Abstract
Background
The heavy use of conventional mineral fertilization considerably increased wheat growth and yield components. However, the excessive nitrogen fertilization accounts for large expenses on farmers’ budget and has negative environmental drawbacks to soil and water ecosystems. Recently, establishing wheat plants associations with a variety of N2-fixing cyanobacteria and/or growth promoting microorganisms in farming systems as nutrients source is seen as ecofriendly and economically feasible solution.
Results
In this work, the influence of different nitrogen (N) levels (100% N, 75% N, and 50% N as urea) and cyanobacteria (Cy) and/or yeast (Y) inoculation each alone or both in combination with different nitrogen levels were assessed on two different wheat (Triticumae stivum L.) genotypes (Sids-14 and Giza-171) through field experiments over two consecutive seasons (2019/2020 and 2020/2021). Although, the full recommended dose (FRD) (100% N) showed the highest chlorophyll (Chl) a, b content during tillering stage, grains quality (NPK-uptake, protein%), and wheat yield components in both genotypes. The integrated inoculation of cyanobacteria and yeast with 50% N, 75% N-fertilization enhanced wheat growth characteristic (Chl a,b) and upgraded soil microbial community (nitrogenase activity and CO2 evolution) in Sids-14 and Giza-171. Cy combined with Y and partial N-fertilization levels enhanced NPK-uptake Kg/fed and protein% in wheat grains in the two wheat genotypes. Moreover, this combination recorded a wheat yield components (plant height, number of spikes/m2, number of grains/spike, 1000 grains wt (gm) and grains yield (tonne/fed) insignificantly different from those obtained by FRD in wheat cultivation.
Conclusion
The integrated application of cyanobacteria with yeast and 50% N or 75% N-fertilization improved soil fertility index and promoted NPK- uptake, protein% and wheat yield components, showing comparable values to conventional chemical fertilization. Cyanobacteria combined with yeast inoculation had socio-economic benefits as it can save about 25–50% of the required mineral nitrogen fertilizers for wheat crop production.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.1 Background
Wheat accounts for 33.5% of total world food grain production and is a significant source of carbohydrate and protein in the human diet, wheat straw also is one of the major sources of animal fodder. The recent report of USDA in 2021 indicated that the estimated wheat production in Egypt reached 9.0 million metric tonnes in 2021/2022 from an average area 1.4 million hectare [1]. However, population growth resulted in a progressive local consumption of wheat in food and industrial use. Therefore, government seeks to increase production area through introducing new varieties with high grain yields, optimizing fertilization's practices and planting methods that can significantly increase the wheat production per unit area [2].
In modern agriculture, wheat production has been traditionally improved by using specific ratios of mineral fertilizers such as nitrogen and phosphorus [3]. However, the frequent application of chemical fertilizers imposes multiple ecological risks through denitrification, volatilization and leaching of these agrochemicals into soil and water webs. Moreover, the extensive use of mineral N as NH4+ (ammonium) induces toxic effect to plant by decreasing ATP production and disrupts the development of many important plants [4]. Thus, finding viable alternatives as nutrient supplements is a serious demand [5, 6]
Recently, cyanobacteria-based biofertilizers are gaining much concern as a viable alternative to conventional chemical fertilizers. The remarkable returns of biofertlizers application include enhanced crop yields per unit area in a relatively short time, low energy consumption and minimum level of soil and water contamination. Besides increasing soil fertility and biological control of some soil borne plant pathogens through stimulated antagonism [7]. Among myriads of nitrogen (N2)-fixing microorganisms, cyanobacteria are one of the most efficient N2-fixers in a diverse agricultural soils [8]. The free-living cyanobacteria can fix 10–30 kg of N/ha/year besides the added organic matter to soil rhizosphere. Thereby, they are considered as a key component of naturally available biofertilizers [9].
Cyanobacteria have been demonstrated to produce a wide array of secondary metabolites such asplant growth regulators (PGR) (cytokinin and indole-3-acetic acid) [10], polysaccharides, vitamins [11, 12], amino acids [13, 14], and antimicrobial agents [15]. These extracellular substances significantly contribute in plant growth improvement and subsequent yield. Cyanobacteria induce plant growth by increasing availability of macronutrient elements such as nitrogen (N), phosphorus (P) and potassium (K) as well as essential elements to plant or through upgrading levels of plant hormones [16, 17].
Nostoc is a widespread heterocystous filamentous cyanobacterial genus has great potential of N2 fixation under variable ecological and agricultural conditions [9]. Nostoc can establish N2-fixing symbiotic association with broad range of host plants such as liverworts and hornworts [18], or form a free-living (non-symbiotic) association. Creation of artificial association between Nostoc strains and rice plant revealed a positive chemotaxis of Nostoc toward plant roots [19]. Nostoc muscorum and Nostoc commune were also found to produce a wide range of beneficial and bioactive substances to plant growth [9, 14]. Consequently, many trials have been made to form intact associations between the free-living N2-fixing cyanobacteria and wheat plant [20,21,22].
