Abstract
Background
Measurement of beta-amyloid (Aβ) and phosphorylated tau (p-tau) levels offers the potential for early detection of neurocognitive impairment. Still, the probability of developing a clinical syndrome in the presence of these protein changes (A+ and T+) remains unclear. By performing a systematic review and meta-analysis, we investigated the risk of mild cognitive impairment (MCI) or dementia in the non-demented population with A+ and A- alone and in combination with T+ and T- as confirmed by PET or cerebrospinal fluid examination.
Methods
A systematic search of prospective and retrospective studies investigating the association of Aβ and p-tau with cognitive decline was performed in three databases (MEDLINE via PubMed, EMBASE, and CENTRAL) on January 9, 2024. The risk of bias was assessed using the Cochrane QUIPS tool. Odds ratios (OR) and Hazard Ratios (HR) were pooled using a random-effects model. The effect of neurodegeneration was not studied due to its non-specific nature.
Results
A total of 18,162 records were found, and at the end of the selection process, data from 36 cohorts were pooled (n= 7,793). Compared to the unexposed group, the odds ratio (OR) for conversion to dementia in A+ MCI patients was 5.18 [95% CI 3.93; 6.81]. In A+ CU subjects, the OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64]. Cerebrospinal fluid Aβ42 or Aβ42/40 analysis and amyloid PET imaging showed consistent results. The OR for conversion in A+T+ MCI subjects (11.60 [95% CI 7.96; 16.91]) was significantly higher than in A+T- subjects (2.73 [95% CI 1.65; 4.52]). The OR for A-T+ MCI subjects was non-significant (1.47 [95% CI 0.55; 3.92]). CU subjects with A+T+ status had a significantly higher OR for conversion (13.46 [95% CI 3.69; 49.11]) than A+T- subjects (2.04 [95% CI 0.70; 5.97]). Meta-regression showed that the ORs for Aβ exposure decreased with age in MCI. (beta = -0.04 [95% CI -0.03 to -0.083]).
Conclusions
Identifying Aβ-positive individuals, irrespective of the measurement technique employed (CSF or PET), enables the detection of the most at-risk population before disease onset, or at least at a mild stage. The inclusion of tau status in addition to Aβ, especially in A+T+ cases, further refines the risk assessment. Notably, the higher odds ratio associated with Aβ decreases with age.
Trial registration
The study was registered in PROSPERO (ID: CRD42021288100).
Similar content being viewed by others
Background
Affecting 55 million people worldwide, dementia is one of the leading causes of years spent with disability and one of the costliest long-term illnesses in society. The most common cause of dementia is Alzheimer's disease (AD), responsible for 60-80% of cases [1, 2].
Two specific protein aggregates play a crucial role in the pathophysiology of AD. One is the amyloid plaque formation in the extracellular space, predominantly by Aβ aggregation. These plaques, among other pathological effects, inhibit the signaling function of neurons [3]. The other protein change is the appearance of neurofibrillary tangles within the neurons, which are formed by the phosphorylation of tau proteins (p-tau) and inhibit the axonal transport inside the cell [4]. Whereas the specific pathology could only be confirmed by autopsy in the past, in vivo tests are available today. Parallelly to this development, the diagnostic definitions of AD have evolved significantly over time, moving from purely clinical assessments and post-mortem examinations to the integration of in vivo amyloid and later p-tau biomarkers, emphasizing the role of preclinical stages [5,6,7,8]. Accordingly, researchers are increasingly trying to link the diagnosis of the disease to biological parameters. However, in general, the clinical practice only considers the quality of the symptoms of dementia and the fact of neurodegeneration confirmed by radiology when establishing an AD diagnosis.
The International Working Group (IWG) [5] emphasizes that diagnosis should align with clinical symptoms. However, for researchers in the field, the U.S. National Institute on Aging – Alzheimer’s Association (NIA-AA) has issued a new framework recommendation [6]. This recommendation defines AD purely in terms of specific biological changes based on the Aβ (A) and p-tau (T) protein status, while neurodegeneration (N) is considered a non-specific marker that can be used for staging. In the recommendation, the category ‘Alzheimer’s disease continuum’ is proposed for all A+ cases, ‘Alzheimer’s pathological changes’ for A+T- cases, and ‘Alzheimer’s disease’ for A+T+ cases. A-(TN)+ cases are classified as ‘non-Alzheimer pathological changes’.
Aβ and p-tau proteins have long been known to be associated with AD development, and their accumulation can begin up to 15-20 years before the onset of cognitive symptoms [9]. Pathological amyloid changes are highly prevalent in dementia: 88% of those clinically diagnosed with AD and between 12 and 51% of those with non-AD are A+, according to a meta-analysis [10]. At the same time, the specificity of the abnormal beta-amyloid level for AD and its central role in its pathomechanism have been questioned [11]. Their use as a preventive screening target is a subject of ongoing discourse [12]. Yet it is still unclear to what extent their presence accelerates cognitive decline. What are the predictive prospects for an individual with abnormal protein levels who is otherwise cognitively healthy or with only mild cognitive impairment (MCI), meaning cases where there is a detectable decline in cognitive ability with maintained ability to perform most activities of daily living independently? [13] Research on non-demented populations shows substantial variation; for example, studies have shown OR values for conversion to dementia ranging from 2.25 [95% CI 0.71; 7.09] [14] to 137.5 [95% CI 17.8; 1059.6] [15]. Comparing conversion data systematically is necessary to provide a clearer picture.
In the CU population over 50 years, the prevalence of being A+ ranges from 10 to 44%, while in MCI it ranges from 27 to 71%, depending on age. Taking this into consideration [16], we aim to investigate the effect of Aβ alone and in combination with p-tau on the conversion to MCI and dementia, through a systematic review and meta-analysis of the available literature. Knowing the prognostic effect can highlight the clinical potential of this current research framework, given that, at present, the therapy of MCI or dementia can only slow down the decline. Prevention starting at an early stage or even before symptoms appear, provides the best chance against the disease.
