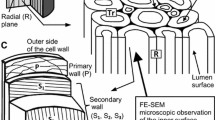
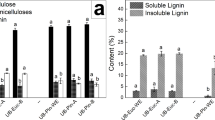
Abstract
Dilute acid hydrolysis (DAH) and auto-hydrolysis (AU) have demonstrated to be optimal pre-treatments for the generation of biofuels from wood. Recent studies have highlighted the importance of ensuring the accessibility of cellulose enzymes during the enzymatic hydrolysis (EH) of pre-treated materials. In this work, the microscopic and nanoscopic structures of Eucalyptus globulus samples pre-treated by AU and DAH were evaluated by different techniques to understand the effect of the ultrastructure of samples on the enzymatic conversion and cellulose accessibility for bioethanol production. Microscopic techniques revealed changes in the physical characteristics of pre-treated fibers, coalescence at microscopic level, and differences in the chemical distribution of lignocellulosic components depending on the severity and type of pre-treatment. The atomic force microscopy-based nanoscopic study of samples showed differences in the effect of the pre-treatments on the ultrastructure of samples, with DAH pre-treatment producing major changes in the secondary cell wall with respect to AU samples at comparable severities, and a positive effect of the DAH ultrastructure changes to increase the EH yield.







Similar content being viewed by others
References
Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, Frederick WJ, Hallett JP, Leak DJ, Liotta CL, Mielenz JR, Murphy R, Templer R, Tschaplinski T (2006) The path forward for biofuels and biomaterials. Science 311(5760):484–489
Emmel A, Mathias AL, Wypych F, Ramos LP (2003) Fractionation of Eucalyptus grandis chips by dilute acid-catalysed steam explosion. Bioresour Technol 86(2):105–115
Castillo RDP, Baeza J, Rubilar J, Rivera A, Freer J (2012) Infrared spectroscopy as alternative to wet chemical analysis to characterize Eucalyptus globulus pulps and predict their ethanol yield for a simultaneous saccharification and fermentation process. Appl Biochem Biotechnol 168(7):2028–2042
Jeoh T, Santa-Maria MC, O’Dell PJ (2013) Assessing cellulose microfibrillar structure changes due to cellulase action. Carbohyd Polym 97(2):581–586
Chandra R, Ewanick S, Hsieh C, Saddler JN (2008) The characterization of pretreated lignocellulosic substrates prior to enzymatic hydrolysis, part 1: a modified Simons’ staining technique. Biotechnol Prog 24(5):1178–1185
Meng X, Ragauskas AJ (2014) Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr Opin Biotech 27:150–158
Ju X, Engelhard M, Zhang X (2013) An advanced understanding of the specific effects of xylan and surface lignin contents on enzymatic hydrolysis of lignocellulosic biomass. Bioresour Technol 132:137–145
Kumar R, Hu F, Sannigrahi P, Jung S, Ragauskas AJ, Wyman CE (2013) Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng 110(3):737–753
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13):4851–4861
Foston M, Ragauskas AJ (2012) Biomass characterization: recent progress in understanding biomass recalcitrance. Ind Biotechnol 8(4):191–208
Geleynse S, Alvarez-Vasco C, Garcia K et al (2014) A multi-level analysis approach to measuring variations in biomass recalcitrance of Douglas fir tree samples. Bioenerg Res 7:1411. doi:10.1007/s12155-014-9483-z
Jeoh T, Ishizawa CI, Davis MF, Himmel ME, Adney WS, Johnson DK (2007) Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol Bioeng 98(1):112–122
Rollin JA, Zhu Z, Sathitsuksanoh N, Zhang YHP (2011) Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol Bioeng 108(1):22–30
Wang QQ, He Z, Zhu Z, Zhang YHP, Ni Y, Luo XL, Zhu JY (2012) Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol Bioeng 109(2):381–389
Felby C, Thygesen LG, Kristensen JB, Jørgensen H, Elder T (2008) Cellulose–water interactions during enzymatic hydrolysis as studied by time domain NMR. Cellulose 15(5):703–710
Li T-Q, Häggkvist M, Ödberg L (1997) Porous structure of cellulose fibers studied by Q-space NMR imaging. Langmuir 13(13):3570–3574
Chen Y, Wang Y, Wan J, Ma Y (2010) Crystal and pore structure of wheat straw cellulose fiber during recycling. Cellulose 17(2):329–338
Esteghlalian AR, Bilodeau M, Mansfield SD, Saddler JN (2001) Do enzymatic hydrolyzability and Simons’ stain reflect the changes in the accessibility of lignocellulosic substrates to cellulase enzymes? Biotechnol Prog 17(6):1049–1054
Hong J, Ye X, Zhang YHP (2007) Quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications. Langmuir 23(25):12535–12540
Tetard L, Passian A, Farahi RH, Kalluri UC, Davison BH, Thundat T (2010) Spectroscopy and atomic force microscopy of biomass. Ultramicroscopy 110(6):701–707
Lupoi JS, Singh S, Simmons BA et al (2014) Assessment of lignocellulosic biomass using analytical spectroscopy: an evolution to high-throughput techniques. Bioenerg Res 7:1. doi:10.1007/s12155-013-9352-1
