Abstract
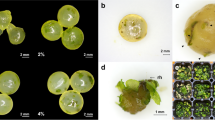
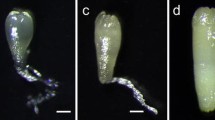
A suspension culture capable of producing a high quantity of proembryogenic masses (PEMs) was evaluated to provide adequate support for synthetic seed production in saltmarsh species Spartina alterniflora. Using immature inflorescences, callus was induced on MS medium supplemented with 2 mg 1−1 2,4-dichlorophenoxyacetic acid (2,4-D). Prior to initiation of suspension culture, calli were proliferated on a modified R4 medium (R4+) for 2–4 weeks. Suspension culture was carried out in a liquid General Medium modified by adding FeSO4 · 7H20, myo-inositol, thiamine · HCl, pyridoxine · HCl, and casein acid hydrolysate. Suspension cells were subcultured weekly by replacing the spent medium with fresh medium. The amount of PEMs tripled in 1 week. Cells from suspension culture had an average regeneration rate of 90% for 6 months of culture. About 40 g of PEMs can be harvested from 1 l of suspension culture in a weekly basis. Plated on R4+ medium, these PEMs produced somatic embryos (SEs) that gave rise to 2,000 plantlets. Encapsulation of SEs or microplantlets (MPs) derived from suspension cells may facilitate direct delivery of micro-propagated S. alterniflora to field planting. Evaluated under in vitro conditions, both SEs and MP-derived synthetic seeds had high conversion rates.



Similar content being viewed by others
Abbreviations
- SE:
-
Somatic embryo
- MP:
-
Microplantlet
- PEM:
-
Proembryogenic mass
References
Agarwal S, Kanwar K, Sharma DR (2004) Factors affecting secondary somatic embryogenesis and embryo maturation in Morus alba L. Sci Hortic 102:359–368
Asano Y, Ito Y, Ohara M, Sugiura K, Fujiie A (1994) Improved regeneration response of creeping bentgrass and japonica rice by maltose and lactose. Plant Cell Tiss Org 39:101–103
Baustian JJ (2006) Restoration success of backfilling canals in coastal louisiana marshes. Restor Ecol 14:636–644
Brischia R, Piccioni E, Standardi A (2002) Micropropagation and synthetic seed in M.26 apple rootstock (II): a new protocol for production of encapsulated differentiating propagules. Plant Cell Tiss Org 68:137–141
Broome SW, Seneca ED, Woodhouse Jr. WW (1988) Tidal salt marsh restoration. Aquat Bot 32:1–22
Bruno JF (2000) Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology 81:1179–1192
Castillo B, Smith MAL, Yadava UL (1998) Plant regeneration from encapsulated somatic embryos of Carica papaya L. Plant Cell Rep 17:172–176
Chaleff RS, Stolarz A (1981) Factors influencing the frequency of callus among cultured Oryza sativa anthers. Physiol Plant 51:201–206
Chen Y (1986) Anther and pollen culture in rice. In: Hu H, yang HY (eds) Haploid of higher plants in vitro. China Acad Pub and Springer-Verlag, Berlin, pp 1–25
Craig NJ, Turner RE, Day Jr. JW (1979) Land loss in coastal Louisiana (USA). Environ Manage 3:133–144
Ganapathi TR (2001) Regeneration of plants from alginate-encapsulated somatic embryos of banana cv. Rasthali (Musa spp. AAB group). In Vitro Cell Devel Biol Plant 37(2):178–181
Iraqi D, Tremblay FM (2001) The role of sucrose during maturation of black spruce (Picea mariana) and white spruce (Picea glauca) somatic embryos. Physiol Plant 111:381–388
Janick J, Kitto SL, Kim Y (1989) Production of synthetic seed by desiccation and encapsulation. In Vitro Cell Devel Biol Plant 25:1167–1172
Jerome LE (1979) Marsh restoration: economic rewards of a healthy salt marsh. Ocean 12:57–61
Kishinami I, Widholm JM (1986) Selection of copper and zinc resistant Nicotiana plumbaginifolia cell suspension cultures. Plant Cell Physiol 27:1263–1268
Kumar MBA, Vakeswaran V, Krishnasamy V (2005) Enhancement of synthetic seed conversion to seedlings in hybrid rice. Plant Cell Tiss Organ 81:97–100
