Abstract
Objectives
The relationships of manipulation of culture temperature and medium circulation rate on the metabolic parameters were regressed by multiple linear regression analysis in hollow fiber bioreactors (HFB).
Results
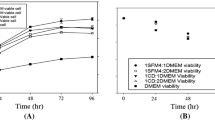
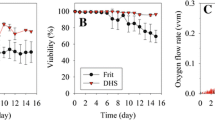
The high circulation rate could significantly enhance the oxygen consumption of the hybridoma cells and the medium’s oxidation–reduction potential. A mildly hypothermic condition of 36 °C and a circulation rate of 182.5 mL/min could support the hybridoma had the maximal antibody titer of 60.75 μg/mL for 20 days. When the ammonium ion was 65 ppm or lactate close to 2.6 g/L, the medium was replaced to maintain the stable and healthy cells at the high cell concentration of 3.33 × 108/mL for continuous antibody production. Two serum-free media could be successfully applied to this perfusion system and maintain hybridoma growth and antibody production.
Conclusion
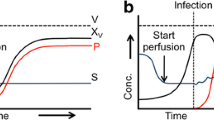
The single-use HFBs could provide the advantages including high cell density, low shear stress, and continuous antibody production.



Similar content being viewed by others
References
Becerra S, Berrios J, Osses N, Altamirano C (2012) Exploring the effect of mild hypothermia on CHO cell productivity. Biochem Eng J 60:1–8
Cadwell JJS (2004) New developments in hollow-fiber cell culture. Am Biotechnol Lab 22(8):14
Chen ZL, Wu BC, Liu H, Liu XM, Huang PT (2004) Temperature shift as a process optimization step for the production of pro-urokinase by a recombinant Chinese hamster ovary cell line in high-density perfusion culture. J Biosci Bioeng 97:239–243
Coronel J, Klausing S, Heinrich C, Noll T, Figueredo-Cardero A, Castilho LR (2016) Valeric acid supplementation combined to mild hypothermia increases productivity in CHO cell cultivations. Biochem Eng J 114:101–109
Croughan MS, Konstantinov KB, Cooney C (2015) The future of industrial bioprocessing: batch or continuous? Biotechnol Bioeng 112(4):648–651
De Napoli IE, Zanetti EM, Fragomeni G, Giuzio E, Audenino AL, Catapano G (2014) Transport modeling of convection-enhanced hollow fiber membrane bioreactors for therapeutic applications. J Membr Sci 471:347–361
Del Val IJ, Kontoravdi C, Nagy JM (2010) Towards the implementation of quality by design to the production of therapeutic monoclonal antibodies with desired glycosylation patterns. Biotechnol Prog 26(6):1505–1527
Diban N, Stamatialis D (2014) Polymeric hollow fiber membranes for bioartificial organs and tissue engineering applications. J Chem Technol Biotechnol 89(5):633–643
Donini M, Marusic C (2019) Current state-of-the-art in plant-based antibody production systems. Biotechnol Lett 41(3):335–346
Eghbali H, Nava MM, Mohebbi-Kalhori D, Raimondi MT (2016) Hollow fiber bioreactor technology for tissue engineering applications. Int J Artif Organs 39(1):1–15
Eisenstein M (2006) Thinking outside the dish. Nat Methods 3(12):1035–1043
Even MS, Sandusky CB, Barnard ND (2006) Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol 24(3):105–108
Fontova A, Lecina M, López-Repullo J, Martínez-Monge I, Comas P, Bragós R, Cairó JJ (2018) A simplified implementation of the stationary liquid mass balance method for on-line OUR monitoring in animal cell cultures. J Chem Technol Biotechnol 93(6):1757–1766
García Münzer DG, Ivarsson M, Usaku C, Habicher T, Soos M, Morbidelli M, Pistikopoulos EN, Mantalaris A (2015) An unstructured model of metabolic and temperature dependent cell cycle arrest in hybridoma batch and fed-batch cultures. Biochem Eng J 93:260–273
Grainger RK, James DC (2013) CHO cell line specific prediction and control of recombinant monoclonal antibody n-glycosylation. Biotechnol Bioeng 110(11):2970–2983
Gramer MJ, Maas J, Lieberman MM (2003) Use of hollow fiber systems for rapid and direct scale up of antibody production from hybridoma cell lines cultured in CL-1000 flasks using BD Cell MAb medium. Cytotechnology 42:155–162
Higareda AE, Possani LD, Ramírez OT (1997) The use of culture redox potential and oxygen uptake rate for assessing glucose and glutamine depletion in hybridoma cultures. Biotechnol Bioeng 56:555–563
Khaparde SS, Roychoudhury PK (2012) Effect of low culture temperature on urokinase production in hollow fiber reactor. Appl Biochem Biotechnol 168:1655–1663