The yeast species Saccharomyces cerevisiae is an ecofriendly and natural biological stimulator for plant growth. Previous reports elucidated the high efficiency of yeast extract to increase plant growth, yield components and grains quality of different crops such faba bean [23], rice [24], sugar beet [25] and maize [26]. As a result, the development of biofertilizers using a combination of two or more microorganisms such as cyanobacteria-bacteria or bacteria-bacteria has been found to enhance wheat height, dry weight, and grains yield [16, 17, 21, 27]. However using cyanobacteria-yeast association with different N-levels is still uncovered. Therefore, this study aims to (1) explore the effect of cyanobacteria and yeast inoculation, either each applied alone or both in combination with different N-levels on wheat growth, grains quality and wheat yield components. (2) to reduce the environmental risk induced by excessive mineral nitrogen used in wheat cultivation. (3) to explore the effect of both cyanobacteria and yeast inoculation on soil fertility status through measuring nitogenase activity as N2-fixation index, and CO2 evolution as an indicator of soil microbial activity.
2 Methods
2.1 Experimental design and field preparation
The field experiments were conducted at Sids Agricultural Research Station, Agricultural Research Center, Beni-Suef Governorate, Egypt (Latitude: 29º 04 N, Longitude: 31º05 E) during two consecutive seasons (2019/2020 and 2020/2021). The initial soil analyses of the experimental area during two seasons indicted slightly alkaline clay soil, pH (7.96 and 7.83) with low organic matter (1.6 and 1.8%), low salinity (1.02 and 1.15 dS/m), and available nitrogen contents (22.1, 16.6 µg/g soil) [28].
To study the effect of both cyanobacteria (Cy) and yeast (Y), Saccharomyces cerevisiae inoculation each applied alone or both in combination with the mineral fertilization on two different genotypes of wheat plant (Triticumae stivum cv. Sids 14and Giza 171). The experimental field was prepared by plowing and puddling, and then divided into 60 plots (2 m × 1 m each) to represent 10 different treatments for each wheat genotype in three replicates. The experiment plots were laid out in randomized complete blocks design. All agronomic practices were kept uniformly according to the Crop Field Research Institute, Agricultural Research Center (Fig. 1). Urea (46.5% N) was used as mineral nitrogen fertilizer, and was applied according to the requirement of each treatment. Where the full recommended nitrogen dose (100% N) is (180 kg/ fed N), 75% N (135 kg/ fed) and 50% N (90 kg/fed). The biological amendments, Cy, Y, and their combination with mineral fertilization (75% N, 50% N) are represented by different seven treatments for each genotype. Super phosphate (15.5% P2O5) as phosphorus source (30 kg/fed) and potassium phosphate (48% K2O) as source of potassium (50 kg/fed) were uniformly applied to all plots.
2.2 Preparation of the microbialinoculants
Cyanobacteria inoculation (Cy) consists of a microalgal mixed culture of Nostoc spongiaeforme EMCCN 3074, Nostoc mucorum EMCCN 3075 and Nostoc commune EMCCN 3076, and yeast inoculation (Y) of Saccharomyces cerevisiae were used as biological amendments. Both cyanobacterial and yeast inoculation were applied twice as soil drench at the rate of 119 L/ha (50 L/fed) after 30 and 55 days of sowing wheat seeds. The cyanobacteria strains were isolated from Egyptian agricultural lands and were identified using molecular tools [29]. The microalgal strains were deposited in the Egyptian Microbial Culture Collection Network (EMCCN) at Agricultural Research Centre, Giza, Egypt. The cyanobacterial strains were maintained and propagated in liquid BG110 medium [30] until the stationary phase, and the microalgal mixed culture was done by mixing equal volumes from each species. The commercial baking yeast Saccharomyces cerevisiae was propagated in standard yeast medium (10 g/L yeast extract, 20 g/L peptone, 200 g/L D-glucose), and was incubated for 48 h at 30 °C to ensure the required cell viable count (109 cfu/ml).
2.3 Soil microbial activity
2.3.1 CO2 evolution
The soil microbial activity represented by CO2 evolution was evaluated in rhizosphere of wheat soil after 65 days of plant growth using the method of Pramer and Schmidt [31]. In 500 ml serum bottles tightly closed with rubber stoppers, ten grams from soil samples were fitted into a cylindrical polyethylene bag and hanged over a mixture of 100 ml, 0.05 N NaOH and 3 ml 50% ml BaCl2, and incubated at 30 oC for 3 days. The amount of CO2 in mg/100 g soil was deduced by titrating the residual NaOH with 0.05 N HCl (1 ml HCl = 1 mg CO2). A control bottle without soil sample was used as blank.
2.3.2 Nitrogenase activity
The activity of nitrogenase enzyme, as indicator of free living N2-fixation potential in rhizosphere of wheat soil (µmole C2H4/g dry soil /h) was determined according the method described by Dilworth [32]. Briefly, 15 gm from each soil sample was homogenized with 2 ml, 10% glucose to activate soil microbes [33]. Soil samples were placed separately in 100 ml serum bottles stoppered with tight rubber silicon caps and were incubated at 30 °C for 24 h. 10% (v/v) of head space gas was replaced by equal volume of acetylene gas (C2H2) by aid of sharp needle syringes. The injected bottles were re-incubated for another 4 h. One ml from the head space gas was analyzed to determine the content of developed ethylene gas (C2H4) by gas chromatography (Hewlett Packard, HP 5890 series 2 plus) fitted with dual flame detector and capillary colum1 (cross linked Al2O3) 50 m × 0.53 cm × 15.0 µm film thickness. The applied temperature of injector, column and detector was 170 oC, 120 oC and 170 °C, respectively. Flow gas rate was 10 ml/min. Ethylene–air mixture was used for generation of standard ethylene curve. Results of N-ase activity were calculated as ethylene produced/gdry soil/ hr.