Donaldson L (2007) Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci Technol 41(5):443
Hanley SJ, Giasson J, Revol J-F, Gray DG (1992) Atomic force microscopy of cellulose microfibrils: comparison with transmission electron microscopy. Polymer 33(21):4639–4642
Simmons B, Dibble D, Singh S, Auer M, Jorgens D, Faulon JL (2008) Utilization of atomic force microscopy, confocal microscopy, and electron microscopy to evaluate biomass pretreatment. Microsc Microanal 14(S2):1492–1493
Österberg M, Schmidt U, Jääskeläinen A (2006) Combining confocal Raman spectroscopy and atomic force microscopy to study wood extractives on cellulose surfaces. Colloids Surface A 291(1–3):197–201
Medeiros RG, Silva LP, Azevedo RB, Silva FG Jr, Filho EXF (2007) The use of atomic force microscopy as a tool to study the effect of a xylanase from Humicola grisea var. thermoidea in kraft pulp bleaching. Enzym Microb Technol 40:723–731
Kristensen JB, Thygesen LG, Felby C, Jorgensen H, Elder T (2008) Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol Biofuels 1
Shahin, V., Barrera, N.P. (2008). Chapter six—providing unique insight into cell biology via atomic force microscopy. In: International review of cytology (Ed.) W.J. Kwang, Vol. 265, Academic Press, pp. 227–252
Frybort S, Obersriebnig M, Müller U, Gindl-Altmutter W, Konnerth J (2014) Variability in surface polarity of wood by means of AFM adhesion force mapping. Colloids Surface A 457:82–87
Meincken M, Evans PD (2009) Nanoscale characterization of wood photodegradation using atomic force microscopy. Eur J Wood Wood Prod 67(2):229–231
Obersriebnig M, Konnerth J, Gindl-Altmutter W (2013) Evaluating fundamental position-dependent differences in wood cell wall adhesion using nanoindentation. Int J Adhes Adhes 40:129–134
Zhang M, Chen G, Kumar R, Xu B (2013) Mapping out the structural changes of natural and pretreated plant cell wall surfaces by atomic force microscopy single molecular recognition imaging. Biotechnol Biofuels 6(1):147
Pietak A, Korte S, Tan E, Downard A, Staiger MP (2007) Atomic force microscopy characterization of the surface wettability of natural fibres. Appl Surf Sci 253(7):3627–3635
Li C, Sun L, Simmons BA et al (2013) Comparing the recalcitrance of Eucalyptus, pine, and switchgrass using ionic liquid and dilute acid pretreatments. Bioenerg Res 6:14. doi:10.1007/s12155-012-9220-4
Marzialetti T, Salazar JP, Ocampos C, Chandra R, Chung P, Saddler J, Parra C (2014) Second-generation ethanol in Chile: optimisation of the autohydrolysis of Eucalyptus globulus. Biomass Conver Bioref 4(2):125–135
Amenaghawon AN, Okieimen CO, Ogbeide CO (2014) Modeling and statistical optimization of dilute acid hydrolysis of Eucalyptus wood chips using response surface methodology. Pacific J Sci Technol 15(1):245–256
Romaní A, Garrote G, Alonso JL, Parajó JC (2010) Experimental assessment on the enzymatic hydrolysis of hydrothermally pretreated Eucalyptus globulus wood. Ind Eng Chem Res 49(10):4653–4663
Castillo RDP, Araya J, Troncoso E, Vinet S, Freer J (2015) Fourier transform infrared imaging and microscopy studies of Pinus radiata pulps regarding the simultaneous saccharification and fermentation process. Anal Chim Acta 866:10–20
Popescu C-M, Popescu M-C, Singurel G, Vasile C, Argyropoulos DS, Willfor S (2007) Spectral characterization of Eucalyptus wood. Appl Spectrosc 61(11):1168–1177
Garcia R, Magerle R, Perez R (2007) Nanoscale compositional mapping with gentle forces. Nat Mater 6(6):405–411
Wiedenhoeft A.C. Structure and function of wood. In: Handbook of wood chemistry and wood composites. Ed. By Roger M. Rowell. 2013.CRC Press. Boca Raton. Pg. 19 (pp. 686).
Araya F, Troncoso E, Mendonça RT, Freer J (2015) Condensed lignin structures and re-localization achieved at high severities in autohydrolysis of Eucalyptus globulus wood and their relationship with cellulose accessibility. Biotechnol Bioeng 112(9):1783–1791
Valenzuela R, Priebe X, Troncoso E, Ortega I, Parra C, Freer J (2016) Fiber modifications by organosolv catalyzed with H2SO4 improves the SSF of Pinus radiata. Ind Crop Prod 86:79–86
Acknowledgements
The authors thank CONICYT: Fondecyt 11130388, ICM P10-035F, and PIA-CONICYT ECM-12 project for funding this project. J. Araya thanks CONICYT-PCHA/Doctorado Nacional/2013-2113064 scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arévalo, C., Freer, J., Naulin, P.A. et al. Study of the Ultrastructure of Eucalyptus globulus Wood Substrates Subjected to Auto-Hydrolysis and Diluted Acid Hydrolysis Pre-treatments and Its Influence on Enzymatic Hydrolysis. Bioenerg. Res. 10, 714–727 (2017). https://doi.org/10.1007/s12155-017-9833-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-017-9833-8