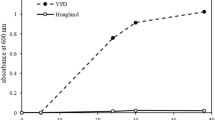
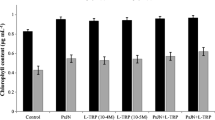
Abstract
A plant-growth-promoting isolate of the yeast Williopsis saturnus endophytic in maize roots was found to be capable of producing indole-3-acetic acid (IAA) and indole-3-pyruvic acid (IPYA) in vitro in a chemically defined medium. It was selected from among 24 endophytic yeasts isolated from surface-disinfested maize roots and evaluated for their potential to produce IAA and to promote maize growth under gnotobiotic and glasshouse conditions. The addition of l-tryptophan (L-TRP), as a precursor for auxins, to the medium inoculated with W. saturnus enhanced the production of IAA and IPYA severalfold compared to an L-TRP-non-amended medium. The introduction of W. saturnus to maize seedlings by the pruned-root dip method significantly (P<0.05) enhanced the growth of maize plants grown under gnotobiotic and glasshouse conditions in a soil amended with or without L-TRP. This was evident from the increases in the dry weights and lengths of roots and shoots and also in the significant (P<0.05) increases in the levels of in planta IAA and IPYA compared with control plants grown in L-TRP-amended or non-amended soil. The plant growth promotion by W. saturnus was most pronounced in the presence of L-TRP as soil amendment compared to seedlings inoculated with W. saturnus and grown in soil not amended with L-TRP. In the glasshouse test, W. saturnus was recovered from inside the root at all samplings, up to 8 weeks after inoculation, indicating that the roots of healthy maize may be a habitat for the endophytic yeast. An endophytic isolate of Rhodotorula glutinis that was incapable of producing detectable levels of IAA or IPYA in vitro failed to increase the endogenous levels of IAA and IPYA and failed to promote plant growth compared to W. saturnus, although colonization of maize root tissues by R. glutinis was similar to that of W. saturnus. Both endophytic yeasts, W. saturnus and R. glutinis, were incapable of producing in vitro detectable levels of gibberellic acid, isopentenyl adenine, isopentenyl adenoside or zeatin in their culture filtrates. This study is the first published report to demonstrate the potential of an endophytic yeast to promote plant growth. This is also the first report of the production of auxins by yeasts endophytic in plant roots.


Similar content being viewed by others
References
Abd El-Hafez AE, Shehata SF (2001) Field evaluation of yeasts as a biofertilizer for some vegetable crops. Arab Univ J Agric Sci 9:169–182
Andrews JH, Buck JW (2002) Adhesion of yeasts to leaf surfaces. Phyllosphere microbiology. American Phytopathological Society, St. Paul, pp 53–68
Arras G, Scherm B, Migheli Q (2002) Improving biocontrol activity of Pichia guillermondii against post-harvest decay of oranges in commercial packing-houses by reduced concentrations of fungicides. Biocontrol Sci Technol 12:547–553
Arsac JF, Lamothe C, Mulard D, Fages J (1990) Growth enhancement of maize (Zea mays L.) through Azospirillum lipoferum inoculation: effect of plant genotype and bacterial concentration. Agronomie 10:649–654
Arshad M, Frankenberger WT Jr (1998) Plant growth regulating substances in the rhizosphere: microbial production and functions. Adv Agron 62:45–151
Bab'eva IP, Belyanin AI (1966) Yeasts of the rhizosphere. Mikrobiologia 35:712–720
Bacon CW, Hinton DM (2002) Endophytic and biological control potential of Bacillus mojavensis and related species. Biol Control 23:274–284
Barea JM, Navarro E, Montoya E (1976) Production of plant growth regulators by rhizosphere phosphate-solubilizing bacteria. J Appl Bacteriol 40:129–134
Bashan Y, Holguin G (1997) Azospirillum-plant relationships: environmental and physiological advances. Can J Microbiol 43:103–121
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50:521–577
Bastian F, Cohen A, Piccoli P, Luna V, Baraldi R, Bottini R (1998) Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically-defined culture media. Plant Growth Regul 24:7–11
Bressan W, Borges MT (2004) Delivery methods for introducing endophytic bacteria into maize. Biocontrol 49:315–322
Buck JW, Lachance MA, Traquair JA (1998) Mycoflora of peach bark: population dynamics and composition. Can J Bot 76:345–354
Cao LX, You JL, Zhou SN (2002) Endophytic fungi from Musa acuminata leaves and roots in south China. World J Microbiol Biotechnol 18:169–171