2.4 Plant analyses
2.4.1 Pigments content
At the vegetative growth stage, after 65 days of growth, random samples of three plants from each plot were collected. The pigment contents (chlorophyll a,b and carotenoids) were determined according to Lichtenthaler [34]. 50 mg from middle leaf tissue was soaked in 10 ml acetone 80% (v/v), samples were frozen for 48 h in darkness. The pigment extract was separated by centrifugation for 10 min at 3000 rpm, chlorophyll (Chl) a,b and carotenoids contents (mg/g fresh wt) were determined by measuring absorbance at 663 nm and 647 nm and 470 nm using the following equations.
2.4.2 Determination of nitrogen, phosphorus and potassium (NPK) uptake
At harvest stage, wheat plants were cut just above the soil surface, plants were oven dried at 70 °C up to a constant dry weight. Percentages of NPK in wheat grains were determined using the Kjeldahl method as described by Jackson [35]. 0.5 g of grinded wheat grains were digested by a mixture of concentrated sulfuric acid and perchloric acid (1:1, v/v) under 400 °C in the electrical digestor (2020 Tecator analytical company). Samples were then cooled down and diluted by distilled water to a know volume up to 100 ml. The digested solutions were used for the determination of NPK% in grains which were then used in calculating the N, Pand K-uptake (Kg/fed) according to Yoshida [36]. Nitrogen content was measured by transferring 10 ml of the digested samples to the distillation tubewith 20 ml of 40% NaOH in distilling unit (Kjeltec system 1002, Tecator). The steam of distilled ammonia was trapped in 100-ml flask containing 10 ml Boric acid (pH 5.0). The absorbed ammonia content was determined by titration against N/70 HCl. Phosphorus was determined by measuring the absorbance of the formed complex of ammonium phosphomolybdate with SnCl2 at 640 nm[37] using spectrophotometer (Spectronic 21D), KH2PO4 was used to generate a standard phosphorus curve. Potassium was measured by the flame photometer as described by Jackson [35] using Jenway flame photometer (PfP7, UK). Protein content was determined by using nitrogen-to-protein conversion factors (NPCF, 5.7) according to Jones [38]. A blank-free sample was done for every analysis, and all measurements were conducted in 3 independent replicates.
2.5 Wheat yield components
Plant samples in 1.0 m2 were randomly selected from each plotat harvest stage to determine plant height (cm), number of spikes/m2, number of grains/spike, weight of 1000 grains wt (gm) and grains yield (tonne/fed).
2.6 Statistical analyses
The obtained data were statistically analyzed using analysis of variance procedure according to Gomez and Gomez [39]. Means were compared using Gen-Stat software at 5% level of probability.
3 Results
3.1 Effects of biofertilizers on N2-fixation efficiency and soil microbial activity
The interactive effects of cyanobacteria and yeast either each inoculated alone or both in combination with different levels of mineral nitrogen fertilization on soil microbial activity (e.g., nitrogenase activity and CO2 evolution) are shown in Table (1). Compared to FRD (100% N), the biological amendment with cyanobacteria significantly increased nitrogenase activity (N-ase) and CO2 evolution in soil rhizosphere of the two wheat genotypes. For instance, N50 + Cy and N75 + Cy significantly increased (P ≤ 0.05) N-ase activity in Sids-14 by 576% and 688%, and in Giza-171 by 1508% and 1427%, respectively, during 1st season. Similarly, N-ase activity was found to considerably increase in Sids-14 (196% and 202%) and in Giza-171 (100% and 93.2%) during 2nd season. Furthermore, the integrated biofertilization using N50 + Y + Cy and N75 + Y + Cy showed the highest increase in N-ase activity in Sids-14 (710%, 1258%) and Giza-171(5047%, 3345%) at the 1st season, and also at 2nd season in Sids-14 (270%, 279%) and Giza-171(141%,124%). N50 + Y + Cy and N75 + Y + Cy significantly increased CO2 evolution in soil rhizosphere of Sids-14 (58%, 56%) and Giza-171(151%, 80%) at the 1st season, and in Sids-14 (71%, 74%) and Giza-171(85%,72%) at the 2nd season. Although cyanobacteria amendment increased the soil microbial activity, however, the integrated application of cyanobacteria with yeast had the highest inductive effects on N2-fixation process and the soil microbial activity of the two wheat genotypes through field experiments.
3.2 Effects of biofertilizer on wheat growth
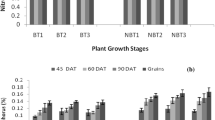
Nitrogen is a structural element of chlorophyll molecules and affects its accumulation in the plant leaves. Our results indicated that the Chl a,b contents were progressively decreased with reduction in applied mineral nitrogen levels (N 100˃N75˃N50%). The FRD resulted in high pigmentation patterns in Sids-14 and Giza-171 genotypes over the two seasons. Meanwhile, integrated biofertilization (N50 + Y + Cy and N75 + Y + Cy) significantly increased Chl a, b contents in two wheat genotypes when compared with individual Cy or Y treatments. Integrated biofertilization had statistical insignificant results (P ≤ 0.05) compared to FRD, and sometimes Chl a, b values surpassed those obtained by 100% N (e.g., Chl a of Sids-14 during 2nd season, Chl b of Sids-14 during 1st and 2nd and Chl b of Giza-171 at 2nd season). On contrast, the individual biological treatments such as Cy, N50 + Cy, N75 + Cy, N50 + Yand N75 + Y marginally decreased Chl a contents in Sids-14 by 6.3–31.3% at 1st and 2nd season. Similar results were also found in Giza-171 where Cy, N50 + Cy, N75 + Cy, N50 + Y and N75 + Y decreased Chl a contents by 6.3–43.8 at 1st and by 6.25 at 2nd season. At the level of genotype, slight fluctuations were observed in Chl a, b contents of each wheat strain during 1st and 2nd seasons, and both showed comparable pigmentation patterns in response to biofertilization (Table 2).
