Abstract
Background
Acute kidney injury (AKI) is a common complication in the critically ill patients and associated with a substantial morbidity and mortality. Severe AKI may be associated with up to 60% hospital mortality. Over the years, renal replacement therapy (RRT) has emerged as the mainstay of the treatment for AKI. However, the exact timing of initiation of RRT for better patient outcome is still debatable with conflicting data from randomized controlled trials. Thus, a systematic review and meta-analysis was performed to assess the impact of “early” versus “late” initiation of RRT.
Methods
All the published literature through the major databases including Medline/Pubmed, Embase, and Google Scholar were searched from 1970 to October 2016. Reference lists from the articles were reviewed to identify additional pertinent articles. Retrieved papers concerning the effect of “early/prophylactic” RRT versus “late/as and when required” RRT were reviewed by the authors, and the data were extracted using a standardized data collection tool. Randomized trials (RCTs) comparing early initiation of RRT or prophylactic RRT with late or as and when required RRT were included. The primary outcome measures were all cause mortality and dialysis dependence on day 90. The secondary outcome measures were: length of ICU stay, length of hospital stay, recovery of renal function and adverse events.
Results
Of the 547 citation retrieved, full text of 44 articles was assessed for eligibility. Of these a total of 10 RCTs with 1,636 participants were included. All the trials were open label; six trials have unclear or high risk of bias for allocation concealment while four trials have low risk of bias for allocation concealment. There was a variable definition of early versus late in different studies. Thus, the definition of early or late was taken according to individual study definition. Compared to late RRT, there was no significant benefit of early RRT on day 30 mortality [6 studies; 1301 participants; RR, 0.92;95% CI: 0.76, 1.12); day 60 mortality [3 trials;1075 participants; RR, 0.94; 95% CI: 0.78, 1.14)]; day 90 mortality [3 trials; 555 participants; RR,0.94;95% CI: 0.67, 1.33)]; overall ICU or hospital mortality; dialysis dependence on day 90 [3 trials; (RR, 1.06; 95% CI:0.53, 2.12)]. There was no significant difference between length of ICU or hospital stay or recovery of renal functions. A subgroup analysis based on modality of RRT or mixed medical and surgical vs. surgical or based on severity of illness showed no difference in outcome measure. The trials with high or unclear risk of bias for allocation concealment showed benefit of early RRT (RR, 0.74; 95% CI: 0.59, 0.91) while the trials with low risk of bias for allocation concealment showed no difference in the mortality (RR, 1.02; 95% CI: 0.89, 1.17). Grade evidence generated for most of the outcomes was “low quality”.
Conclusion
This updated meta-analysis showed no added benefit of early initiation of RRT for patients with AKI. The grade evidence generated was of “low quality” and there was a high heterogeneity in the included trials.
PROSPERO registration number
Similar content being viewed by others
Background
Acute kidney injury (AKI) is a common complication in the critically ill patients and associated with a substantial morbidity and mortality [1,2,3]. Severe AKI may be associated with up to 60% hospital mortality [4]. Over the years, renal replacement therapy (RRT) has emerged as the mainstay of the treatment for AKI. Intermittent hemodialysis (IHD), peritoneal dialysis (PD) and continues renal replacement therapy (CRRT) are various modalities to conduct RRT. Early initiation of RRT helps in the removal of uremic toxins, allow fluid and electrolyte balance and prevent life threatening complications such as metabolic encephalopathy, hyperkalemia, pulmonary oedema [5].
The timing of initiation of RRT for better patient outcome is still debatable with conflicting data from randomized controlled trials [6,7,8,9]. Two meta-analysis concluded that early RRT improves survival in critically ill patients [10, 11] . However, a recent meta-analysis [12] concluded that “early” initiation of RRT in critical illness complicated by AKI does not improve patient survival or confer reductions in intensive care unit (ICU) or hospital length of stay (LOS). This meta-analysis included both RCTs, and cohort studies. Moreover, after publication of this meta-analysis, two large studies have been published. We conducted an updated systematic review including RCTs and Quasi-RCTs (no observational studies) to support or refute the earlier evidence on the initiation of early versus late RRT. We have also performed a robust subgroup and sensitivity analysis as well as graded the quality of evidence and strength of recommendations by using GRADE approach which is lacking in previous systematic reviews and metanalysis.
