Abstract
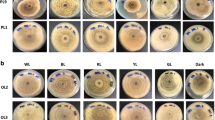
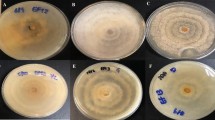
The regulation of fungicidal and hydrolytic enzyme activity was investigated in a set of cyanobacterial strains belonging to the genus Anabaena (Anabaena laxa RPAN8, Anabaena iyengarii RPAN9, Anabaena variabilis RPAN59 and Anabaena oscillarioides RPAN69), with A. variabilis RPAN16 serving as negative control. Time course studies undertaken with cultures incubated under different light and temperature conditions revealed enhancement in growth and fungicidal activity under continuous light (CL) and light dark (LD, 16:8) conditions and temperature of 30 °C and 40 °C. A significant increase of 3–18 % in chitosanase activity was recorded in all the 4-week-old cultures under CL condition and at 40 °C. Endoglucanase activity of RPAN8 and 9 was twofolds higher than the other strains under all light/dark conditions and temperature in the 4-week-old cultures, while continuous dark (CD) enhanced CMCase activity in RPAN69. This study provided useful information regarding the most suitable conditions of light and temperature for maximizing hydrolytic enzyme activity and fungicidal activity, as a prelude to their effective use as biocontrol agents.






Similar content being viewed by others
References
Abed RMM, Dobretsov S, Sudesh K (2009) Applications of cyanobacteria in biotechnology. J Appl Microbiol 106:1–12
Ando A, Saito A, Arai S, Usuda S, Furuno M, Kaneko N, Shida O, Nagata Y (2008) Molecular characterization of a novel family-46 chitosanase from Pseudomonas sp. A-01. Biosci Biotech Bioch 72:2074–2081
Chaudhary V, Prasanna R, Gupta V, Singh SB, Natarajan C, Nain L (2010) Development of microtitre plate based assay for evaluation of fungicidal potential and microscopic analyses of cyanobacterial metabolites. Arch Phytopathol Plant Prot 43:1435–1444
Chernin L, Chet I (2002) Microbial enzymes in biocontrol of plant pathogens and pests. In: Burns R, Dick R (eds) Enzymes in the environment: activity, ecology and applications. Marcel Dekker Inc., New York, pp 171–225
Commonwealth Agricultural Bureaux (1968) Plant pathologist’s pocketbook. Commonwealth Mycological Institute, Kew, p 239
Dukare AS, Prasanna R, Dubey SC, Chaudhary V, Nain L, Singh R, Saxena AK (2011) Evaluating novel microbe amended composts as biocontrol agents in tomato. Crop Protect 30:436–442
El-Katatny MH, Somitsch W, Robra KH, El-Katatny MS, Gubitz GM (2000) Production of chitinase and β-1, 3- glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii. Food Technol Biotechnol 38(3):173–180
Fukamizo T, Honda Y, Toyoda H, Ouchi S, Goto S (1996) Chitinous component of the cell wall of Fusarium oxysporum, its structure deduced from chitosanase digestion. Biosci Biotechnol Biochem 60:1705–1708
Ghosh TK, Bailey HJ, Bisaria VS, Enari TM (1983) Measurement of cellulase activities—final recommendations, Commission of Biotechnology. Intern Union Pure App Chem 59:1–13
Gupta V, Prasanna R, Natarajan C, Srivastava AK, Sharma J (2010) Identification, characterization and regulation of a novel antifungal chitosanase gene (cho) in Anabaena spp. Appl Environ Microbiol 76:2769–2777
Gupta V, Natarajan C, Kumar K, Prasanna R (2011a) Identification and characterization of endoglucanases for fungicidal activity in Anabaena laxa. J Appl Phycol 23:73–81
Gupta V, Prasanna R, Srivastava AK, Sharma J (2011b) Purification and characterization of a novel antifungal endo type chitosanase from Anabaena fertilissima. Ann Microbiol. doi:10.1007/s13213-011-0350-2
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds), Methods in Microbiology, Vol. 5B: London, Academic Press, pp 209-344
Kulik MM (1995) The potential for using cyanobacteria (blue green algae) and algae in the biological control of plant pathogenic bacteria and fungi. Eur J Plant Pathol 101:585–599
MacKinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140:315–322