3.3 Influence of biofertilizer on wheat grain quality
NPK-uptake by wheat grains implies quality of wheat grains. As anticipated, FRD resulted in the highest NPK-uptake (Kg/fed) in the two genotypes over the 1st and 2nd seasons. Results indicated that the NPK-uptake was significantly decreased with decreasing of applied mineral nitrogen levels (N 100%˃ N 75%˃N 50%). Our results also indicated that the treatment with Cy as a sole biofertilizers or yeast with partial mineral nitrogen fertilization (e.g., N50 + Y, N75 + Y) induced remarkable decreases in NPK-uptake of wheat grain in both genotypes. However, the integrated biofertilization of cyanobacteria and yeast with partial mineral nitrogen fertilization showed similar and insignificant values of NPK-uptake obtained by 100% N (Table 3). Among seven biological amendments, N75 + Y + Cy recorded the highest averages of NPK-uptake of wheat grains in Sids-14 (52.8, 7.7 and 15.9 kg/fed) and in Giza-171 (63.9, 11.3 and18.9 kg/fed) during 1st season. The efficiency of N75 + Y + Cy was also confirmed by results of 2nd season where NPK-uptake was 53.1, 7.6 and 15.1 kg/fed in Sids-14 and 64.1, 7.7 and 17.6 kg/fed in Giza-171.
Protein content of wheat grains supported the results of photosynthetic pigments, and indicated a significant positive relationship between protein content and N-fertilization. The highest protein content in wheat grains was detected at 100% N as well as under treatments of integrated biofertilization. Giza-171 showed the highest protein content (10.2%, 10.5%) at the 1st season and (10.0%, 10.3%) at the 2nd season under N50 + Y + Cy and N75 + Y + Cy treatments, respectively.
3.4 Influence of biofertilizers on wheat yield components
The interactive effect of inoculation with cyanobacteria (Cy), yeast (Y) and mineral fertilization on wheat yield components is shown in Tables 4 and 5. Results indicated that crop growth indices during stage of vegetative growth (e.g., heading, plant height and number of spikes/m2 were N-level dependent. Reducing mineral nitrogen fertilization by 75% N significantly decreased plant height, and this reduction was more pronounced at 50% N in Sids-14 (14.5%, 6.4%) and in Giza-171 (13.1%, 11.2%) at 1st and 2nd season. On the other hand, treatment with Cy as a sole biofertilizers or N50 + Y remarkably decreased plant height in Sids-14 (12%, 11%) at 1st season and (10.3%, 7.3%) at 2nd season, and in Giza-171 (9.3%, 14%) and (3.7%, 6.5%) at 1st and 2nd seasons, respectively, when compared with FRD. Contrarily, the integrated biofertilization represented by N50 + Y + Cy and N75 + Y + Cy showed comparable results of plant heights with that of 100% N which, attained the highest plant height.
Results of number of spikes/m2 interpreted the level of soil nitrogen content, and showed similar pattern to previous results. Where 75% N significantly decreased number of spikes/m2 and this reduction was further strengthen by 50% N in Sids-14 (26.8%, 31.2%) and in Giza-171 (20.2%, 14.2%) during 1st and 2nd seasons.
Treatment with Cy as a sole biofertilizers or N50 + Cy and N50 + Y was also not enough for wheat growth and significantly decreased number of spikes/m2 in Sids-14 (23%, 14.5% and 16.2%) in the 1st season and (30%, 16% and 23%) in the 2nd season. Giza-171 exhibited also a comparable reduction patterns of spikes/m2 (15.8%, 6.4% and 13%) at 1st season and (10.2%, 0.7% and 6.1%) at 2nd season. On contrast, the integrated inoculation of N50 + Y + Cy and N75 + Y + Cy significantly enhanced the number of spikes/m2 compared to individual treatments of Cy or Y alone, and showed equivalent results to those of 100% N particularly at 2nd season for both genotypes.
At the harvest stage, results of wheat yield index represented by number of grains/spike, weight of 1000 grains and grains yield (tonne/ fed) were consistent with the photosynthesis and growth indices results, and reflected the positive relationship between wheat yield components and N-fertilization levels (Table 5). Treatment with 100% N recorded the highest number of grains/spike, weight of 1000 grains and the greatest wheat yields for both Sids-14 and Giza-171 over two seasons. However, 75% N treatment decreased number of grains/spike, weight of 1000 grains and wheat yields, this reduction was more pronounced under 50% N level in Sids-14 (38%, 3.8% and 18.6%) at the 1st season and (21.3%, 10.1% and 23.3%) at the 2nd season. Giza-171 also showed a similar reduction patterns (28.7%, 16.7% and 24.9%) at 1st season and (21.2%, 17.4% and 31.5%) at 2nd season. At the level of genotype, Giza-171 was more vulnerable to N-level deficiency and showed higher reduction percentages of number of grains/spike, weight of 1000 grains and wheat yields (tonne/fed) when compared to Sids-14.