Methods
Study registration
Our study was registered in the PROSPERO database (ID: CRD42021288100), with a pre-defined research plan and detailed objectives, is reported strictly in accordance with the recommendation of the PRISMA 2020 guideline and was performed following the guidance of the Cochrane Handbook [17].
We aimed to determine the change in odds of progression to MCI or dementia among non-demented subjects based on abnormal Aβ levels alone, or in combination with abnormal p-tau levels.
Search and selection
We included longitudinal prospective and retrospective studies that used the NIA-AA 2018 recommended measurement of Aβ and p-tau (for Aβ: amyloid PET, CSF Aβ42, or Aβ42/40 ratio; for p-tau: tau PET, or CSF p-tau) and investigated the role of Aβ and +/- p-tau in CU and MCI subjects in progression to MCI or dementia. Case reports and case series were excluded. Overlapping populations were taken into account during the data extraction. Our search key was run in the Medline, Embase, and Central databases on 31 October 2021, and the search was updated on 9 January 2024 (see Supplementary Material, Appendix 1). After removing duplicates, we screened publications by title and abstract, and in the second round by full text. Two independent reviewers conducted the selection (ZH, MP), and a third reviewer (GC) resolved disagreements. The degree of the agreement was quantified using Cohen’s kappa statistics at each selection stage.
As part of the selection process, articles that only examined the ADNI database [18] were excluded, as patient-level data were used instead (see Supplementary Material Appendix 2 for details of the patient-level data analysis of the ADNI).
A standardized Excel (Microsoft Corporation, Redmond, Washington, USA) document sheet was used for data extraction (for one special case of data extraction see Supplementary Material Appendix 3). Where data were available in graphical form only, we used an online software (Plot Digitizer) [19, 20]. The following data were extracted: source of data used in the studies (place of clinical trial or name of database), baseline characteristics of the population (age, gender, APOE status, and education level), type of exposure (Aβ, p-tau, and neurodegeneration), measurement technique of the exposure, data on cognitive impairment separately for the different exposure groups).
Data synthesis
Generally, where several studies used the same population sample or cohort, only data from the study with the largest sample size were used. Conversion to Alzheimer’s dementia and to unspecified dementia was assessed together, as the definition of Alzheimer’s dementia varied between the studies, and the diagnosis was based on neurocognitive tests. If conversion to both types of dementia was given, the value of the conversion to unspecified dementia was used. The population with subjective cognitive symptoms was scored jointly with the CU population, as these subpopulations could not be differentiated objectively.
Odds ratio and hazard ratio values were used or calculated based on the available information (for details on the methodology, see Supplementary Material Appendix 4). Considering that studies report their results on different age groups, a meta-regression analysis was performed to investigate how age affects the likelihood of developing dementia based on Aβ levels.
Studies applied different analysis methods to identify Aβ positivity. Where multiple amyloid categories were being considered, the preferred method was amyloid PET. When relying on CSF analysis, the Aβ42/40 ratio was given precedence over Aβ42 since the 42/40 ratio has a higher concordance with amyloid PET [21]. To estimate the confounding effect caused by different amyloid measurement techniques a subgroup analysis was performed. For the assessment of p-tau, studies measured p-tau181 levels from CSF samples, or employed tau PET. While there is also a limited number of tau PET measurements in the ADNI, in order to ensure consistency in the analyses, we used exclusively the CSF p-tau181 levels from the ADNI database.
For the OR analysis, studies with varying follow-up times were pooled. To estimate the resulting bias, a meta-regression analysis was performed to explore how follow-up time affected the results.
Statistical analysis
Statistical analyses were performed in the R programming environment (version 4.1.2) using the “meta” software package version 5.2-0. To visualize synthesized data, we used forest plots showing ORs or HRs and corresponding confidence intervals for each individual study and pooled effect sizes in terms of ORs and HRs. For dichotomous outcomes, odds ratios and hazard ratios with 95% confidence intervals (CI) were used as effect measures. To calculate odds ratios, the total number of patients in each study and the number of patients with the event of interest in each group were extracted from each study. Raw data from the selected studies were pooled using a random-effects model with the Mantel-Haenszel method [22,23,24]. The random-effects model was used as we assumed that the true effect would vary between studies due to differences in demographics and clinical measures, such as age or baseline cognitive impairment.
Heterogeneity was assessed by calculating I2, tau2, and the prediction interval. I2 is defined as the percentage of variability in the effect size that is not caused by sampling error, whereas tau2 is the square root of the standard deviation of the true effect size. As I2 is heavily dependent on the precision of the studies and tau2 is sometimes hard to interpret (as it is insensitive to the number of the studies and their precision), the prediction interval has also been calculated. The great advantage of the prediction interval is that this measure is easy to interpret: if the interval does not include zero, further studies are expected to show a similar result.
Sensitivity analysis
We performed outlier detection according to Viechtbauer et al. [25]. A study is considered an outlier if the confidence interval of the study does not overlap with the confidence interval of the pooled effect. The idea behind is to detect effect sizes that differ significantly from the overall effect. As a sensitivity analysis, we repeated the analyses after removing any outliers and then we compared the pooled effects before and after the exclusion, in order to detect if outliers would have a substiantial impact on the overall effect.
Risk of bias assement
The risk of bias was assessed according to the recommendation of the Cochrane Collaboration; using the QUIPS tool [26], two investigators (ZH and YS) independently assessed the quality of the studies, and a third author solved disagreements. Publication bias was examined using the Peter’s regression test [27] and visual inspection of the adjusted Funnel-plots.
