Abstract
Purpose of Review
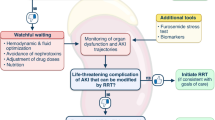
Aside from absolute indications, the optimal timing of renal replacement therapy (RRT) in critical care is unknown. In this review, we discuss initiation of RRT in relation to both severity of acute kidney injury (AKI) and fluid accumulation.
Recent Findings
Results from studies of early vs. late RRT are conflicting, and no definitive conclusions have been made. Observational data points to fluid accumulation as a detrimental factor in critical illness and recent studies have shown that early fluid removal with RRT is feasible and could potentially improve survival.
Summary
There is a gap in the knowledge regarding when to initiate RRT in the absence of acute life-threatening complications. Recent studies of fluid accumulation in critically ill patients indicate the importance of avoiding fluid overload, and RRT might play an increasing role in the management of fluid balance in critical care.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–23. https://doi.org/10.1007/s00134-015-3934-7.
Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1. https://doi.org/10.1038/kisup.2012.1.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. https://doi.org/10.1186/cc2872.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. https://doi.org/10.1186/cc5713.
Beall D, Bywaters EG, Belsey RH, Miles JA. Crush injury with renal failure. Br Med J. 1941;1:432–4.
Nisula S, Kaukonen K-M, Vaara ST, Korhonen A-M, Poukkanen M, Karlsson S, et al. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420–8. https://doi.org/10.1007/s00134-012-2796-5.
Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT. One-year mortality among Danish intensive care patients with acute kidney injury: a cohort study. Crit Care. 2012;16:R124. https://doi.org/10.1186/cc11420.
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–8. https://doi.org/10.1681/ASN.2014070724.
Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med. 2008;36:S198–203. https://doi.org/10.1097/CCM.0b013e318168ccd5.
Levey AS, Stevens LA, Frcp C, Schmid CH, Zhang YL, Iii AFC, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Levey AS, Inker LA. Assessment of glomerular filtration rate in health and disease: a state of the art review. Clin Pharmacol Ther. 2017;102:405–19. https://doi.org/10.1002/cpt.729.
Stevens LA, Coresh J, Greene T, Levey a S. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83.
KDIGO. KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2012;2013:3.
Jensen J-US, Hein L, Lundgren B, Bestle MH, Mohr T, Andersen MH, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2:e000635. https://doi.org/10.1136/bmjopen-2011-000635.
Itenov TS, Berthelsen RE, Jensen J-U, Gerds TA, Pedersen LM, Strange D, et al. Predicting recovery from acute kidney injury in critically ill patients: development and validation of a prediction model. Crit Care Resusc. 2018;20:54–60.
Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care. 2011;1:13. https://doi.org/10.1186/2110-5820-1-13.
Griffin BR, Gist KM, Faubel S. Current status of novel biomarkers for the diagnosis of acute kidney injury: a historical perspective. J Intensive Care Med. 2019;17:088506661882453. https://doi.org/10.1177/0885066618824531.
Fayad AI, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev. 2016;10:CD010613. https://doi.org/10.1002/14651858.CD010613.pub2.
Rabindranath KS, Adams J, MacLeod AM, Muirhead N. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev 2007:CD003773. doi:https://doi.org/10.1002/14651858.CD003773.pub3.
Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RLC. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20:122. https://doi.org/10.1186/s13054-016-1291-8.
•• Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–9. https://doi.org/10.1001/jama.2016.5828Recent, large randomized clinical trial.
• Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Verney C, Pons B, et al. Timing of renal support and outcome of septic shock and acute respiratory distress syndrome. A post hoc analysis of the AKIKI randomized clinical trial. Am J Respir Crit Care Med. 2018;198:58–66. https://doi.org/10.1164/rccm.201706-1255OCFollow-up study on recent large randomized clinical trial.
•• Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379:1431–42. https://doi.org/10.1056/NEJMoa1803213. Recent, large randomized clinical trial.
•• Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–33. https://doi.org/10.1007/BF01123134Recent, large randomized clinical trial.
Bagshaw SM, Lamontagne F, Joannidis M, Wald R. When to start renal replacement therapy in critically ill patients with acute kidney injury: comment on AKIKI and ELAIN. Crit Care. 2016;20:245. https://doi.org/10.1186/s13054-016-1424-0.
Mavrakanas TA, Aurian-Blajeni DE, Charytan DM. Early versus late initiation of renal replacement therapy in patients with acute kidney injury: a meta-analysis of randomised clinical trials. Swiss Med Wkly. 2017;147:w14507. https://doi.org/10.4414/smw.2017.14507.
Xu Y, Gao J, Zheng X, Zhong B, Na Y, Wei J. Timing of initiation of renal replacement therapy for acute kidney injury: a systematic review and meta-analysis of randomized-controlled trials. Clin Exp Nephrol. 2017;21:552–62. https://doi.org/10.1007/s10157-016-1316-2.
Yang X, Tu G, Zheng J, Shen B, Ma G, Hao G, et al. A comparison of early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. BMC Nephrol. 2017;18:264. https://doi.org/10.1186/s12882-017-0667-6.
Moreira FT, Palomba H, Chaves RC de F, Bouman C, Schultz MJ, Serpa Neto A. Early versus delayed initiation of renal replacement therapy for acute kidney injury: an updated systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Rev Bras Ter Intensiva. 2018;30:376–84. https://doi.org/10.5935/0103-507X.20180054.
Wang X, Jie Yuan W. Timing of initiation of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Ren Fail. 2012;34:396–402. https://doi.org/10.3109/0886022X.2011.647371.
Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury—a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol. 2017;18:78. https://doi.org/10.1186/s12882-017-0486-9.