Objective
To evaluate the impact of “early” versus “late” initiation of RRT.
Methods
The review has been registered at the PROSPERO register: CRD42016043092
Type of studies
Randomized controlled trials and quasi-randomized trials (RCTs) were included.
Participants
Hospitalized patients with AKI were included. Patients with preexisting chronic kidney disease (estimated glomerular filtration rate [GFR] <30 mL/min) on long term dialysis, previous renal replacement therapy, AKI resulting from vascular malformations (occlusion of the renal artery), glomerulonephritis, interstitial nephritis, vasculitis, post-renal obstruction, hemolytic uremic syndrome (HUS) or thrombotic thrombocytopenic purpura, post renal transplant AKI and confirmed or suspected pregnancy, malignancy and HIV were excluded.
Interventions
The interventions consist of administration of early/prophylactic or as and when required/late RRT in patients with AKI. The definition of early and late RRT was taken as described in the individual study.
Types of outcome measures
Primary outcomes
-
1.
Mortality rate
-
2.
Dialysis Dependence at 3 month
Secondary outcomes
-
1.
Length of ICU stay
-
2.
Length of hospital stay
-
3.
Recovery of renal function
-
4.
Adverse events
Search methods for identification of studies
Cochrane Central Register of Controlled Trials (CENTRAL), PubMed/MEDLINE, Google Scholar, Cochrane renal group were searched from 1970 to October 2016. Following search strategy was applied: (((((((((renal replacement therapy) OR Renal Dialysis) OR dialysis) OR Hemodialysis) OR Hemodiafiltration) OR Hemofiltration)) AND (((((acute kidney injury) OR Acute Renal Injury) OR Acute Renal Insufficiency) OR Acute Renal Failure) OR Acute Kidney Failure)) AND (((((((timing) OR time) OR Initiation) OR start) OR early) OR Earlier) OR Late)) AND ((randomized controlled trial) OR Controlled Clinical Trial). To identify unpublished trial results, we searched the US National Institutes of Health, Department of Health and Human Services trials registry (http://www.clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform (ICTRP) trial registry.
Data extraction
Data was extracted using a pilot tested data extraction form. Two authors independently extract data including author, type of participants, exposure and intervention (modality of RRT, timing), results (clinical outcomes and adverse events).
Risk of bias (quality) assessment
Two review authors (GC and RD) independently assessed the methodological quality of the selected trials by using Cochrane risk of bias tool [13].
Strategy for data synthesis
The data from various studies was pooled and expressed as mean difference (MD) with 95% confidence interval (CI) in case of continuous data, and risk ratio (RR) with 95% CI in case of categorical data. P-value <0.05 was considered significant. Heterogeneity was assessed by I-squared statistics. In case of high level heterogeneity (>50%), we tried to explore the cause. A fixed effects model was initially conducted. If, significant heterogeneity was found between trials, potential sources of heterogeneity were considered and where appropriate, a random effects model was used. RevMan (Review Manager) version 5.3 was used for all the analyses.
Subgroup analysis
We performed the following subgroup analysis:
-
1.
Surgical versus mixed medical admission.
-
2.
Modality of RRT
-
3.
Severity of illness
-
4.
Risk of bias for allocation concealment
Publication bias
This was looked by construction of the inverted funnel plot as suggest by Egger et al.[14].
Grade of evidence
For assessment of the quality of evidence we used GRADE Profiler software (version 3.2). The software uses five parameters for rating the quality of evidence. The parameters used were - limitations to design of randomized controlled trials, inconsistency of results or unexplained heterogeneity, indirectness of evidence, imprecision of results, and publication bias. The rating was done as – no, serious, and very serious limitation.