Results
Search results
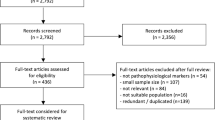
During the systematic search (Fig. 1), 18,162 records were found, and finally, 46 eligible articles were obtained (Supplementary Material eTable 1); While some of the articles analyzed the same cohorts, we were able to pool data from 36 different cohorts or centres. The Cohens’s kappa was 0.91 for the title and abstract, and 0.86 for the full-text selection. Given the amount of data found, we decided to examine the targeted outcomes separately and focus only on the conversion data in this report.
The investigated studies expressed their results in different ways. They calculated unadjusted or adjusted hazard ratios or presented the number of conversions for the different follow-up periods. In the latter case, we calculated odds ratios for the defined time periods. The measured exposures also differed: data were given only for Aβ or in combination with p-tau or neurodegeneration. There were also differences in the techniques used to measure exposure, with CSF sample being used in some cases and PET scan in others.
During data extraction, one [28] article was excluded because of inconsistently reported conversion data, and four [15, 29,30,31] were excluded from the A/T analysis because the definition of the pathologic Aβ and p-tau was based on Aβ/p-tau ratio, which did not comply with the NIA-AA 2018 recommendation.
Data synthesis
The eligible studies investigated three groups: CU, MCI, and mixed - in which the results were collectively expressed for both the MCI and CU groups. The CU group comprised either cognitively healthy subjects or individuals with only subjective cognitive complaints. To define the MCI group, all studies followed the Petersen criteria [32]. Four studies examined mixed groups. Since all of them studied large samples (n>180), it was considered more valuable to jointly analyze them with MCI, since the outcome was also the conversion to dementia. As a result of the joint analysis, our findings are based on a substantially larger sample. To support this decision, we performed a subgroup analysis comparing the Aβ positive MCI and mixed population studies. The OR differed significantly from the unexposed group in both the MCI (OR 5.83 [3.80; 8.93]) and the mixed (4.64 [95% CI 1.16; 18.61]) subgroups, and there was no significant difference between the two subgroups (p=0.55) (Supplementary Material eFigure 1).
Conversion from MCI to dementia
Aβ exposition - in OR
Based on a mixed model meta-analysis of 3,576 subjects (Table 1), we observed a significant association between Aβ positivity and higher conversion rates. Compared to the unexposed, the OR for conversion to dementia in the amyloid positives were 5.18 [95% CI 3.93; 6.81]; t(21)=12.47; (p<0.0001). The I2- test for heterogeneity revealed that 44.8% of the variance across studies was due to heterogeneity (Fig. 2A). As a result of the outlier detection we excluded the Balassa study and found a very similar overall effect and a reduced heterogeneity (5.05 [95% CI 3.98; 6.40]; t(20) = 14.2; p < 0.0001; I2 = 31.4%). Meta-regression analysis of mean age showed a statistically significant decrease in OR values with increasing age (R2 = 59.05%, beta = -0.04, SE = 0.019, [95% CI = -0.03 to -0.083], df = 18, t = -2.27, p = 0.036) (Fig. 2B). The Hartunk-Knapp method was applied to adjust test statistics and confidence intervals to reduce the risk of false positives.
Conversion of Aβ exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A OR for Aβ exposition; B meta-regression of age and ORs for conversion regarding Aβ exposure. The size of the circle is proportional to the weight of each study in the meta-analysis. The line corresponds to meta-regression with age as covariate, and beta represents the slope of ORs by mean age
Beta-amyloid was determined by CSF Aβ42, CSF Aβ42/40 ratio or amyloid PET. When the three groups were compared in a subgroup analysis, the OR was 5.87 (2.83; 12.19) for CSF Aβ42, 5.00 (3.31; 7.55) for CSF Aβ42/40 ratio, and 5.32 (2.53; 11.18) for amyloid PET. The difference between the subgroups was not significant (p=0.88) (Supplementary Material eFigure 2).
The meta-regression analysis performed to examine the role of follow-up time showed no association with respect to the ORs (R2 = 0%, beta = -0.002, SE = 0.07, [95% CI = -0.02 - 0.01], df = 11, p = 0.77) (Supplementary Material eFigure 3A).
We used a funnel plot to examine publication bias (Supplementary Material eFigure 4A). Most of the studies with large sample sizes lie close to the midline, which confirms that the pooled effect size seems valid. However, the visual inspection of the plot raised the possibility of some publication bias in two ways: (1) Studies in the bottom right corner of the plot have significant results despite having large standard errors (2) The absence of studies in the bottom left corner (blank area in the figure) may indicate that studies with nonsignificant results were not published. In order to quantify funnel plot asymmetry, the Peter’s regression test was applied. The test results were not significant (t = 1.7, df = 20, p = 0.11) so no asymmetry was proven in the funnel plot.
The effect of Aβ exposition in terms of HR
Several studies reported their results in HRs instead of or in addition to ORs (Supplementary Material eTable 2). The advantage of the HR value is that this measure is independent of the length of follow-up times of the studies. For these reasons, we also considered it important to analyze the results expressed in HR. Based on pooled data of patients studied (n=1,888), the HR for conversion to dementia was 3.16 [95% CI 2.07; 4.83], p < 0.001 (Fig. 3A).
Conversion of Aβ exposed MCI groups to dementia in HR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A HR for Aβ exposition; B sub-group analysis of studies with adjusted and unadjusted HR values
To investigate the effect of adjustment, we conducted a subgroup analysis between the unadjusted and adjusted measurements. Although there was a trend for higher unadjusted HR values compared to the adjusted HRs, the difference did not reach statistical significance (unadjusted HR : 5.07 [95% CI 2.77 - 9.26], adjusted HR 2.86 [95% CI 1.70 - 4.83] p=0.055) (Fig. 3B). We could not analyze HR in the A+T-, A+T+, and A-T+ subgroups, due to the low number of available studies.