Kishishita S, Nishikawa T, Shinoda Y, Nagashima H, Okamoto H, Takuma S, Aoyagi H (2015) Effect of temperature shift on levels of acidic charge variants in IgG monoclonal antibodies in Chinese hamster ovary cell culture. J Biosci Bioeng 119(6):700–705
Legazpi L, Díaz J, Laca A, Díaz M (2005) Kinetic analysis of hybridoma cell culture in a protein-free medium: substrate and agitation effects. Biochem Eng J 26(2–3):122–130
Li F, Vijayasankaran N, Shen A, Kiss R, Amanullah A (2010) Cell culture processes for monoclonal antibody production. mAbs 2(5):466–479
Liu H, Gaza-Bulseco G, Chumsae C, Newby-Kew A (2007) Characterization of lower molecular weight artifact bands of recombinant monoclonal IgG1 antibodies on non-reducing SDS-PAGE. Biotechnol Lett 29(11):1611–1622
Liu CG, Hao XM, Lin YH, Bai FW (2016) Redox potential driven aeration during very-high-gravity ethanol fermentation by using flocculating yeast. Sci Rep 6:25763
Liu CH, Liu YX, Wu WC (2018a) Facile development of medium optimization for antibody production: implementation in spinner flask and hollow fiber reactor. Cytotechnology 70:1631–1642
Liu M, Wang J, Tang H, Fan L, Zhao L, Wang HB, Zhou Y, Tan WS (2018b) Cell culture medium supplemented with taurine decreases basic charge variant levels of a monoclonal antibody. Biotechnol Lett 40(11–12):1487–1493
Mercille S, Massie B (1994) Induction of apoptosis in nutrient-deprived cultures of hybridoma and myeloma cells. Biotechnol Bioeng 44:1140–1152
Sen S, Roychoudhury PK (2013) Step-up/step-down perfusion approach for increased mAb 520C9 production by a hybridoma cell line. Biotechnol Lett 35(2):153–163
Sung KY, Sun OH, Gyun ML (2004) Enhancing effect of low culture temperature on specific antibody productivity of recombinant Chinese hamster ovary cells: clonal variation. Biotechnol Prog 20(6):1683–1688
Sunley K, Butler M (2010) Strategies for the enhancement of recombinant protein production from mammalian cells by growth arrest. Biotechnol Adv 28(3):385–394
Templeton N, Young JD (2018) Biochemical and metabolic engineering approaches to enhance production of therapeutic proteins in animal cell cultures. Biochem Eng J 136:40–50
Vermasvuori R, Hurme M (2011) Economic comparison of diagnostic antibody production in perfusion stirred tank and in hollow fiber bioreactor processes. Biotechnol Prog 27(6):1588–1598
von Stosch M, Davy S, Francois K, Galvanauskas V, Hamelink JM, Luebbert A, Mayer M, Oliveira R, O’Kennedy R, Rice P, Glassey J (2014) Hybrid modeling for quality by design and PAT-benefits and challenges of applications in biopharmaceutical industry. Biotechnol J 9(6):719–726
Yang ST, Luo J, Chen C (2004) A fibrous-bed bioreactor for continuous production of monoclonal antibody by hybridoma. Adv Biochem Eng Biotechnol 87:61–96
Yolmeh M, Jafari SM (2017) Applications of response surface methodology in the food industry processes. Food Bioprocess Technol 10(3):413–433
Yun JW, Lee SY, Choi BW, Oh HK, Kim SH, Byun TH, Park SY (2000) Continuous stable production of von Willebrand factor monoclonal antibody in spin filter bioreactor with bleeding technology. Biotechnol Bioprocess Eng 5(2):130–135
Zhang X, Sun YT, Tang H, Fan L, Hu D, Liu J, Liu X, Tan WS (2015) Culture temperature modulates monoclonal antibody charge variation distribution in Chinese hamster ovary cell cultures. Biotechnol Lett 37(11):2151–2157
Acknowledgements
We express gratitude to Ministry of Science and Technology (MOST 106-2221-E-182-050, 108-2221-E-182-039), Chang Gung University (BMRP 758) and Chang Gung Memorial Hospital (CMRPD2G0282, 2H0071, 2H0072) for funding and supporting this research. We thank the valuable suggestions for bioreactor operation from Frank R. H. Wang, at United BioPharma, Hsinchu, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that no conflicting financial interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, CH., Liu, YX., Kumari, M. et al. Multivariate analysis of metabolic parameters and optimization of antibody production using high cell density hybridoma in hollow fiber bioreactors. Biotechnol Lett 41, 963–977 (2019). https://doi.org/10.1007/s10529-019-02712-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02712-3