Results
Description of the studies
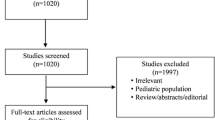
Of the 547 citation retrieved, full text of 44 articles was assessed for eligibility (Fig. 1). Of these a total of 10 RCTs with 1,672 participants were included. Thirty three studies were excluded due to following reasons: Non RCT/review articles (n = 18), comparing different modalities of RRT (n = 09), comparing different doses/drugs during RRT (n = 05); Ongoing studies (n = 02). All the trials were open label with most of the trials having unclear or high risk of bias for allocation concealment. There was a variable definition of early versus late in different studies. Thus, the definition of early or late was taken according to individual study definition. A summary of the studies is provided in Table 1.
Primary outcome measure
Overall Mortality: Ten studies with 1672 participants reported 662 deaths. Compared with the patients assigned to late RRT, patients assigned to early RRT had 7% reduction in mortality rate. However, pooled results showed no significant difference between the two groups (RR, 0.93;95% CI: 0.75, 1.15) (Fig. 2). Since there was a significant heterogeneity (I2 = 50%;p = 0.17), we tried to explore the heterogeneity based on pre-specified subgroups analysis such as: Surgical versus mixed patients, severity of illness, modality of RRT and risk of bias for allocation concealment. We also performed a period wise mortality analysis to address the heterogeneity in the included trials.
Day 30 mortality: This was reported in 6 trials [5,8,, 7–9, 15, 16] with 1301 participants. The pooled results showed 8% decrease in mortality with early initiation of RRT. However, there was no significant difference between the early and late RRT (RR, 0.92;95% CI: 0.68, 1.06], (Additional file 1: Figure S1 a)
Day 60 mortality: This was reported in 3 trials [8, 9, 15] with 1075 participants. The pooled results showed no significant difference between the two strategies (RR, 0.94; 95% CI:0.78, 1.14) (Additional file 1: Figure S1a).
Day 90 mortality: This was reported in three trials [9, 15, 17] with 555 participants. The pooled results showed no significant difference between the two strategies (RR, 0.94; 95% CI:0.67, 1.33) (Additional file 1: Figure S1a) .
Overall ICU mortality: Overall ICU mortality was reported in 3 trials [7, 15, 17]. Pooled mortality showed no significant reduction in ICU mortality with initiation of early RRT (RR, 1.08; 95% CI:0.84, 1.39) (Additional file 1: Figure S1a).
Overall hospital mortality: This was reported in 6 trials and the pooled results showed no significant difference between the mortality rates between the two groups (RR, 1.07; 95% CI: 0.81, 1.42) (Additional file 1: Figure S1a).
Dialysis Dependence at Day 90: 3 trials [6, 9, 17] reported dialysis dependence at 90 days in the two groups. Pooled data showed no significant difference between the two groups (RR, 1.06 95% CI: 0.53, 2.12) (Additional file 1: Figure S1c)
Subgroup based on Surgical versus mixed patients
Overall 30 day mortality: 2 trials reported this outcome [15, 16]. Overall there was no significant difference in overall 30 day mortality (RR,0.51;95% CI: 0.09, 3.08.) (Additional file 1: Figure S1b)
Overall 60 day mortality: Only 1 trial [15] reported this outcome. Overall there was no significant difference between the two groups (RR, 1.14;95% CI: 0.83, 1.58)
Overall 90 day mortality: 1 trial [15] reported this outcome without any significant difference between the two strategies.
Overall ICU: There was no significant difference between ICU (RR,1.11;95% CI:0.82, 1.52) or hospital mortality (RR,1.01;95% CI:0.74, 1.36) in the surgical patients undergoing early versus late initiation of RRT.
Subgroup analysis based on severity of illness
There was no significant difference in overall day 30 mortality (5 trials, 1257 patients;RR,0.91;95% CI:0.73, 1.15);day 60 mortality (3 trials, 1075 participants; RR, 0.90; 95% CI:0.64, 1.27); day 90 mortality (3 trial, 555 participants, RR, 0.90; 95% CI:0.49, 1.64), hospital mortality (RR, 1.12; 95% CI: 0.76, 1.65) and ICU mortality (RR, 1.12;95% CI:0.75, 1.68) in critically ill undergoing early RRT as compared to late RRT.