The effect of Aβ and p-tau exposition in terms of OR
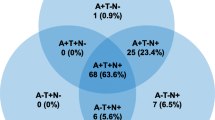
We examined the combined effect of p-tau and Aβ (Table 2), and compared A+T+, A+T-, and A-T+ exposures to A-T-. Based on pooled data for patients studied (n=1,327), the OR for conversion to dementia in A+T- was 2.73 [95% CI 1.65; 4.52], and the odds ratio was significantly higher in the presence of both exposures (A+T+) (p<0.001), with an OR of 11.60 [95% CI 7.96; 16.91]. The effect of A-T+ exposure on conversion was not significant (OR: 1.47 [0.55; 3.92]) (Fig. 4A).
Conversion of Aβ and p-tau exposed MCI groups to dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares’ area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ and p-tau expositions in OR; B sub-group analysis of comparisons between the A+T+ and A+T- groups; C sub-group analysis of comparisons between the A+T- and A-T+ groups
Subgroup analyses showed that the A+T+ group had a significantly higher odds of conversion compared to the A+T- group (p <0.001), while the A+T- and A-T+ groups did not differ significantly (p=0.15) (Fig. 4B and C).
Conversion from CU to MCI or dementia
The effect of Aβ exposition in terms of OR
Analyses on the CU population (n = 4,217) yielded very similar results to the MCI sample. The OR for conversion to MCI or dementia was 5.79 [95% CI 2.88; 11.64] (t(13) = 5.43; p = 0.0001), the results of the studies did however show a high degree of heterogeneity (I2= 73% [55%; 84%]) (Table 3, Fig. 5A). As a result of the outlier detection we removed the Aruda study and found a very similar overall effect (6.33 [95% CI 3.42; 11.71]; t(12) = 6.54; p < 0.0001; I2 = 72.1%).
Conversion of Aβ and p-tau exposed CU groups to MCI or dementia in OR. The squares and bars represent the mean values and 95% CIs of the effect sizes, and the squares' area reflects the weight of the studies. Diamonds represent the combined effects, and the vertical dotted line represents the line of no association. A Aβ exposition in OR. B Aβ and p-tau expositions in OR
Meta-regression analysis of mean age did not show a significant association with OR. (R2 = 8.22%, beta = -0.05, SE = 0.05, [95% CI = -0.17 – 0.7], df = 11, t =, p = 0.37).
Meta-regression analysis also showed no association between follow-up time and ORs (R2 = 0.35%, beta = -0.014, SE = 0.024, [95% CI = -0.07 - 0.04], df = 8, p = 0.58) (Supplementary Material eFigure 3B).
We applied a funnel plot to examine publication bias (Supplementary Material eFigure 4B).Most of the studies with large sample sizes lie close to the midline, which reaffirms the pooled effect size’s validity. In order to quantify funnel plot asymmetry, Peter’s regression test was applied. The test results were not significant (t = 0.9, df = 12, p = 0.31) indicating that no asymmetry was demonstrated in the funnel plot.
The effect of Aβ exposition in terms of HR
Four cohorts provided HRs for the CU population (n=2700) with one cohort (ADNI) representing the 55.3% of the total sample (weight: 78.5%) (Supplementary Material eTable 3). The pooled HR for conversion was 2.33 [95% CI 1.88; 2.88] (p=0.001) (Supplementary Material eFigure 5)
The combined effect of Aβ and p-tau exposition in terms of OR
Using data from a total of 2228 subjects, we investigated the effect of p-tau in combination with Aβ (Table 4) in the CU population. The OR for conversion is 2.04 [95% CI 0.70; 5.97] for A+T-, and 13.46 [95% CI 3.69; 49.11] for the A+T+, compared to the A-T- group The OR shows a trend level increased risk (t=2.1, P=0.12) for the A+T- group compared to the A-T- group.
Similarly to the MCI population, subgroup analyses showed that the A+T+ group had significantly higher OR for conversion compared to the A+T- group (p <0.01). The analysis could not be performed for A-T+ due to the low number of these cases.
Risk of bias assessment
The risk of bias was assessed separately for the analyses discussed above. The overall risk of the studies ranged from low to moderate, except in three cases: twice we found a high risk of bias due to attrition of above 50% [59, 60], and once due to a focus on monozygotic twins [61] (Supplementary Material, eFigure 6). These articles (n=197) were excluded from all analyses.
Discussion
Summary and context
A pathological Aβ state are strongly correlated with the risk of clinical progression. The odds ratio for conversion is 5.18 in the MCI population and 5.79 in the CU population. Therefore, measuring Aβ levels alone can identify a population at high risk. The OR for conversion to dementia differs significantly between the A+T+ and A+T- groups in both the MCI and CU populations: while the OR is 2.73 [95% CI 1.65; 4.52] for MCI and 2.04 [95% CI 0.70; 5.97] for CU subjects in the A+T- group, it increases to 11.60 [95% CI 7.96; 16.91] for MCI and 14.67 [95% CI 3.69; 49.11] for CU in the A+T+ group. Note that in the case of A+T- at CU population, only a trend-level statistical correlation is visible.
The results of the meta-regression show a decrease in OR with mean age (Fig. 2B). Based on this result it seems that the impact of Amyloid positivity on conversion is decreasing with age. The fact that age is a risk factor for dementia and vascular and other neurodegenerative damage are more frequent in elderly age is a possible explanation to this finding. Our findings combined with the results of Rodrigue et al. [62] suggests that amyloid burden increases with age, while its impact on conversion rates slightly decreases with age.
The appearance of Aβ is assumed to be one of the earliest signs of AD [63, 64]. Our results fit into this picture by showing that only the A+T+ and A+T- groups showed an increased risk for conversion compared to A-T-, the A-T+ group did not. Thus, Aβ alone is suitable for detecting the population at risk, while p-tau alone is not as effective in the prediction conversion. Our result is in line with previous studies showing that the A-T+ group has a weaker association with cognitive decline compared to the A+T- or A+T+ groups [65, 66]. However, it is important to emphasize that previous results showing that T+ status is closely associated with neurodegeneration and the A-T+ group is related to frontotemporal dementia [67]. More research is needed to fully explain the significance of the A-T+ group.
