cord injury. Exp Neurol 219:424–429
Von Schack D, Agostino MJ, Murray BS, Li Y, Reddy PS, Chen J, Choe SE, Strassle BW, Li C, Bates B, Zhang L, Hu H, Kotnis S, Bingham B, Liu W, Whiteside GT, Samad TA, Kennedy JD, Ajit SK (2011) Dynamic changes in the microRNA expression profile reveal multiple regulatory mechanisms in the spinal nerve ligation model of neuropathic pain. PLoS ONE 6:e17670
Sakai A, Suzuki H (2013) Nerve injury-induced upregulation of miR-21 in the primary sensory neurons contributes to neuropathic pain in rats. Biochem Biophys Res Commun 435:176–181
Aldrich BT, Frakes EP, Kasuya J, Hammond DL, Kitamoto T (2009) Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 164:711–723
Waxman SG, Kocsis JD, Black JA (1994) Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is re-expressed following axotomy. J Neurophysiol 72:466–471
Shoji T, Nakasa T, Yamasaki K, Kodama A, Miyaki S, Niimoto T, Okuhara A, Kamei N, Adachi N, Ochi M (2012) The effect of intra-articular injection of microRNA-210 on ligament healing in a rat model. Am J Sports Med 40:2470–2478
Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N, Kawamata M, Kelnar K, Bader AG, Brown D, Ochiya T (2010) Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol Ther 18:181–187
Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17:1031–1036
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Ou S, Zhao YD, Xiao Z, Wen HZ, Cui J, Ruan HZ (2011) Effect of lappaconitine on neuropathic pain mediated by P2X3 receptor in rat dorsal root ganglion. Neurochem Int 58:564–573
Chen HP, Fan J, Cui S (2006) Detection and estrogen regulation of leptin receptor expression in rat dorsal root ganglion. Histochem Cell Biol 126:363–369
Ren YS, Qian NS, Tang Y, Liao YH, Yang YL, Dou KF, Toi M (2012) Sodium channel Nav1.6 is up-regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Res Bull 87:244–249
Katz N (2000) Neuropathic pain in cancer and AIDS. Clin J Pain 16:S41–S48
Shimoshige Y, Enomoto R, Aoki T, Matsuoka N, Kaneko S (2010) The involvement of aldose reductase in alterations to neurotrophin receptors and neuronal cytoskeletal protein mRNA levels in the dorsal root ganglion of streptozotocin-induced diabetic rats. Biol Pharm Bull 33:67–71
Zhou XF, Deng YS, Xian CJ, Zhong JH (2000) Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci 12:100–105
Sakurai E, Kurihara T, Kouchi K, Saegusa H, Zong S, Tanabe T (2009) Upregulation of casein kinase 1epsilon in dorsal root ganglia and spinal cord after mouse spinal nerve injury contributes to neuropathic pain. Mol Pain 5:74
Samad OA, Tan AM, Cheng X, Foster E, Dib-Hajj SD, Waxman SG (2013) Virus-mediated shRNA knockdown of Na(v)1.3 in rat dorsal root ganglion attenuates nerve injury-induced neuropathic pain. Mol Ther 21:49–56
Sapunar D, Kostic S, Banozic A, Puljak L (2012) Dorsal root ganglion—a potential new therapeutic target for neuropathic pain. J Pain Res 5:31–38
Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, Liu XG (2013) Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. doi:10.1016/j.expneurol.2013.01.018
Cheng KI, Lai CS, Wang FY, Wang HC, Chang LL, Ho ST, Tsai HP, Kwan AL (2011) Intrathecal lidocaine pretreatment attenuates immediate neuropathic pain by modulating Nav1.3 expression and decreasing spinal microglial activation. BMC Neurol 11:71
Kim CH, Oh Y, Chung JM, Chung K (2001) The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Res Mol Brain Res 95:153–161
Black JA, Cummins TR, Plumpton C, Chen YH, Hormuzdiar W, Clare JJ, Waxman SG (1999) Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J Neurophysiol 82:2776–2785
Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG (2004) Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci 24:4832–4839
Lindia JA, Kohler MG, Martin WJ, Abbadie C (2005) Relationship between sodium channel NaV1.3 expression and neuropathic pain behavior in rats. Pain 117:145–153
Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G (2011) Differential expression of microRNAs in mouse pain models. Mol Pain 17:17
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 30973030, 30800424, 81260318 and 81371967), Natural Science Foundation (No. 20122BAB205061) and Science and Technology Support Program (No. 2009JX01268) of Jiangxi Province, China. Educational Department Foundation (No. GJJ13155) of Jiangxi Province, China. We are grateful for critical reading of the manuscript by Michael Sun, USA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hong-Ping Chen, Wei Zhou and Lu-Mei Kang have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Chen, HP., Zhou, W., Kang, LM. et al. Intrathecal miR-96 Inhibits Nav1.3 Expression and Alleviates Neuropathic Pain in Rat Following Chronic Construction Injury. Neurochem Res 39, 76–83 (2014). https://doi.org/10.1007/s11064-013-1192-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1192-z