Abstract
Background
The genus Sanicula L. is a unique perennial herb that holds important medicinal values. Although the previous studies on Sanicula provided us with a good research basis, its taxonomic system and interspecific relationships have not been satisfactorily resolved, especially for those endemic to China. Moreover, the evolutionary history of this genus also remains inadequately understood. The plastid genomes possessing highly conserved structure and limited evolutionary rate have proved to be an effective tool for studying plant phylogeny and evolution.
Results
In the current study, we newly sequenced and assembled fifteen Sanicula complete plastomes. Combined with two previously reported plastomes, we performed comprehensively plastid phylogenomics analyses to gain novel insights into the evolutionary history of this genus. The comparative results indicated that the seventeen plastomes exhibited a high degree of conservation and similarity in terms of their structure, size, GC content, gene order, IR borders, codon bias patterns and SSRs profiles. Such as all of them displayed a typical quadripartite structure, including a large single copy region (LSC: 85,074–86,197 bp), a small single copy region (SSC: 17,047–17,132 bp) separated by a pair of inverted repeat regions (IRs: 26,176–26,334 bp). And the seventeen plastomes had similar IR boundaries and the adjacent genes were identical. The rps19 gene was located at the junction of the LSC/IRa, the IRa/SSC junction region was located between the trnN gene and ndhF gene, the ycf1 gene appeared in the SSC/IRb junction and the IRb/LSC boundary was located between rpl12 gene and trnH gene. Twelve specific mutation hotspots (atpF, cemA, accD, rpl22, rbcL, matK, ycf1, trnH-psbA, ycf4-cemA, rbcL-accD, trnE-trnT and trnG-trnR) were identified that can serve as potential DNA barcodes for species identification within the genus Sanicula. Furthermore, the plastomes data and Internal Transcribed Spacer (ITS) sequences were performed to reconstruct the phylogeny of Sanicula. Although the tree topologies of them were incongruent, both provided strong evidence supporting the monophyly of Saniculoideae and Apioideae. In addition, the sister groups between Saniculoideae and Apioideae were strongly suggested. The Sanicula species involved in this study were clustered into a clade, and the Eryngium species were also clustered together. However, it was clearly observed that the sections of Sanicula involved in the current study were not respectively recovered as monophyletic group. Molecular dating analysis explored that the origin of this genus was occurred during the late Eocene period, approximately 37.84 Ma (95% HPD: 20.33–52.21 Ma) years ago and the diversification of the genus was occurred in early Miocene 18.38 Ma (95% HPD: 10.68–25.28 Ma).
Conclusion
The plastome-based tree and ITS-based tree generated incongruences, which may be attributed to the event of hybridization/introgression, incomplete lineage sorting (ILS) and chloroplast capture. Our study highlighted the power of plastome data to significantly improve the phylogenetic supports and resolutions, and to efficiently explore the evolutionary history of this genus. Molecular dating analysis explored that the diversification of the genus occurred in the early Miocene, which was largely influenced by the prevalence of the East Asian monsoon and the uplift of the Hengduan Mountains (HDM). In summary, our study provides novel insights into the plastome evolution, phylogenetic relationships, taxonomic framework and evolution of genus Sanicula.
Similar content being viewed by others
Background
Sanicula L. is a unique genus of perennial woodland herbs belonging to Apiaceae subfamily Saniculoideae [1]. The genus consists of about forty species worldwide that are widely distributed in Asia, North America, Europe and Africa [1,2,3,4], with two distribution centers: China and North America. The relatively widespread distribution of this genus is mainly due to the hooked prickles on the fruit that can stick to animals, which facilitate the spread of these species to further areas [5].
The genus, known as black snakeroot or sanicle, was established by Linnaeus in 1753 with the type species S. europaea L [1, 6]. The main morphological features of its members are characterized by spiny or bristly simple leaves, simple (rarely compound) umbels or heads, showy bracts, conspicuously prominent and persistent calyx, two persistent styles and fruits covered with scales, bristles, or hooked prickles, which can easily distinguish them from other genera of the Apiaceae [1, 7]. However, the genus always exhibits great variability in habit, foliage, flowers and fruits [8], thus some researchers were prone to divide the genus into more smaller classification units. For example, De Candolle separated the genus into two sections: Sanicla DC. and Sanicoria DC. [9, 10] relied on leaf and fruit morphology. However, Drude divided the genus into three subgenera (Sanicula L., Sanicoria DC. and Erythrosana Drude) and seven sections (Rosiflorae Drude, Flaviflorae Drude, Orthospermae Drude, Camplospermae Drude, Pinnatae Drude, Tuberculatae Drude and Tuberosae Drude) based on the petal color, seed surface shape and fruit prickles [11]. On the basis of Drude, Wolff divided the genus into ten sections [12]. Later, Shan and Constance proposed a completely revised treatment for this genus, who reduced the four sections of Wolff’s classification to the synonymy taxonomy in sect. Sanicoria DC and formed five sections (Tuberculatae Drude, Pseudopetagnia H.wolff, Sanicula DC., Sandwicenses Shan and Constance, Sanicoria DC.) based on differences in habit, flower, fruit and distribution [8]. Therefore, it can be seen that the classification system of genus Sanicula has long been disputed. In addition, species delimitation of this genus was also blurred, which was also mainly caused by the high variability of morphological characteristics. For example, S. orthacantha var. stolonifera Shan & S.L.Liou has long rhizomes with distinct nodes, so it was considered to be a variety of S. orthacantha S. Moore [13]. Li et al. observed that S. pengshuiensis M. L. Sheh & Z. Y. Liu was morphologically similar to S. lamelligera Wolff ex Kretsch, and thus treated it as a synonymy of the latter [14]. From previous studies, it can be seen that the traditional methods of distinguishing these species were mainly focused on their morphological features, whereas many species of the genus have always shown great morphological instability, which have lead to extreme difficulties in species classification and delimitation [8,9,10,11,12]. Therefore, more evidence are needed to re-evaluate the taxonomic system of the genus.