The PET scan is known to be a more sensitive tool for detecting Amyloid positivity compared to CSF sampling [68]. However, from a prognostic point of view, our results did not show a significant difference (p=0.73) between PET measurements (OR: 6.02) and the more cost-effective but invasive CSF Aβ42 measurements (OR: 5.11). It is important to note here that the present meta-analysis is underpowered for detecting prognostic differences between these methods. Due to the heterogeneity among studies, the impact of confounding factors, and standardised studies are required to evaluate the comparative prognostic value of these biomarkers accurately.
Our results based on ORs are further strengthened by the HR analyses giving similar results for Aβ exposure in the MCI (HR: 3.16) and CU (HR: 2.33) populations. It should be noted that in the HR analysis of the CU group, ADNI accounts for 78.5% of the weight, which is a limitation of this meta-analysis. This disproportionate representation may affect the overall result. Regarding the statistical trend-level association with a higher unadjusted HR, it should be noted that in the presence of a random distribution of other risk factors (e.g. baseline MMSE score or educational level), the unadjusted value may overestimate the HR. As in the case of a non-random distribution, the adjusted value underestimates the HR. With this in mind, we recommend reporting both values in the future.
Our analyses were performed on CU and MCI populations. Including mixed populations with the MCI population was a practical simplification, as several studies with a large number of cases gave their results combining MCI subjects with CU subjects, and we aimed to answer the set of questions based on the largest population. To investigate the potential bias of this method, we performed subgroup analysis comparing the mixed and MCI populations, and the result was not significant. The Aβ OR based on the mixed-only group is 4.64 [95% CI 1.16; 18.61], and the OR calculated on the MCI-only studies is 5.83 [95% CI 3.80; 8.93]. Thus, the inclusion of the mixed population in the pool decreases the OR of the main analysis (5.21 [95% CI 3.93; 6.90]) slightly (Supplementary Material eFigure 1).
Strengths and limitations
There are several limitations to consider when interpreting our results. The study populations differ in several aspects; for cognitive status, the population ranges from those with no cognitive symptoms through those with subjective cognitive symptoms (these two groups were considered CU) to MCI groups. Therefore, the distance from the cognitive state corresponding to MCI or dementia also varies. Due to the different cut-offs used in the studies, subjects with grey area scores may oscillate between A- and A+ groups, increasing heterogeneity. Our study could not examine the role of other risk factors such as education, cardiovascular status, obesity, diabetes, depression, social and physical activity [69], or genetic status [70, 71], which may also contribute to heterogeneity. Furthermore, there is a considerable heterogeneity by mean age, and our meta-regression analysis of MCI group showed a significant decreasing effect of mean age on ORs.
In the OR analysis of Aβ in the CU group, in the context of the outlier value of the Arruda study, the possibility of a statistical extreme value can be assumed due to the small number of A+ subjects and the much larger A- group. Similarly, in the case of the Grontvedt [14] and Hanseeuw [41] studies, which show exceptionally high values, the A+ and A- groups show a similar uneven distribution. Similarly, the outliers in the MCI amyloid OR analysis are also associated with small sample sizes. For the Aβ HR analysis in the CU group, the interpretability of the result is strongly influenced by one specific cohort (ADNI), which accounts for 78% of the overall weight. In the A+T+/A+T-/A-T+ analyses, no outliers were found in either the MCI or CU groups.
Furthermore, we note that although the Aβ OR analyses could be confirmed by also calculating the HRs, the inability to analyze the effect of p-tau on HR due to the low number of studies limits the completeness of the A/T analysis.
We pooled studies reporting AD-type dementia conversion and studies reporting conversion to unspecified dementia. This simplification was necessary because different studies defined Alzheimer’s dementia differently, generally considering the amnestic clinical symptoms rather than biomarkers.
The fact that the studies used different neuropsychology tests to define MCI may contribute to the heterogeneity in the pooled sample. Another contributing factor would be the heterogeneity in the definition of MCI, however among the studies in our pool, only one, by Riemschneider et al. [48] (sample size = 28), precedes the 2003 ‘Key Symposium’ [72] that transformed the MCI concept. All other studies were published subsequent to it. While MCI subgroups were deifned after the 2003 Symposium, the definition of MCI (objective cognitive impairment, essentially preserved general cognitive functioning, preserved independence in functional abilities) did not change afterwards. Furthermore, most of the studies pooled in the analyses were published after 2010.
Another source of heterogeneity is the relatively small sample size of some studies, leading to a higher variability of results. However, we thought that including studies with lower sample sizes was also important to get a complete picture.
It is essential to discuss the difference in the follow-up times between studies. The follow-up times ranged from 20 months to more than 10 years. Follow-up times were given in different ways, either as mean, median or up to a certain point. While naturally, the odds of conversion increase over time, our meta-regression analysis suggests that there is no significant difference in the odds ratios over (follow-up) time. The moderate heterogeneity of the studies also points in this direction. We also note here that hazard ratios independent of follow-up time showed similar results to OR analyses. Finally, yet importantly, we would like to point out that pathological protein changes can begin up to 20 years before the appearance of symptoms [6]. Such an extended follow-up is very difficult to carry out; therefore, all studies were shorter than that.
The results for Aβ are based on 7,793 individuals, and the combined analyses of Aβ and p-tau are based on data of over 3,500 individuals. Studies using CSF sampling or amyloid/tau PET to detect Aβ and p-tau were pooled together, despite using different kits and thresholds for positivity, contributing to the heterogeneity of results. This variation is acknowledged in Tables 1, 2, 3 and 4, where the cut-off values are provided. Previous large population studies have indicated that amyloid and tau PET scans exhibit slightly higher sensitivity compared to CSF sampling techniques [73, 74, 68]. Nonetheless, the concordance between these diagnostic methods remains substantial. Moreover, findings from prior research (Lee et al. [75], Toledo et al. [76], Palmqvist et al. [77]) demonstrating high concordance across different amyloid CSF and amyloid PET measurements suggest that the impact of methodological differences on heterogeneity may be limited, All techniques are recommended by the National Institute on Aging-Alzheimer’s Association (NIA-AA) [6] for measurement.