Subgroup analysis based on modality of RRT
There was no significant difference in overall day 30 mortality in the patients undergoing continuous renal replacement therapy (CRRT) (3 trials, 413 participants; RR, 0.90;95% CI:0.65, 1.26); patients undergoing intermittent hemodialysis (IHD) (1 trial,44 participants; RR, 0.16;95% CI:0.02, 1.17); patients undergoing either CRRT or IHD (1 trial,620 participants; RR, 0.95;95% CI:0.79, 1.14): day 60 mortality in patients undergoing CRRT (2 trials, 455 participants; RR, 0.93;95% CI:0.62, 1.38) or patients undergoing either CRRT or IHD (1 trial,620 participants; RR, 0.97;95% CI: 0.82, 1.14): day 90 mortality in patients undergoing CRRT (2 trials, 455 participants; RR, 0.92;95% CI:[0.56, 1.50) or patients undergoing either CRRT or IHD (1 trial,100 participants; RR, 1.03;95% CI:0.62, 1.71): Overall hospital mortality in the patients undergoing CRRT (2 trials, 330 participants; RR, 1.14;95% CI:0.88, 1.48) or IHD (1 trial,44 participants; RR, 0.16;95% CI:0.02, 1.17) and overall ICU mortality in the patients undergoing CRRT (2 trial, 330 participants; RR, 1.13;95% CI: 0.85, 1.49) or patients undergoing either CRRT or IHD (1 trial, 100 participants; RR, 0.88;95% CI: 0.47, 1.63) (Fig. 3)
Subgroup analysis based on risk of bias for allocation concealment
Six trials have low risk of bias for allocation concealment [6, 8, 9, 15, 17] while 4 [5, 7, 16, 18, 19] have unclear or high risk of bias for allocation concealment. There was a significant reduction in overall mortality in the patients assigned to early RRT in the studies with high or unclear risk of bias (RR, 0.74; 95% CI:0.59, 0.91) as compared to those with low risk of bias for allocation concealment (RR, 1.02;95% CI:0.89, 1.17) (Fig. 4)
Secondary Outcomes
Length of ICU stay: Six studies reported this outcome [8,16,, 9, 15–17]. Out of these, 5 trials reported this outcome as median (interquartile range) [7,8,9, 15, 17] and found no significant difference between ICU stay in the two groups. Another trial [16] reported a significant reduction in ICU stay in the patients undergoing early RRT as compared to late RRT (MD,-45.87;95% CI:-75.54,–16.20).
Length of Hospital stay: Seven trials reported this outcome [8,16,, 9, 15–17] and 5 reported them as median (Interquartile range). In 4 trials there was no significant difference in the length of hospital stay between the two groups while 1 trial has shown significant difference between hospital stay in patients receiving early RRT. Two trials [7, 18] have given this outcome as mean (SD) and were pooled. The pooled results no difference in the length of hospital stay (MD,-3.62; 95% CI :-8.91, 16.16).
Recovery of renal function by day 90: 2 trials reported recovery of renal functions on day 90 [9, 15]. Pooled data showed no significant difference between two groups (RR, 1.04;95% CI:0.80, 1.35) (Additional file 1: Figure S1d).
Adverse events
Bleeding: 7 trials with 1520 participants reported this outcome [6,7,8,9, 15, 17, 18]. On pooling the data no significant difference in the adverse event was observed between the two groups (RR, 0.92;95% CI:0.67, 1.25) (Fig. 5).
Catheter related complications: Four trials reported this outcome [6, 9, 15, 17]. The pooled results showed no significant difference between the two groups (RR, 1.41: 95% CI: 0.59, 3.37) with point estimate favouring late strategy (Fig. 5).