On the other hand, treatment with Cy as a sole biofertilizer significantly decreased number of grains/spike, weight of 1000 grains and wheat yield in Sids-14 (29%, 1.8% and 25.7%) during the 1st season and (18.7%, 10.7% and 31.2%) in the 2nd season, and also in Giza-171 (29.9%, 12.2% and 30.1%) at 1st season and (27.1%, 12.3% and 23.5%) at 2nd season. Similarly, N50 + Cy and N50 + Y did not fulfill nitrogen requirement needed for wheat growth and resulted in significant decreases in number of grains/spike, weight of 1000 grains and wheat yields in the two wheat genotypes. The integrated biofertilization of N50 + Y + Cy and N75 + Y + Cy significantly enhanced number of grains/spike, weight of 1000 grains and wheat yields compared to individual treatments of Cy or Y alone. Moreover, both genotypes treated with N50 + Y + Cy and N75 + Y + Cy showed wheat yields (tonne/fed) similar to those of 100% N over two seasons.
At scale of genotype, Sids-14 treated with N50 + Y + Cy showed significant yield components (number of grains/spike, weight of 1000 grains and wheat yields) compared to Giza-171 over 1st and 2nd season.
4 Discussion
4.1 Integrated biofertilization increased N2-fixation efficiency and soil microbial activity
The remarkable increases in N-ase activity and CO2 evolution after treatment with cyanobacteria indicated their effective role in N2-fixation process. Which can be further strengthen through using a mixed treatments of cyanobacteria with yeast due to existence of growth promoting producer microorganisms in farming systems (Table 1). A similar finding has been reported by Ghazal et al. [16] where the integrated application of cyanobacteria with Rhizobium radiobacter significantly increased soil microbial community with Azotobacter spp. and Azospirillum spp. and increased number of N2-fixing cyanobacteria which led to a consecutive increasing in CO2 evolution and dehyderogenase activity. Cyanobacteria and yeasts have been elucidated to produce phytohormones and extracellular bioactive substances such as enzymes, polysaccharides, phenolic compounds and organic acids which help in improving soil microbial activity and soil health [40, 41]. In the present study, we observed that increasing the nitrogen levels to 100% N significantly decreased N-ase activity and CO2 evolution we attributed that to suppressing the number of free-living nitrogen fixing microorganisms in soil. Application of urea-N significantly decreased nitrogenase activity of the N2-fixing cyanobacterium Stenotrophomonas maltophila with a dose-dependent manner, and completely inhibited at 300 mg/L [42].
4.2 Integrated biofertilization increased wheat growth and improved grains quality
Cyanobacteria association with yeast and partial N-fertilization increased Chl a, b contents in both wheat genotypes (Table 2). Our result is in accordance with Abdel- Rahman et al. [23] and Agamy et al.[25] where, the stimulating effect of yeasts (Saccharomyces sp.) on Chl a and b is due to increasing photosynthetic efficiency. Cyanobacteria-wheat association significantly contributed in improving wheat growth characteristics and grain weight due to the high phytostimulation potential of cyanobacteria besides their high efficiency in N2-fixation [10]. In the present study, cyanobacteria association with yeast and partial N-fertilization (N50 + Y + Cy and N75 + Y + Cy) significantly contributed in increasing protein% and NPK-uptake in wheat grains of Sids-14 and Giza-171 (see Table 3).
Yeast-based biofertilization increased photosynthetic pigments, vegetative growth characteristics and induced protein, sugars, free amino acids and carbohydrate contents in faba bean, sugar beet and corn [23, 25, 43]. A yeast species Williopsis saturnus was found to endophytically produce two auxins molecules indole-3-acetic acid and indole-3-pyruvic acid when inoculated to Zea mays roots and led to significant increases in plant growth profile (e.g., dry weights, roots and shoots length)[26].
Thus, we assume that combination of N2-fixing cynanobacteria with yeast as biofertilizer could synergically increase availability of macronutrient and micronutrient elements beside their photosimulation potential to wheat growth. Yeast application has been proved to reduce the use of synthetic fertilizers by 20–30% [44, 45].
Our result also showed that application of the FRD (180 kg/fed) significantly induced wheat growth, Chl a, b contents in leaves and improved grains quality. This finding is supported by results of Rusek et al. [46] and Mussarat et al.[3]. However, from the economic point of view the excessive mineral fertilization is not economically feasible.
4.3 Integrated biofertilization increased wheat yield components
Results showed that integrated biofertilization significantly improved wheat yield components when compared with individual biofertilization (Tables 4, 5). Our results are in accordance with Ghazal et al.[16] where combination of cyanobacteria with R. radiobacter and different levels of nitrogen fertilization achieved a greater grain yield comparable to 100% N and induced the highest NPK-uptake in wheat plants either grains and straw. Generally, inoculation of N2-fixing cyanobacteria to cereals such as wheat and rice has been proven to induce N-availability in soil and consequently to plant leading to vigor seedlings with optimal yields at harvest stage [10, 16, 47]. Cyanobacteria-based biofertilization has been reported also to increased yields of fruits (tomato), legumes and cotton by 5–25% [21, 27, 48, 49]. Similarly, application of cyanobacteria with 50% N of mineral fertilizer significantly increased rice yield components (e.g., number and weight of grains/ spike and total weight of 100 grains [38]. Hussain and Hasnain [10] explained the high production yield of wheat plant inoculated with cyanobacterial species (Chroococcidiopsis sp. Ck4 and Anabaena sp. Ck1) to the endogenous phytohormones (cytokinin and indole-3-acetic acid) during the plant–cyanobacteria association in the rhizosphere.