Chanway CP, Radley RA, Holl FB (1991) Inoculation of conifer seed with plant growth promoting Bacillus strains causes increased seedling emergence and biomass. Soil Biol Biochem 23:575–580
Cleland RE (1990) Auxin and cell elongation. In: Davies PJ (ed) Plant hormones and their role in plant growth and development. Kluwer, Dordrecht, pp 132–148
di Menna ME (1957) The isolation of yeasts from soil. J Gen Microbiol 17:678–688
El-Abyad MS, El-Sayed MA, El-Shanshoury AR, Farid M (1994) Optimization of culture conditions for indole-3-pyruvic acid production by Streptomyces griseoflavus. Can J Microbiol 40:754–760
El-Tarabily KA (2004) Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J Appl Microbiol 96:69–75
El-Tarabily KA, Hardy GEStJ, Sivasithamparam K, Kurtböke ID (1996) Microbiological differences between limed and unlimed soils and their relationship with cavity spot disease of carrots (Daucus carota L.) caused by Pythium coloratum in Western Australia. Plant Soil 183:279–290
El-Tarabily KA, Nassar AH, Hardy GEStJ, Sivasithamparam K (2003) Fish emulsion as a food base for rhizobacteria promoting growth of radish (Raphanus sativus L. var sativus) in a sandy soil. Plant Soil 252:397–411
Fallik E, Okon Y, Epstein E, Goldman A, Fischer M (1989) Identification and quantification of IAA and IBA in Azospirillum brasilense-inoculated maize roots. Soil Biol Biochem 21:147–153
Fisher PJ, Petrini O, Scott HM (1992) The distribution of some fungal and bacterial endophytes in maize (Zea mays L.). New Phytol 122:299–305
Floch G, Rey P, Benizri E, Benhamou N, Tirilly Y (2003) Impact of auxin-compounds produced by the antagonistic fungus Pythium oligandrum or the minor pathogen Pythium group F on plant growth. Plant Soil 257:459–470
Frankenberger WT Jr, Poth M (1987) Biosynthesis of indole-3-acetic acid by the pine ectomycorrhizal fungus, Pisolithus tinctorius. Appl Environ Microbiol 53:2908–2913
Frankenberger WT Jr, Chang AC, Arshad M (1990) Response of Raphanus sativus to the auxin precursor, l-tryptophan applied to soil. Plant Soil 129:235–241
Gomes NCM, Fagbola O, Costa R, Rumjanek NG, Buchner A, Mendona-Hagler L, Smalla K (2003) Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol 69:3758–3766
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192–195
Guinn G, Brummett DL, Beier RC (1986) Purification and measurement of abscisic acid and indole-acetic acid by high performance liquid chromatography. Plant Physiol 81:997–1002
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Kaldorf M, Ludwig-Muller J (2000) AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol Plant 109:58–67
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480
Larran S, Monaco C, Alippi HE (2001) Endophytic fungi in leaves of Lycopersicon esculentum Mill. World J Microbiol Biotechnol 17:181–184
Larran S, Perello A, Simon MR, Moreno V (2002) Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J Microbiol Biotechnol 18:683–686
Lu H, Zou WX, Meng JC, Hu J, Tan RX (2000) New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci 151:67–73
Maria GL, Sridhar KR (2003) Endophytic fungal assemblage of two halophytes from west coast mangrove habitats, India. Czech Mycol 55:241–251
Millonig G (1976) Laboratory manual of biological electron microscopy. Saviolo M (ed) Vercelli, Italy
Mucciarelli M, Scannerini S, Bertea C, Maffei M (2003) In vitro and in vivo peppermint (Mentha piperita) growth promotion by nonmycorrhizal fungal colonization. New Phytol 158:579–591
Musson G, McInroy JA, Kloepper JW (1995) Development of delivery systems for introducing endophytic bacteria into cotton. Biocontrol Sci Technol 5:407–416
Nakamura T, Murakami T, Saotome M, Tomita K, Kitsuwa T, Meyers SP (1991) Identification of indole-3-acetic acid in Pichia spartinae, an ascosporogenous yeast from Spartina alterniflora marshland environments. Mycologia 83:662–664
Nassar AH, El-Tarabily KA, Sivasithamparam K (2003) Growth promotion of bean (Phaseolus vulgaris L.) by a polyamine-producing isolate of Streptomyces griseoluteus. Plant Growth Regul 40:97–106
Nieto KF, Frankenberger WT Jr (1989) Biosynthesis of cytokinins in soil. Soil Sci Soc Am J 53:735–740
Normanly J, Cohen JD, Fink GR (1993) Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci U S A 90:10355–10359
Payne C, Bruce A (2001) The yeast Debaryomyces hansenii as a short-term biological control agent against fungal spoilage of sawn Pinus sylvestris timber. Biol Control 22:22–28
Perondi NL, Luz WC, Thomas R (1996) Microbiological control of Gibberella in wheat. Fitopatol Bras 21:243–249