• Meersch M, Küllmar M, Schmidt C, Gerss J, Weinhage T, Margraf A, et al. Long-term clinical outcomes after early initiation of RRT in critically ill patients with AKI. J Am Soc Nephrol. 2018;29:1011–9. https://doi.org/10.1681/ASN.2017060694Follow-up study on recent large randomized clinical trial.
Butcher B, Liu K. Fluid overload in AKI-epiphenomenon or putative effect on mortality? Curr Opin Crit Care. 2012;18:593–8. https://doi.org/10.1097/MCC.0b013e32835a1c44.Fluid.
Combes A, Bréchot N, Amour J, Cozic N, Lebreton G, Guidon C, et al. Early high-volume hemofiltration versus standard care for post-cardiac surgery shock. The HEROICS study. Am J Respir Crit Care Med. 2015;192:1179–90. https://doi.org/10.1164/rccm.201503-0516OC.
Zhang L, Chen Z, Diao Y, Yang Y, Fu P. Associations of fluid overload with mortality and kidney recovery in patients with acute kidney injury: a systematic review and meta-analysis. J Crit Care. 2015;30:860.e7–860.e13. https://doi.org/10.1016/j.jcrc.2015.03.025.
Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, et al. An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med. 2012;40:1753–60. https://doi.org/10.1097/CCM.0b013e318246b9c6.
Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–7. https://doi.org/10.1038/ki.2009.159.
Fülöp T, Pathak MB, Schmidt DW, Lengvárszky Z, Juncos JP, Lebrun CJ, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J. 2010;56:333–7. https://doi.org/10.1097/MAT.0b013e3181de35e4.
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD, National Heart, Lung and BIARDSN. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–73. https://doi.org/10.2215/CJN.08781010.
Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO. Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant. 2012;27:956–61. https://doi.org/10.1093/ndt/gfr470.
Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. https://doi.org/10.1186/cc6916.
Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40:1256–66. https://doi.org/10.1007/s00134-014-3360-2.
Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, et al. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care. 2014;18:624. https://doi.org/10.1186/s13054-014-0624-8.
Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14. https://doi.org/10.1186/cc12484.
Vaara ST, Korhonen A-M, Kaukonen K-M, Nisula S, Inkinen O, Hoppu S, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16:R197. https://doi.org/10.1186/cc11682.
Neyra JA, Li X, Canepa-Escaro F, Adams-Huet B, Toto RD, Yee J, et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med. 2016;44:1891–900. https://doi.org/10.1097/CCM.0000000000001835.
Berthelsen RE, Perner A, Jensen AK, Jensen J-U, Bestle MH. Fluid accumulation during acute kidney injury in the intensive care unit. Acta Anaesthesiol Scand. 2018;62:780–90. https://doi.org/10.1111/aas.13105.
Balakumar V, Murugan R, Sileanu FE, Palevsky P, Clermont G, Kellum JA. Both positive and negative fluid balance may be associated with reduced long-term survival in the critically ill. Crit Care Med. 2017;45:e749–57. https://doi.org/10.1097/CCM.0000000000002372.
Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. https://doi.org/10.1097/SLA.0b013e318256be72.
Cruces P, Salas C, Lillo P, Salomon T, Lillo F, Hurtado DE. The renal compartment: a hydraulic view. Intensive Care Med Exp. 2014;2:26. https://doi.org/10.1186/s40635-014-0026-x.
Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet (London, England). 1988;1:1033–5. https://doi.org/10.1016/S0140-6736(88)91851-X.
Malbrain MLNG, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anestezjol Intens Ter. 2014;46:361–80. https://doi.org/10.5603/AIT.2014.0060.
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43:155–70. https://doi.org/10.1007/s00134-016-4573-3.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettilä V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–705. https://doi.org/10.1007/s00134-016-4500-7.
Rosner MH, Ostermann M, Murugan R, Prowle JR, Ronco C, J a K, et al. Indications and management of mechanical fluid removal in critical illness. Br J Anaesth. 2014;113:764–71. https://doi.org/10.1093/bja/aeu297.
• Berthelsen RE, Perner A, Jensen AK, Rasmussen BS, Jensen JU, Wiis J, et al. Forced fluid removal in intensive care patients with acute kidney injury: the randomised FFAKI feasibility trial. Acta Anaesthesiol Scand. 2018;62:936–44. https://doi.org/10.1111/aas.13124. Study of goal directed fluid removal in AKI—a possible indication for RRT.
Murugan R, Balakumar V, Kerti SJ, Priyanka P, Chang C-CH, Clermont G, et al. Net ultrafiltration intensity and mortality in critically ill patients with fluid overload. Crit Care. 2018;22:223. https://doi.org/10.1186/s13054-018-2163-1.
Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207. https://doi.org/10.1186/cc13015.
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–51. https://doi.org/10.1056/NEJMra1208627.
ADQI XII Investigators Group. ADQI 12 Figures. Acute Dial Qual Initiat 12 2013. http://www.adqi.org (accessed January 7, 2019).
Hoste EA, Maitland K, Brudney CS, Mehta R, Vincent J-L, Yates D, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113:740–7. https://doi.org/10.1093/bja/aeu300.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Morten H. Bestle, Theis Skovsgaard Itenov, and Rasmus E. Berthelsen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Critical Care Anesthesia
Rights and permissions
About this article
Cite this article
Bestle, M.H., Itenov, T.S. & Berthelsen, R.E. Renal Replacement Therapy in Critical Care: When to Start?. Curr Anesthesiol Rep 9, 135–143 (2019). https://doi.org/10.1007/s40140-019-00325-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-019-00325-0