Manjunath M, Prasanna R, Nain L, Dureja P, Singh R, Kumar A, Jaggi S, Kaushik BD (2010) Biocontrol potential of cyanobacterial metabolites against damping off disease caused by Pythium aphanidermatum in solanaceous vegetables. Archiv Phytopathol Plant Prot 43:666–677
Matsuda Y, Iida Y, Shinogi T, Kakutani K, Nonomura T, Toyodai H (2001) In vitro suppression of mycelial growth of Fusarium oxysporum by extracellular chitosanase of Sphingobacterium multivorum and cloning of the chitosanase gene csnSM1. J Gen Plant Pathol 67:318–324
Mur LR (1983) Some aspects of the ecophysiology of cyanobacteria. Ann Microbiol Inst Pasteur 134B:61–72
Ohtakara A (1988) Chitosanase and β-N-acetyl hexosamine from Pycnosporus cinnabarinus. Methods Enzymol 168:464–468
Ordentlich A, Elad Y, Chet I (1988) The role of chitinase of Serratia marcescens in the biocontrol of Sclerotium rolfsii. Phytopathol 78:84–88
Prasanna R, Lata TR, Gupta V, Middha S, Joshi M, Ancha R, Kaushik BD (2008) Evaluation of fungicidal activity of extracellular filtrates of cyanobacteria—possible role of hydrolytic enzymes. J Basic Microbiol 48:186–194
Prasanna R, Nain L, Ancha R, Shrikrishna J, Joshi M, Kaushik BD (2009) Rhizosphere dynamics of inoculated cyanobacteria and their growth promoting role in rice crop. Egypt J Biol 11:26–36
Prasanna R, Gupta V, Natarajan C, Chaudhary V (2010a) Allele mining for chitosanases and microcystin-like compounds in Anabaena strains. World J Microbiol Biotechnol 26:717–724
Prasanna R, Joshi M, Rana A, Nain L (2010b) Modulation of IAA production in cyanobacteria by tryptophan and light. Polish J Microbiol (Formerly Acta Microbiologica Polonica) 59:99–105
Prasanna R, Sood A, Jaiswal P, Nayak S, Gupta V, Chaudhary V, Joshi M, Natarajan C (2010c) Rediscovering cyanobacteria as valuable sources of bioactive compounds. Appl Biochem Microbiol 46:133–147
Radhakrishnan B, Prasanna R, Jaiswal P, Nayak S, Dureja P (2009) Modulation of biocidal activity of Calothrix sp. and Anabaena sp. by environmental factors. Biologia 64:881–889
Ray S, Bagchi SN (2001) Nutrients and pH regulate algicide accumulation in cultures of cyanobacterium Oscillatoria laetevirens. New Phytol 149:455–460
Riegman R, Mur LR (1985) Effects of photoperiodicity and light irradiance on phosphate limited Oscillatoria agardhii in chemostat cultures. Arch Microbiol 142:72–76
Sivonen K, Jones G (1999) Cyanobacterial Toxins. In: Chorus I, Batram JA (eds) Toxic cyanobacteria in water–guide to their Public Health consequences, monitoring and management. E and FN Spon, London, pp 41–111
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue green algae (Order: Chroococcales). Bacteriol Rev 35:171–305
Volk RB, Mundt S (2007) Cytotoxic and non-cytotoxic exometabolites of the cyanobacterium Nostoc insulare. J Appl Phycol 19:55–62
Wang SY, Ye XY, Rao PF (2009) Isolation and biochemical characterization of a novel leguminous defense peptide with antifungal and antiproliferative potency. Appl Microbiol Biotechnol 82:79–86
Acknowledgments
This study was supported by the AMAAS Network Project on Microorganisms, which was supported by the Indian Council of Agricultural Research (ICAR), New Delhi, India. We thank the authorities of the Division of Microbiology, Indian Agricultural Research Institute, New Delhi, for providing facilities for this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Fungicidal activity of cell free filtrates of three and four weeks old cultures of A. laxa (RPAN8), A. iyengarii (RPAN9), A. variabilis (RPAN59), A. oscillarioides (RPAN69) and A. variabilis (RPAN16), incubated under different light and temperature conditions (expressed as the size of the zone of inhibition for F. moniliforme, F. oxysporum lycopersici, R. solani and P. debaryanum) (DOC 57 kb)
Rights and permissions
About this article
Cite this article
Chaudhary, V., Prasanna, R. & Bhatnagar, A.K. Modulation of fungicidal potential of Anabaena strains by light and temperature. Folia Microbiol 57, 199–208 (2012). https://doi.org/10.1007/s12223-012-0114-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0114-9