On the other hand, yeast acts as biological stimulator to growth due to production of growth promoting substances, and has high ability to make active association with the non-leguminous crops such as rice, sugar beet and wheat [24, 25]. Foliar spray using yeast (Saccharomyces cerevisiae) increased rice yield components, improved seed quality and decreased seed-borne fungi [24]. Yeasts also have high potential to decompose complex organic matter residues releasing essential macronutrient elements (N, P and K) and increase their availability to plant [43, 44, 50]. Additionally, the cellular content of yeast from macronutrients and micronutrients, and vitamins was proposed to have stimulant effect on plant to build up dry matters [51, 52]
Previous reports demonstrated the positive effect of applied nitrogen levels on wheat yield components [2, 46]. Where, the maximum N-availability in the soil by 100% N resulted in the highest heading, plant height, number of spikes/m2, number of grains /spike, 1000 grains wt (gm) and grains yield (tonne/fed). Nevertheless, the integrated biofertilization showed similar values besides increasing soil fertility which suggests this technology for sustainable agricultural technology.
5 Conclusion
Saving of available nitrogen source to wheat plant is a substantial requirement for optimal crop yield with high grains quality. However, chemical fertilization imposes several environmental problems. Therefore, seeking for ecofriendly and cheap biotechnology-driven methods to supply wheat plants with nitrogen requirements is a reasonable solution. In the current study, application of integrated inoculation of cyanobacteria with yeasts along with partial mineral nitrogen fertilization (50% N, 75% N), over two independent field experiments, significantly increased soil microbial activity indices such as nitrogenase activity and CO2 evolution. Compared to individual amendments of cyanobacteria or yeast alone, integrated biofertilization significantly improved plant growth characteristics (Chl a,b), grains quality (NPK uptake and protein%), and increased wheat yield components (e.g., plant height, number of spikes/m2, number of grains/spike, 1000 grains wt (gm) and grains yield (tonne/fed)) in two wheat genotypes. Results of integrated biofertilization are comparable to those obtained by FRD with non-significant differences. Cyanobacteria-yeast combination with adjusted levels of mineral N-fertilizers could provide sustainable, cheap and safe solutions for modern agriculture technology, and may lead to sustainable economic development.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- PGR:
-
Plant growth promoting
- EMCCN:
-
Egyptian microbial culture collection network
- AOAC:
-
Association of official agricultural chemists
- FRD:
-
Full recommended dose
- NPCF:
-
Nitrogen-to-protein conversion factors
- Cy:
-
Cyanobacteria
- Y:
-
Yeast
- Cfu:
-
Colony forming unit
- N:
-
Mineral nitrogen fertilization
- N2 :
-
Fixation: nitrogen fixation
- N-ase:
-
Nitrogenase activity
- NPK- uptake:
-
Nitrogen, phosphorus and potassium uptake
- Chlrophyll a:
-
Chla
- Chlrophyll b:
-
Chlb
- Fed:
-
Feddan
- Hectare:
-
Ha
References
USDA (United States Department of Agriculture), Foreign agricultural services (2021). Egypt is able to secure a steady supply of grains during the COVID-19 pandemic, grain and feed annual, gain global agricultural information network, report number EG2021–0004
El-Sheref ES (2020) Effects of planting methods and nitrogen fertilization rates on the productivity of some wheat varieties. Fayoum J Agric Res Dev 34(2):97–111
Mussarat M, Shair M, Muhammad D, Mian IA, Khan S, Adnan M, Fahad S, Dessoky ES, Sabagh EL (2021) Accentuating the role of nitrogen to phosphorus ratio on the growth and yield of Wheat crop. Sustainability 13:2253. https://doi.org/10.3390/su13042253
Elhanafi L, Houhou M, Rais C, Mansouri I, Elghadraoui L, Greche H (2019) Impact of excessive nitrogen fertilization on the biochemical quality, phenolic compounds, and antioxidant power of sesamum indicum l seeds. J Food Qual 2019:1–6. https://doi.org/10.1155/2019/9428092
Choudhury ATMA, Kennedy IR (2005) Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun Soil Sci Plant Anal 36(11–12):1625–1639. https://doi.org/10.1081/CSS-200059104
Babu S, Prasanna R, Bidyarani N, Singh R (2015) Analysing the colonisation of inoculated cyanobacteria in wheat plants using biochemical and molecular tools. J Appl Phycol 27:327–338. https://doi.org/10.1007/s10811-014-0322-6
Win TT, Barone GD, Secundo F, Pengcheng F (2018) Algal biofertilizers and plant growth stimulants for sustainable agriculture. Indus Biotechnol 14(4):203–211. https://doi.org/10.1089/ind.2018.0010