Future directions
Conversion to Alzheimer’s disease could not be analyzed specifically, as most of the articles examining conversion either did not define Alzheimer’s disease or the definition was based on neuropsychological testing but not on biomarkers (i.e., Aβ and p-tau status were assessed only at baseline). According to the NIA-AA guideline [6] and our results, we recommend biomarker-based studies to assess conversion rates to Alzheimer’s disease.
Conclusions
In view of the Aβ and p-tau status, the most endangered population can be identified before the appearance of cognitive symptoms or at least at a mild stage. While the significance of Aβ in conversion is clear, it appears that its ability to predict the onset decreases with age. If we consider the current therapeutic limitations and the importance of early prevention, we believe that the initiation of non-pharmacological and pharmacological treatments should be related to Aβ and p-tau status rather than cognitive status.
Identifying the most endangered population also makes research more effective. The efficacy of different dementia prevention approaches can be more accurately assessed by knowing the Aβ and p-tau status of the patient. As the population targeted by the interventions can be more homogeneous, the effectiveness can be measured more precisely by identifying the population most at risk of conversion.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A-:
-
Non-pathologic levels of beta-amyloid
- A+:
-
Pathologic levels of beta-amyloid
- Aβ:
-
Beta-amyloid
- AD:
-
Alzheimer’s disease
- ADNI:
-
Alzheimer’s Disease Neuroimaging Initiative
- CI:
-
Confidance interval
- CU:
-
Cognitively unimpaired
- CSF:
-
Cerebrospinal fluid
- HR:
-
Hazard ratio
- MCI:
-
Mild cognitive impairment
- N-:
-
Absence of neurodegeneration
- N+:
-
Presence of neurodegeneration
- NIA-AA: :
-
National Institute on Aging Alzheimer’s Association
- OR:
-
Odds ratio
- PET:
-
Positron emission tomography
- p-tau:
-
Phosphorylated tau
- T-:
-
Non-pathologic levels of phosphorylated tau
- T+:
-
Pathologic levels of phosphorylated tau
References
Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization; 2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542796/.
Gauthier S, Rosa-Neto P, Morais JA, & Webster C. 2021. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer’s Disease International.
De Strooper B. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–15. https://doi.org/10.1016/j.cell.2015.12.056.
Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. The Lancet. 2021;397(10284):1577–90. https://doi.org/10.1016/s0140-6736(20)32205-4.
Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the international working group. Lancet Neurol. 2021;20(6):484–96. https://doi.org/10.1016/s1474-4422(21)00066-1.
Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research framework: toward a biological definition of alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. 1984;34(7):939–44. https://doi.org/10.1212/wnl.34.7.939.
McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–83. https://doi.org/10.1016/j.neurobiolaging.2010.04.007.
Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes. JAMA. 2015;313(19):1939. https://doi.org/10.1001/jama.2015.4669.
Morris GP, Clark IA, Vissel B. Questions concerning the role of amyloid-β in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol. 2018;136(5):663–89. https://doi.org/10.1007/s00401-018-1918-8.
Van Der Flier WM, Scheltens P. The ATN framework—moving preclinical Alzheimer disease to clinical relevance. JAMA Neurology. 2022;79(10):968. https://doi.org/10.1001/jamaneurol.2022.2967.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. https://doi.org/10.1001/archneur.56.3.303.
Grøntvedt GR, Lauridsen C, Berge G, et al. The amyloid, tau, and neurodegeneration (A/T/N) classification applied to a clinical research cohort with long-term follow-up. J Alzheimers Dis. 2020;74(3):829–37. https://doi.org/10.3233/jad-191227.
Balasa M, Sánchez-Valle R, Antonell A, et al. Usefulness of biomarkers in the diagnosis and prognosis of early-onset cognitive impairment. J Alzheimer’s Di. 2014;40(4):919–27. https://doi.org/10.3233/JAD-132195.
Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313(19):1924–38. https://doi.org/10.1001/jama.2015.4668.
Page MJ, McKenzie JE, Bossuyt PM, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021: n71. https://doi.org/10.1136/bmj.n71.
Weiner MW. Alzheimer’s disease neuroimaging initiative. Available from: https://adni.loni.usc.edu/.
Aydin O, Yassikaya MY. Validity and reliability analysis of the plotdigitizer software program for data extraction from single-case graphs. Perspect Behav Sci. 2022;45(1):239–57. https://doi.org/10.1007/s40614-021-00284-0.
Huwaldt, J. A., & Steinhorst, S. (2020). Plot digitizer 2.6.9.PlotDigitizer-Software. http://plotdigitizer.sourceforge.net/.
Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal Fluid Aβ42/40 Corresponds better than Aβ42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis. 2017;55(2):813–22. https://doi.org/10.3233/jad-160722.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–48.
Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124(5):719–23. https://doi.org/10.1093/oxfordjournals.aje.a114447.
Thompson SG, Turner RM, Warn DE. Multilevel models for meta-analysis, and their application to absolute risk differences. Stat Methods Med Res. 2001;10(6):375–92. https://doi.org/10.1177/096228020101000602.
Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–25. https://doi.org/10.1002/jrsm.11.
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295(6):676–80. https://doi.org/10.1001/jama.295.6.676.
Kemppainen NM, Scheinin NM, Koivunen J, et al. Five-year follow-up of 11C-PIB uptake in Alzheimer’s disease and MCI. Eur J Nucl Med Mol Imaging. 2014;41(2):283–9. https://doi.org/10.1007/s00259-013-2562-0.
Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1–42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98–106. https://doi.org/10.1001/archgenpsychiatry.2011.155.
Forlenza OV, Radanovic M, Talib LL, et al. Cerebrospinal fluid biomarkers in Alzheimer’s disease: diagnostic accuracy and prediction of dementia. Alzheimers Dement (Amst). 2015;1(4):455–63. https://doi.org/10.1016/j.dadm.2015.09.003.
Hansson O, Buchhave P, Zetterberg H, Blennow K, Minthon L, Warkentin S. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2009;30(2):165–73. https://doi.org/10.1016/j.neurobiolaging.2007.06.009.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. https://doi.org/10.1111/j.1365-2796.2004.01388.x.
Arruda F, Rosselli M, Mejia Kurasz A, et al. Stability in cognitive classification as a function of severity of impairment and ethnicity: a longitudinal analysis. Article in Press. Appl Neuropsychol Adult. 2023:1-14. https://doi.org/10.1080/23279095.2023.2222861.
Baldeiras I, Silva-Spínola A, Lima M, et al. Alzheimer’s disease diagnosis based on the amyloid, tau, and neurodegeneration scheme (ATN) in a real-life multicenter cohort of general neurological centers. J Alzheimer’s Dis. 2022;90(1):419–32. https://doi.org/10.3233/JAD-220587.
Bos I, Verhey FR, Ramakers I, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther. 2017;9(1):101. https://doi.org/10.1186/s13195-017-0328-9.
Cerami C, Della Rosa PA, Magnani G, et al. Brain metabolic maps in Mild cognitive impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2015;7:187–94. https://doi.org/10.1016/j.nicl.2014.12.004.
de Wilde A, Reimand J, Teunissen CE, et al. Discordant amyloid-β PET and CSF biomarkers and its clinical consequences. Alzheimers Res Ther. 2019;11(1):78. https://doi.org/10.1186/s13195-019-0532-x.
Eckerström C, Svensson J, Kettunen P, Jonsson M, Eckerström M. Evaluation of the ATN model in a longitudinal memory clinic sample with different underlying disorders. Alzheimers Dement (Amst). 2021;13(1): e12031. https://doi.org/10.1002/dad2.12031.
Frölich L, Peters O, Lewczuk P, et al. Incremental value of biomarker combinations to predict progression of mild cognitive impairment to Alzheimer’s dementia. Alzheimers Res Ther. 2017;9(1):84. https://doi.org/10.1186/s13195-017-0301-7.
Groot C, Cicognola C, Bali D, et al. Diagnostic and prognostic performance to detect alzheimer’s disease and clinical progression of a novel assay for plasma p-tau217. Article Alzheimer’s Res Ther. 2022;14(1):67. https://doi.org/10.1186/s13195-022-01005-8.
Hanseeuw BJ, Malotaux V, Dricot L, et al. Defining a Centiloid scale threshold predicting long-term progression to dementia in patients attending the memory clinic: an [(18)F] flutemetamol amyloid PET study. Eur J Nucl Med Mol Imaging. 2021;48(1):302–10. https://doi.org/10.1007/s00259-020-04942-4.
Herukka SK, Hallikainen M, Soininen H, Pirttilä T. CSF Aβ42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Article Neurology. 2005;64(7):1294–7. https://doi.org/10.1212/01.WNL.0000156914.16988.56.
Jiménez-Bonilla JF, Quirce R, De Arcocha-Torres M, et al. A 5-year longitudinal evaluation in patients with mild cognitive impairment by 11C-PIB PET/CT: a visual analysis. Nucl Med Commun. 2019;40(5):525–31. https://doi.org/10.1097/mnm.0000000000001004.
Lopez OL, Becker JT, Chang Y, et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology. 2018;90(21):E1920–8. https://doi.org/10.1212/WNL.0000000000005549.
Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–60. https://doi.org/10.1212/WNL.0b013e3181b23564.
Orellana A, García-González P, Valero S, et al. Establishing in-house cutoffs of CSF Alzheimer’s disease biomarkers for the AT(N) stratification of the Alzheimer center barcelona cohort. Int J Mol Sci. 2022;23(13):6891. https://doi.org/10.3390/ijms23136891.
Ortega RL, Dakterzada F, Arias A, et al. Usefulness of CSF biomarkers in predicting the progression of amnesic and nonamnesic mild cognitive impairment to Alzheimer’s disease. Curr Aging Sci. 2019;12(1):35–42. https://doi.org/10.2174/1874609812666190112095430.
Riemenschneider M, Lautenschlager N, Wagenpfeil S, Diehl J, Drzezga A, Kurz A. Cerebrospinal fluid tau and beta-amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol. 2002;59(11):1729–34. https://doi.org/10.1001/archneur.59.11.1729.
Rizzi L, Missiaggia L, Schwartz IVD, Roriz-Cruz M. Value of CSF biomarkers in predicting risk of progression from aMCI to ADD in a 5-year follow-up cohort. SN Compr Clin Med. 2020;2(9):1543–50. https://doi.org/10.1007/s42399-020-00437-3.
Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and Outcomes of amyloid positivity among persons without dementia in a longitudinal population-based setting. JAMA Neurol. 2018;75(8):970–9. https://doi.org/10.1001/jamaneurol.2018.0629.
Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–92. https://doi.org/10.1002/ana.22248.
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. https://doi.org/10.1016/s1474-4422(06)70355-6.
Dang C, Harrington KD, Lim YY, et al. Relationship Between amyloid-β positivity and progression to mild cognitive impairment or dementia over 8 years in cognitively normal older adults. J Alzheimers Dis. 2018;65(4):1313–25. https://doi.org/10.3233/jad-180507.
Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline: The SCIENCe project. Neurology. 2020;95(1):e46–58. https://doi.org/10.1212/wnl.0000000000009724.