Petersson S, Jonsson N, Schnürer J (1999) Pichia anomala as a biocontrol agent during storage of high-moisture feed grain under airtight conditions. Postharvest Biol Technol 15:175–184
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Springer, Berlin Heidelberg New York, pp 179–197
Prikryl Z, Vancura V, Wurst M (1985) Auxin formation by rhizosphere bacteria as a factor of root growth. Biol Plant 27:159–163
Rennie RJ, De Freitas JR, Ruschel AP, Vose PB (1982) Isolation and identification of N2-fixing bacteria associated with sugar cane (Saccharum sp.). Can J Microbiol 28:462–467
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S (1992) Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl Environ Microbiol 58:2691–2693
Sarwar M, Frankenberger WT Jr (1994) Influence of l-tryptophan and auxins applied to the rhizosphere on the vegetative growth of Zea mays L. Plant Soil 160:97–104
Sivasithamparam K (1998) Root cortex—the final frontier for the biocontrol of root-rot with fungal antagonists: a case study on a sterile red fungus. Annu Rev Phytopathol 36:439–452
Srinivasan M, Petersen DJ, Holl FB (1996) Influence of indoleacetic-acid-producing Bacillus isolates on the nodulation of Phaseolus vulgaris by Rhizobium etli under gnotobiotic conditions. Can J Microbiol 42:1006–1014
Stone JK, Bacon CW, White JF Jr (2000) An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF Jr (eds) Microbial endophytes. Marcel Dekker, New York, USA, pp 3–29
Strzelczyk E, Pokojska-Burdziej A (1984) Production of auxins and gibberellin-like substances by mycorrhizal fungi, bacteria and actinomycetes isolated from soil and the mycorrhizosphere of pine (Pinus silvestris L.). Plant Soil 81:185–194
Sturz AV, Christie BR, Matheson BG (1998) Associations of bacterial endophyte populations from red clover and potato crops with potential for beneficial allelopathy. Can J Microbiol 44:162–167
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Suslow TV, Schroth MN (1982) Rhizobacteria of sugar beets: effects of seed application and root colonization on yield. Phytopathology 72:199–206
Tian XL, Cao LX, Tan HM, Zeng QG, Jia YY, Han WQ, Zhou SN (2004) Study on the communities of endophytic fungi and endophytic actinomycetes from rice and their antipathogenic activities in vitro. World J Microbiol Biotechnol 20:303–309
Tien TM, Gaskings MH, Hubbell DH (1979) Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl Environ Microbiol 37:1016–1024
Urquhart EJ, Punja ZK (2002) Hydrolytic enzymes and antifungal compounds produced by Tilletiopsis species, phyllosphere yeasts that are antagonists of powdery mildew fungi. Can J Microbiol 48:219–229
Varma A, Verma S, Sudha, Sahay N, Butehorn B, Franken P (1999) Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 65:2741–2744
Wellington EMH, Williams ST (1978) Preservation of actinomycete inoculum in frozen glycerol. Microbios Lett 6:151–157
Wickerham LJ (1951) Taxonomy of yeasts. Tech Bull No 1029. US Department of Agriculture, Washington, DC, pp 1–56
Zahir ZA, Arshad M, Azam M, Hussain A (1997) Effect of an auxin precursor tryptophan and Azotobacter inoculation on yield and chemical composition of potato under fertilized conditions. J Plant Nutr 20:745–752
Zakharova EA, Shcherbakov AA, Brudnik VV, Skripko NG, Bulkhin NS, Ignatov VV (1999) Biosynthesis of indole-3-acetic acid in Azospirillum brasilense: insights from quantum chemistry. Eur J Biochem 259:572–576
Zinniel DK, Lambrecht P, Harris NB, Feng ZY, Kuczmarski D, Higley P, Ishimaru CA, Arunakumari A, Barletta RG, Vidaver AK (2002) Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol 68:2198–2208
Acknowledgements
The authors would like to thank the United Arab Emirates University Research Council for financial support (grant number 03-04-2-11/04). We would like to thank the laboratory and field staff at the University of United Arab Emirates for their valuable assistance. We would like also to thank A. Gbewonyo and S. Tariq from the Electron Microscopy Unit, Faculty of Medicine and Health Sciences, United Arab Emirates University, for their assistance in the preparation of transmission electron microscope specimens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nassar, A.H., El-Tarabily, K.A. & Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fertil Soils 42, 97–108 (2005). https://doi.org/10.1007/s00374-005-0008-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0008-y