Thrombocytopenia: 3 trials reported this outcome [8, 9, 17, 18] and the pooled results showed no significant difference between the two strategies (RR,1.20:95% CI:0.87, 1.65) (Fig. 5).
Arrhythmias: 4 trials reported this outcome [8, 9, 17, 18] and the pooled results showed no significant difference between the two groups (RR, 0.91;95% CI: 0.70, 1.19) (Fig. 5).
Hypotension: 4 trials reported this outcome [6, 9, 15, 17] reported this outcome. Pooled results showed no significant difference with point estimate favouring late RRT (RR, 1.18; 95% CI: 1.00, 1.38) (Fig. 5).
Electrolyte abnormalities: There was no significant difference between the two strategies with respect to hypokalemia (RR, 1.02;95% CI: 0.51, 2.03), hyperkalemia (RR, 0.80;95% CI: 0.45, 1.41) and hypocalcaemia (RR, 0.89;95% CI:0.51, 1.54). Hypophosphatemia was seen more in patients undergoing early dialysis (RR, 1.51; 95% CI: 1.05, 2.18) (Fig. 5).
Publication bias
To assess whether there was a bias in the published literature, funnel plot was constructed using the MD and 1/SE values obtained from trials measuring one of the primary outcome (overall mortality). In the absence of a publication bias, such a plot is expected to have a shape resembling an inverted funnel. From the funnel plot generated, the possibility of publication bias in the analysis is less (Fig. 6).
Grade of evidence
The evidence generated was of “low quality” for all the primary outcomes (GRADE Table 2).
Discussion
Summary of Evidence
After an extensive search of literature we could find 10 trials to be eligible for inclusion. Our results indicates that in patients with AKI there is no benefit of early initiation of renal replacement therapy on overall mortality, dialysis dependence on day 90, length of ICU or hospital stay and renal recovery on day 90. There was no significant difference in the adverse events between early and late group except for hypophosphatemia which was seen more common in the patients undergoing early RRT. The grade evidence generated was low grade for most of the outcomes.
Studies exploring the initiation strategies for RRT have shown conflicting results. Early initiation of RRT, theoretically may allow for better control of fluid and electrolyte status, fasten removal of uremic toxins and prevent complications like gastric hemorrhage and metabolic encephalopathy [20]. On the other hand a delayed strategy of RRT initiation may give sufficient time for spontaneous patient recovery and may avoid the need for RRT, thus minimizing risk associated with RRT [9].
Two recent trials [8, 9] have also shown conflicting results regarding timing of initiation of RRT. Zarbock et al. [9] (ELAIN trial) reported a significant reduction of mortality over 90 days in critically ill patients with AKI undergoing RRT while in the study by Gaudry et al. [8] (AKIKI trial) the authors found no significant reduction in mortality in patients assigned to early RRT as compared to late RRT. This difference may be due to different patient’s characteristics such as inclusion of more ill patients in the ELAIN trial as compared to that in AKIKI trial (SOFA 16 versus SOFA 11) [21]. Another difference was the use of RRT modality in the two studies. In the AKIKI trial 55% of the patients received intermittent hemodialysis as RRT modality while all the patients received CRRT in ELAIN trial. However, we have done a subgroup analysis based on the modality of RRT, severity of illness and type of patients and found no difference in mortality rates among the two groups. A recent systematic review has shown a benefit of early RRT on reduction of all cause mortality [22]. However, greater heterogeneity in the studies and a combined analysis of both RCTs and non- RCTs together may have overestimated the effect. Further, on subgroup analysis based on the type of studies (RCTs versus non RCTs), authors found no statistically significant decrease in the mortality rate in RCT group.
On subgroup analysis based on risk of bias for allocation concealment we found a significant reduction in mortality (26%) in the patients assigned to early RRT. Previous studies have also shown that treatment inadequate allocation concealment may exaggerate treatment effect by 40% and unclear allocation concealment may exaggerate treatment effect by 30%.