Hatashita S, Wakebe D. Amyloid β deposition and glucose metabolism on the long-term progression of preclinical Alzheimer’s disease. Future Sci OA. 2019;5(3):Fso356. https://doi.org/10.4155/fsoa-2018-0069.
Ossenkoppele R, Pichet Binette A, Groot C, et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nature Medicine. 2022;28(11):2381–7. https://doi.org/10.1038/s41591-022-02049-x.
Strikwerda-Brown C, Hobbs DA, Gonneaud J, et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurology. 2022;79(10):975. https://doi.org/10.1001/jamaneurol.2022.2379.
Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–65. https://doi.org/10.1016/s1474-4422(13)70194-7.
Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer’s disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dement Geriatr Cogn Disord. 2009;27(5):458–64. https://doi.org/10.1159/000216841.
Hong YJ, Park JW, Lee SB, et al. The influence of amyloid burden on cognitive decline over 2 years in older adults with subjective cognitive decline: a prospective cohort study. Dement Geriatr Cogn Disord. 2021;50(5):437–45. https://doi.org/10.1159/000519766.
Tomassen J, den Braber A, van der Landen SM, et al. Abnormal cerebrospinal fluid levels of amyloid and tau are associated with cognitive decline over time in cognitively normal older adults: A monozygotic twin study. Alzheimers Dement (N Y). 2022;8(1): e12346. https://doi.org/10.1002/trc2.12346.
Rodrigue KM, Kennedy KM, Devous MD Sr, et al. β-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–95. https://doi.org/10.1212/WNL.0b013e318245d295.
Donohue MC, Jacqmin-Gadda H, Le Goff M, et al. Estimating long-term multivariate progression from short-term data. Alzheimers Dement. 2014;10(5 Suppl):S400–10. https://doi.org/10.1016/j.jalz.2013.10.003.
Young AL, Oxtoby NP, Daga P, et al. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain. 2014;137(Pt 9):2564–77. https://doi.org/10.1093/brain/awu176.
Oberstein TJ, Schmidt MA, Florvaag A, et al. Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain. 2022;145(11):4032–41. https://doi.org/10.1093/brain/awac297.
Wisse LEM, Butala N, Das SR, et al. Suspected non-AD pathology in mild cognitive impairment. Neurobiol Aging. 2015;36(12):3152–62. https://doi.org/10.1016/j.neurobiolaging.2015.08.029.
Pouclet-Courtemanche H, Nguyen TB, Skrobala E, et al. Frontotemporal dementia is the leading cause of “true” A-/T+ profiles defined with Aβ(42/40) ratio. Alzheimers Dement (Amst). 2019;11:161–9. https://doi.org/10.1016/j.dadm.2019.01.001.
Vos SJB, Gordon BA, Su Y, et al. NIA-AA staging of preclinical Alzheimer disease: discordance and concordance of CSF and imaging biomarkers. Neurobiol Aging. 2016;44:1–8. https://doi.org/10.1016/j.neurobiolaging.2016.03.025.
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. https://doi.org/10.1016/s0140-6736(20)30367-6.
Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–7. https://doi.org/10.1001/jama.2019.9879.
Licher S, Ahmad S, Karamujić-Čomić H, et al. Genetic predisposition, modifiable-risk-factor profile and long-term dementia risk in the general population. Nat Med. 2019;25(9):1364–9. https://doi.org/10.1038/s41591-019-0547-7.
Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J Intern Med. 2004;256(3):240–6. https://doi.org/10.1111/j.1365-2796.2004.01380.x.
La Joie R, Bejanin A, Fagan AM, et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–90. https://doi.org/10.1212/wnl.0000000000004860.
Wolters EE, Ossenkoppele R, Verfaillie SCJ, et al. Regional [(18)F]flortaucipir PET is more closely associated with disease severity than CSF p-tau in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2020;47(12):2866–78. https://doi.org/10.1007/s00259-020-04758-2.
Lee J, Jang H, Kang SH, et al. Cerebrospinal fluid biomarkers for the diagnosis and classification of Alzheimer’s disease spectrum. J Korean Med Sci. 2020;35(44):361. https://doi.org/10.3346/jkms.2020.35.e361.
Toledo JB, Brettschneider J, Grossman M, et al. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124(1):23–35. https://doi.org/10.1007/s00401-012-0983-7.
Palmqvist S, Zetterberg H, Mattsson N, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85(14):1240–9. https://doi.org/10.1212/wnl.0000000000001991.
Acknowledgements
Not applicable.
Funding
Open access funding provided by Semmelweis University. 1. Supported by the GINOP-2.3.4-15-2020-00008 project. The project is co-financed by the European Union and the European Regional Development Fund.
2. This is an EU Joint Programme- Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organization under the aegis of JPND - www.jpnd.eu (National Research, Development and Innovation, Hungary, 2019-2.1.7-ERA-NET-2020-00006).
3. Supported by the National Research, Development and Innovation Office (NKFI/OTKA FK 138385).
Role of funding source: The sponsor(s), did not participate in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
ZH: conceptualisation, project administration, methodology, formal analysis, writing – original draft; ME: conceptualisation, methodology, formal analysis, writing – review and editing; MP: conceptualisation, formal analysis, writing - review and editing; TS formal analysis, writing – review and editing; YS: formal analysis, writing – review and editing; BH: writing - review and editing; TT: conceptualisation, writing – review and editing; ZM: conceptualisation, supervision, writing - review and editing; PH: conceptualisation, supervision, writing - review and editing; GCs: conceptualization, methodology, formal analysis, supervision, writing – original draft, visualization. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huszár, Z., Engh, M., Pavlekovics, M. et al. Risk of conversion to mild cognitive impairment or dementia among subjects with amyloid and tau pathology: a systematic review and meta-analysis. Alz Res Therapy 16, 81 (2024). https://doi.org/10.1186/s13195-024-01455-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01455-2