Garlapati D, Chandrasekaran M, Devanesan A, Mathimani T, Pugazhendhi A (2019) Role of cyanobacteria in agricultural and industrial sectors: an outlook on economically important byproducts. Appl Microbiol Biotechnolo 103(12):4709–4721. https://doi.org/10.1007/s00253-019-09811-1
Pathak J, Rajneesh PK, Maurya SP, Singh DP, Sinha RP (2018) Cyanobacterial farming for environment friendly sustainable agriculture practices: Innovations and perspectives. Front Environ Sci. https://doi.org/10.3389/fenvs.2018.00007
Hussain A, Hasnain S (2011) Phytostimulation and biofertilization in wheat by cyanobacteria. J Ind Microbiol Biotechnol 38:85–92. https://doi.org/10.1007/s10295-010-0833-3
Indira P, Biswajit R (2012) Commercial and industrial applications of microalgae. J Algal Biomass Utilizat 3:89–100
Chittora D, Meena M, Barupal T, Swapnil P, Sharma K (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem and Biophys Rep 22:100737. https://doi.org/10.1016/j.bbrep.2020.100737
Hassan SH, Abdel Hameed MS, Hammouda OE, Ghazal FM, Hamed SM (2012) Effect of different growth conditions on certain biochemical parameters of different cyanobacterial strains. Malaysian J Microbiol 8(4):266–272
Li Z, Guo Mn (2017) Healthy efficacy of Nostoc commune Vaucher. Oncotarget 9(18):14669–14679. https://doi.org/10.18632/oncotarget.23620
Tassara C, Zaccaro MC, Storni MM, Palma M, Zulpa G (2008) Biological control of lettuce white mold with cyanobacteria. Int J Agric Biol 10:487–492
Ghazal FM, Moussa LAA, Fetyan NAH (2010) Cyanobacteria and Rhizobium radiobacter as possible biofertilizers in wheat production. J Agric Chemi Biotechnol 1(7):383–399. https://doi.org/10.21608/jacb.2010.90050
RajaaK EAN, Jorge M, Ana PM, Gonzalo S, Olimpio M, Abderrahmane D, Carlos R (2021) A consortium of cyanobacteria and plant growth promoting Rhizobacteria for wheat growth improvement in a hydroponic system. S Afr J Bot 142:247–258. https://doi.org/10.1016/j.sajb.2021.06.035
Duggan PS, Thiel T, Adams DG (2012) Symbiosis between the cyanobacterium Nostoc and the liverwort Blasia requires a CheR-type MCP methyltransferase. Symbiosis 59(2):111–120. https://doi.org/10.1007/s13199-012-0216-9
Nilsson M, Rasmussen U, Bergman B (2005) Competition among symbiotic cyanobacterial Nostoc strains forming artificial associations with rice (Oryza sativa). FEMS Microbiol Lett 245(1):139–144. https://doi.org/10.1016/j.femsle.2005.03.010
Gantar M, Rowell P, Kerby NW, Sutherland IW (1995) Role of extracellular polysaccharide in the colonization of wheat (Triticum vulgare L.) roots by N2-fixing cyanobacteria. Biol Fert Soils 19(1):41–48. https://doi.org/10.1007/BF00336345
Nain L, Rana A, Joshi M, Jadhav SD, Dinesh Kumar YS, Shivay SP, Prasanna R (2010) Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil 331(1–2):217–230. https://doi.org/10.1007/s11104-009-0247-z
Kholssi R, Marks EAN, Miñón J, Montero O, Lorentz JF, Rad DA, C, (2022) Biofertilizing effects of Anabaena cylindrica biomass on the growth and nitrogen uptake of wheat. Commun Soil Sci Plant Anal 53(10):1216–1225. https://doi.org/10.1080/00103624.2022.2043350
Abdel- Rahman MH, Hassan HR, Nassar RMA, Abdel-Aziz HSM (2020) Influence of foliar spray with yeast extract on faba bean plant (Vicia faba L.). Plant Archiv 20(1):1439–1449
Dewedar GA, Ibrahim EAM (2016) Effect of foliar application of yeast on yield and seed quality of some rice cultivars. J Plant Product 7(6):593–601. https://doi.org/10.21608/jpp.2016.45533
Agamy R, Hashem M, Alamri S (2013) Effect of soil amendment with yeasts as bio-fertilizers on the growth and productivity of sugar beet. Afr J Agric Res 8(1):46–56. https://doi.org/10.5897/AJAR12.1989
Nassar AH, El-Tarabily KA, Sivasithamparam K (2005) Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fertil Soils 42:97–108. https://doi.org/10.1007/s00374-005-0008-y
Karthikeyan N, Prasanna R, Sood A, Jaiswal P, Nayak S, Kaushik BD (2009) Physiological characterization and electron microscopic investigation of cyanobacteria associated with wheat rhizosphere. Folia Microbiol 54(1):43–51. https://doi.org/10.1007/s12223-009-0007-8
A.O.A.C. (1986) Official Methods of Analysis 14th Ed. Association of Official Agricultural Chemists, Alingtan, Virginia, USA
Hamed SM, Raut MP, Jaffé SRP, Wright PC (2017) Evaluation of the effect of aerobic–anaerobic conditions on photohydrogen and chlorophyll a production by environmental Egyptian cyanobacterial and green algal species. Int J Hydrogen Energ 42:6567–6577. https://doi.org/10.1016/j.ijhydene.2017.01.058
Allen MM, Stanier RY (1968) Growth and division of some unicellular blue-green algae. J Gen Microbiol 51:199–202. https://doi.org/10.1099/00221287-51-2-199
Pramer and Schmidt (1964) Experimental soil microbiology. Burgess publishing company, Minnesota
Dilworth MJ (1966) Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochimicaet Biophysica Acta 127:285–294. https://doi.org/10.1016/0304-4165(66)90383-7
