Abstract
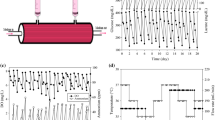
The perfusion mode of a continuous cell culture bioreactor was modified to establish a closed loop system. Eighty percent of the spent medium was re-used twice. The medium cycle bioreactor unit was operated sterile and uncomplicated without a technical retention system for the high molecular weight substances. Therefore, only 20% of the actual medium was necessary to run the recycling process. During seven days culture time in a two liter scale 5 grams of IgG1 type monoclonal antibody was produced. During that period the cell specific productivity was constant. Renewal of proteins was omitted because the protein content in the system persisted at a high level. Therefore, self-conditioning substances of the cells were retained in the system as well as the expensive medium components (proteins with catalytic or stimulating function). Seventy to 80% of medium costs and medium quantity were saved for each medium recycling step. Only cheap metabolites that are consumed by the cells had to be supplemented. Uptake rates of glucose and amino acids were calculated to establish a suitable supplementation mixture for the recirculated medium.
Similar content being viewed by others
References
BüntemeyerH, BödekerBGD and LehmannJ (1987) Membranestirrer-reactor for Bubble Free Aeration and Perfusion. In: Modern Approaches to Animal Cell Technology, SpierRE and GriffithsJB (eds), Butterworth, London, 411–419.
Büntemeyer H (1988) PhD Thesis, University of Hannover, FRG.
BüntemeyerH, BödekerBGD and LehmannJ (1990) Ein Zellkulturfermenter mit integriertem Membransystem für die homogene Kulturführung. Chem.-Ing.-Tech. 62, No. 5, 393–395.
BüntemeyerH, LütkemeyerD and LehmannJ (1991) Optimization of Serum-free Fermentation Processes for Antibody Production. Cytotechnology 5: 57–67.
ChmielH and StrathmannH (1985) Membranen in der Verfahrenstechnik. Chem.-Ing.-Tech. 57, No. 7, 581–596.
HasskarlH (1990a) Gentechnikrecht: Textsammlung (Gentechnikgesetz und Rechtsverordnungen). Editio Cantor Verlag, Aulendorf.
Hasskarl H (1990b) ditto, p. 110, p. 148, p. 154.
Kempken R, Büntemeyer H and Lehmann J (1990a) Cell Culture Medium Detoxification by Electrodialysis. Poster No. 11 at 12th July, 5th European Congress on Biotechnology, Copenhagen, 9th–13th July 1990.
Kempken R, Büntemeyer H and Lehmann J (1990b) The Medium Cycle Fermentor: a New Approach for Medium Recycling and Reduction of Fermentation Process Costs. Proceedings of the German-Japanese Workshop on Animal Cell Culture Technology, Hamburg-Harburg, 5th–6th November 1990, in press.
Kempken R, Büntemeyer H and Lehmann J (1991a) Medium-Kreislauf-Fermenter minimiert Kosten und Abwasser. Poster No. 161, 9. DECHEMA Jahrestagung der Biotechnologen, Ost-Berlin, 30.01.-01.02.
Kempken R, Lütkemeyer D, Heidemann R, Maurer U, Büntemeyer H and Lehmann J (1991b) Elimination of Ammonium and Lactate as Toxic Agents in Animal Cell Cultures. Poster at the 4th Tutzing Symposium on Chemical Engineering in Medicine and Biotechnology, Tutzing (FRG), 25th–28th February 1991.
Kempken R, Büntemeyer H and Lehmann J (1991c) Biological and Technological Aspects of Medium Recycling with the Medium Cycle Fermentor (MCF). Lecture at the 4th Tutzing Symposium on Chemical Engineering in Medicine and Biotechnology, Tutzing (FRG), 25th–28th February 1991.
LehmannJ, VorlopJ and BüntemeyerH (1988) Bubble-free Reactors and Their Development for Continuous Culture with Cell Recycle. In: Animal Cell Biotechnology SpierRE and GriffithsJB (eds), Academic Press, London, Vol. 3, 221–237.
Lehmann J, Kempken R, Lütkemeyer D and Büntemeyer H (1989) Economic Aspects of Medium Recycling. Trends in Animal Cell Culture Technology (Proceedings of the 2nd Annual Meeting of the JAACT, Tsukuba, Japan, 20th–22th November 1989), Murakami H (ed), VCH Publishers 1990, 55–59.
Lütkemeyer D, Heidemann R, Maurer U, Büntemeyer H and Lehmann J (1991) Mediumrezyklisierung mit Ammoniumentgiftung im 201 Perfusionsfermenter. Poster No. 68, 9. DECHEMA Jahrestagung der Biotechnologen, 0st-Berlin, 30.01-01.02.
MizrahiA and AvihooA (1977) Growth Medium Utilization and its Re-use for Animal Cell Cultures. Journal of Biological Standardization 5: 31–37.
NewlandM, GreenfieldPF and ReidS (1990) Hybridoma Growth Limitations: The Roles of Energy Metabolism and Ammonia Production. Cytotechnology 3: 215–229.
Pirt SJ (1985) Principles of Microbe and Cell Cultivation, Blackwell Scientific Publications pp. 209–222.
Rønning OW and Schartum M (1990) Removal of Inhibitory Factors from Hybridoma Cell Cultures by Gel Filtration. Production of Biologicals from Animal Cells in Culture (Proceedings of the 10th ESACT Meeting, Avignon, 7th–11th May 1990), Spier RE, Griffiths JB and Meignier B (eds), Butterworth-Heinemann Ltd. 1991, 218–223.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kempken, R., Büntemeyer, H. & Lehmann, J. The medium cycle bioreactor (MCB): Monoclonal antibody production in a new economic production system. Cytotechnology 7, 63–74 (1991). https://doi.org/10.1007/BF00350912
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00350912