Larkin P, Scowcroft W (1981) Somaclonal variation a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li XY, Huang H, Murphy BJ, Gbur EE Jr (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34:22–26
Lisek A, Olikowska T (2004) In vitro storage of strawberry and raspberry in calcium-alginate beads at 4°C. Plant Cell Tiss Org 78:167–172
Mamiya K, Sakamoto Y (2001) A method to produce encapsulatable units for synthetic seeds in Asparagus oficinalis. Plant Cell Tiss Org 64:27–32
Murashige T, Skoog FA (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Naill MC, Roberts SC (2005) Cell cycle analysis of Taxus suspension cultures at the single cell level as an indicator of culture heterogeneity. Biotechnol Bioeng 90:491–500
Nieves N, Zambrano Y, Tapia R, Cid M, Pina D, Castillo R (2003) Field performance of artificial seed-derived sugarcane plants. Plant Cell Tiss Org 75:279–282
Norgaard JV, Krogstrup P (1991) Cytokinin-induced somatic embryogenesis from immature embryos of Abies nordmanniana Lk. Plant Cell Rep 9:509–513
Onay A, Jeffree CE, Yeoman MM (1996) Plant regeneration from encapsulated embryoids and an embryogenic mass of pistachio, Pistacia vera L. Plant Cell Rep 15:723–726
Patel A, Pusch L, Mix-Wagner G, Vorlop KD (2000) A novel encapsulation technique for the production of artificial seeds. Plant Cell Rep 19:868–874
Pattnaik S, Chand PK (2000) Morphogenic response of the alginate-encapslated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tiss Org 60:177–185
Saha M, Phatak A, Chandra N (2004) Generation of synthetic seeds from different varieties of Carica papaya L. J Tiss Res 4:207–209
Seliskar DM, Gallagher JL (2000) Exploiting wild population diversity and somaclonal variation in the salt marsh grass Distichlis spicata (Poaceae) for marsh creation and restoration. Am J Bot 87:141–146
Seneca ED (1974) Germination and seedling response of Atlantic and Gulf coasts populations of Spartina alterniflora. Am J Bot 61:947–956
Sharp WR, Evans A, Ammirato PV, Yamada Y (1984) Handbook of plant cell culture, vol. 2, Crop Species. Macmillan, New York
Singh AK, Varshney R, Sharma M, Agarwal SS, Bansal KC (2006) Regeneration of plants from alginate-encapsulated shoot tips of Withania somnifera (L.) dunal, a medicinally important plant species. J Plant Physiol 163:220–223
Tang K, Sun X, An D, Power JB, Cocking EC, Davey MR (2000) Plant regeneration from protoplasts of a commercial Asian long-grain javanica rice. Plant Cell Tiss Org 60:79–82
Thompson J.D. 1991. The biology of an invasive plant. Bioscience 41:393–401
Ticha I, Cap F, Pacovska D, Hofman P, Haisel D, Capkova V, Schafer C (1998) Culture on sugar medium enhances photosynthetic capacity and light resistance of plantlets grown in vitro. Plant Physiol 102:155–162
Tonon G, Capuana M, Rossi C (2001) Somatic embryogenesis and embryo encapsulation in Fraxinus angustifolia Vhal. J Hort Sci Biotechnol 76:753–757
Utomo HS, Croughan SS, Croughan TP (1995) Suspension and protoplast culture of US rice cultivars. Plant Cell Rep 15:34–37
Wang J, Seliskar DM, Gallagher J (2003) Tissue culture and plant regeneration of Spatina alterniflora: implications for wetland restoration. Wetlands 23:386–393
West TP, Ravindra MB, Preece JE (2006) Encapsulation, cold storage, and growth of Hibiscus moscheutos nodal segments. Plant Cell Tiss Org 81:97–100
Author information
Authors and Affiliations
Corresponding author
Additional information
Approved for publication by the Director of the Louisiana Agricultural Experiment Station as manuscript number 07-61-0353.
Rights and permissions
About this article
Cite this article
Utomo, H.S., Wenefrida, I., Meche, M.M. et al. Synthetic seed as a potential direct delivery system of mass produced somatic embryos in the coastal marsh plant smooth cordgrass (Spartina alterniflora). Plant Cell Tiss Organ Cult 92, 281–291 (2008). https://doi.org/10.1007/s11240-007-9334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9334-0