Okafor N, Macrea IC (1973) The influence of moisture level, light aeration and glucose upon acetylene reduction by black Earth soil. Soil Biol Biochem 5:181–186. https://doi.org/10.1016/0038-0717(73)90108-9
Lichtenthaler HK (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Jackson ML (1962) Soil Chemical Analysis. Constable, Co., Lt., London
Yoshida SY (1981) Fundamental of rice crop science. International Rice Research Institute, IRRI, Pub. Manila Philippines
Chapman HD Pratt PF (1961) Methods of analysis for soil, plant and water Univ. of California Div. of Agric Sci
Jones DB (1931) Factors for converting percentages of nitrogen in foods and feeds into percentages of proteins. USDA (US Department of Agriculture) Circular Series 183:1–21
Gomez MA, Gomez AA (1993) Statistical procedure for agricultural research (2nded). John Wile & Sons, New York, p 680
Chaudhary V, Prasanna R, Bhatnagar AK (2012) Modulation of fungicidal potential of Anabaena strains by light and temperature. Folia Microbiol 57:199–208. https://doi.org/10.1007/s12223-012-0114-9
Prasanna R, Chaudhary V, Gupta V, Babu S, Kumar A, Singh R, Shivay YS, Nain L (2013) Cyanobacteria mediated plant growth promotion and bioprotection against Fusarium wilt in tomato. Eur J Plant Pathol 136(2):337–353. https://doi.org/10.1007/s10658-013-0167-x
Ayuni N, Radziah O, Naher UA, Panhwar QA, Halimi MS (2015) Effect of nitrogen on nitrogenase activity of diazotrophs and total bacterial population in rice soil. J Anim Plant Sci 25(5):1358–1364
Alzandi AA, Naguib DM (2022) Effect of yeast application on soil health and root metabolic status of corn seedlings under drought stress. Arch Microbiol 204:233. https://doi.org/10.1007/s00203-022-02843-8
Nagib SR, Abdelmageed MM, Haddad SA (2022) Response of durum wheat (Triticum durum, L.) to different combinations of chemical nitrogen fertilizer levels and the red yeast as a biofertilizer. SVU-Int J Agric Scie 4(1):69–82. https://doi.org/10.21608/svuijas.2022.109926.1158
Hernández-Fernández M, Cordero-Bueso G, Ruiz-Muñoz M, Cantoral JM (2021) Culturable yeasts as biofertilizers and biopesticides for a sustainable agriculture: a comprehensive review. Plants 10:822. https://doi.org/10.3390/plants10050822
Rusek P, Mikos-Szymánska M, Karsznia M, Sienkiewicz-Cholewa U, Igras J (2016) The effectiveness of nitrogen phosphorus fertilization in winter wheat (Triticumae stivum L.) cultivation. Bulg J Agri Sci 22:752–755
Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M (2017) Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: a pot experiment. J Appl Phycol 30(1):79–85. https://doi.org/10.1007/s10811-017-1138-y
Prasanna R, Joshi M, Rana A, Shivay Y, Nain L (2012) Influence of co-inoculation of bacteria-cyanobacteria on crop yield and C-N sequestration in soil under rice crop. World J Microbiol Biotechnol 28:1223–1235. https://doi.org/10.1007/s11274-011-0926-9
Prasanna R, Lata TR, Gupta V, Middha S, Joshi M, Ancha R, Kaushik BD (2008) Evaluation of fungicidal activity of extracellular filtrates of cyanobacteria-possible role of hydrolytic enzymes. J Basic Microbiol 48:186–194. https://doi.org/10.1002/jobm.200700199
Mekki BB, Ahmed AG (2005) Growth, yield and seed quality of soybean (Glycine max L.) as affected by organic, bio-fertilizer and yeast application. Res J Agric Biol Sci. 4:320–324
Hesham A-L, Mohamed H (2011) Molecular genetic identification of yeast strains isolated from Egyptian soils for solubilization of inorganic phosphates and growth promotion of corn plants. J Microbiol Biotechnol 21:55–61. https://doi.org/10.4014/jmb.1006.06045
Alonso LM, Kleiner D, Ortega E (2008) Spores of the mycorrhizal fungus Glomus mosseae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza 18:197–204. https://doi.org/10.1007/s00572-008-0172-7
Acknowledgements
The authors wish to thank Soils, Water and Environment Research Institute (SWERI), and Wheat Research Department, Field Crop Research Institute, Agriculture Research Center, Giza, Egypt, for facilities provided to conduct this research work.
Funding
No funding received from any agency.
Author information
Authors and Affiliations
Contributions
SMH and NME-G designed the experimental approach, contributed to full plant and soil analyses, interpreted the results and drafted the manuscript. STE and SMH carried out the field crop study, data analysis and drafting of the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamed, S.M., El-Gaml, N.M. & Eissa, S.T. Integrated biofertilization using yeast with cyanobacteria on growth and productivity of wheat. Beni-Suef Univ J Basic Appl Sci 11, 112 (2022). https://doi.org/10.1186/s43088-022-00288-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00288-y