The strength of present systematic review is:1) we have included both randomized and quasi-randomized controlled trials to strengthen the present evidence2) we have done sensitivity analysis by excluding trials with unclear and high risk of bias for allocation concealment3) we also assigned GRADE evidence to further grade the quality of evidence and recommendations.
Conclusion
This updated meta-analysis showed no added benefit of early initiation of RRT for patients with AKI with respect to all cause mortality, dialysis dependence, and recovery of renal functions or hospital stay. The grade evidence generated was of “low quality” and there was high heterogeneity in the included trials. We need more good quality RCTs in different patient subgroups including children to further strengthen the evidence.
Abbreviations
- AKI:
-
Acute Kidney Injury
- CRRT:
-
Continues renal replacement therapy
- IHD:
-
Intermittent hemodialysis
- RCTs:
-
Randomized controlled trials
- RRT:
-
Renal replacement therapy
References
Hoste EAJ, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73.
Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–709.
Ahlström A, Tallgren M, Peltonen S, Räsänen P, Pettilä V. Survival and quality of life of patients requiring acute renal replacement therapy. Intensive Care Med. 2005;31:1222–8.
Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–8.
Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803–10.
Jamale TE, Hase NK, Kulkarni M, et al. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2013;62:1116–21.
Bouman CSC, Oudemans-Van Straaten HM, Tijssen JGP, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–11.
Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122–33.
Zarbock A, Gerß J, Van Aken H, Boanta A, Kellum JA, Meersch M. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (the ELAIN-trial): study protocol for a randomized controlled trial. Trials. 2016;17:148.
Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52:272–84.
Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72.
Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RLC. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20:122.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysisdetected by a simple, graphical test. BMJ. 1997;315:629–34.
Combes A, Bréchot N, Amour J, et al. Early high-volume hemofiltration versus standard care for post-cardiac surgery shock. The HEROICS study. Am J Respir Crit Care Med. 2015;192:1179–90.
Durmaz I, Yagdi T, Calkavur T, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75:859–64.
Wald R, Adhikari NKJ, Smith OM, et al. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88:897–904.
Pursnani ML, Hazra DK, Singh B, Pandey DN. Early haemodialysis in acute tubular necrosis. J Assoc Physicians India. 1997;45:850–2.
Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodialysis international. International Symposium on Home Hemodialysis. 2004;8:320–5.
Gibney N, Hoste E, Burdmann EA, et al. Timing of initiation and discontinuation of renal replacement therapy in AKI: unanswered key questions. Clin J Am Soc Nephrol. 2008;3:876–80.
Meersch M, Zarbock A. Timing of renal replacement therapy in acute kidney injury-an issue of importance? J Thoracic Dis. 2016;8:2301–4.
Wang C, Lv LS, Huang S, et al. Initiation time of renal replacement therapy on patients with acute kidney injury: a systematic review and meta-analysis of 8179 participants. Nephrology (Carlton). 2017;22:7–18.
Acknowledgements
The authors would like to thank Mrs. Seema, librarian AIIMS Bhopal and Mrs Neelima Chaddha, librarian PGI Chandigarh for providing full text of articles. Our sincere thanks to administration of AIIMS Bhopal and AIIMS Bhubaneswar for providing us necessary infrastructure to conduct this meta-analysis.
Funding
No funding was obtained for this study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author’s Contribution
GC designed the research; GC and RR wrote the paper; GC and RR performed the research; GC and RR analyzed the data; all authors read and approved the final manuscript.
Competing interest
The authors declare that they have no competing interests
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable as this is a systematic review.
Declarations
The abstract of this meta-analysis was presented in the Indian Society of Pediatric Nephrology conference 2016.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Figure S1.
a: Forest plot showing period -wise mortality. b: Forest plot showing subgroup; Mixed Vs surgical patients. c: Forest plot showing dialysis dependence on day 90. d: Forest plot showing recovery of renal functions by day 90. (PDF 309 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bhatt, G.C., Das, R.R. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol 18, 78 (2017). https://doi.org/10.1186/s12882-017-0486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-017-0486-9