A robust phylogenetic framework can provide strong evidence for the taxonomy of the genus Sanicula. Hence, some molecular markers have been applied to the phylogenetic studies of this genus, including single or multiple-locus DNA sequence data (for example ITS sequences, plastid DNA rpl16, rps16 intron and rpoC1 intron, trrnQ-trn K 5’-exon). Among these studies, Vargas P et al. observed that the sect. Sanicoria was not a monophyletic group and suggested that the circumscription of sect. Sanicoria should be enlarged to include the species of sect. Sandwicenses [5]. Valiejo et al. subsequently elucidated the relationships among the main genera of Saniculoidea based on nrITS sequences and found that sec. Pseudopetagnia and sect. Sanicula were also not monophyletic group [15]. In addition, there was much controversy about the interspecific relationships of the genus. For example, Vargas P et al. thought S. orthacantha was more closely related to S. lamelligera [5], while Yang et al. considered S. orthacantha had affinity to S. chinese [16]. However, these phylogenetic trees generated from DNA fragments had weak supports and low resolutions, and failed to provide sufficient information to support the improvement of taxonomy for Sanicula [5, 7, 17,18,19]. The sectional taxonomy system and the interspecific relationships of genus Sanicula are facing a severe challenge. Therefore, additional molecular resources are urgently needed to reconstruct a strongly robust phylogeny of genus Sanicula and re-evaluate the sectional taxonomy and interspecific relationships of the genus.
Besides, the evolutionary history of Sanicula is still poorly understood. Kadereit et al. proposed that the genus originated in the Miocene period of the Tertiary (15.5–6.0 Ma) based on the ITS sequences and plastid fragment rps16, combined with molecular clock [19]. Wen et al. indicated the origin of the genus was occurred at 16.54 Ma (95% HPD: 8.06–27.67 Ma) referred from 79 CDS data [20]. Vargas P et al. estimated the divergence time of sect. Sanicoria and sect. Sandwicenses relied on nrDNA sequences [5]. Although previous studies have laid the foundation for exploring the evolutionary history of the genus, the results obtained may be unreliable due to limited sampling and DNA fragments resulting in low supports and resolutions of the phylogenetic trees. Thus, a reliable phylogenetic framework and time tree are urgently needed to further investigate the evolutionary history of the genus based on expanded sampling.
Additionally, many species of Sanicula are valuable traditional herbs [21]. For example, S. lamelligera, known as “Fei Jingcao”, is a well-known traditional Chinese medicine that is widely used to treat cold, cough, tracheitis, bruises and amenorrhea [13, 22]. Other species such as S. orthacantha S. Moore., S. orthacantha var. brevispina de Boiss., S. caerulescens Franch., S. chinensis Bunge., S. elata Hamilt., and S. astrantiifolia Wolff ex Kretsch. are also Chinese medicinal herbs with the effects of resolving phlegm and cough, activating blood circulation and removing blood stasis [13]. Nevertheless, misuse of species names occurred frequently due to the abundant morphological variations within species, such as S. chinensis and S. orthacantha, as well as S. caerulescens and S. lamelligera [23], which made it difficult to identify species accurately. Hence, it is necessary to develop more efficient and specific DNA barcodes for Sanicula species authentication to ensure medicinal quality.
Plastid is a crucial and special multifunctional organelle involved in processes such as photosynthesis, carbon fixation and numerous major biological metabolic processes [24, 25]. The plastid genome (plastome) of flower plants ranges in size from 120 kb to 170 kb and displays a typical quadripartite structure, comprising a large single copy (LSC: 82–90 kb), a small single copy (SSC: 15–20 kb) and a pair of inverted repeats (IRa & IRb: 22–25 kb) [26]. It always encodes 110–130 unique genes, including about 80 protein-coding genes (PCGs), 30 transfer RNA (tRNAs) genes and four ribosomal RNA (rRNAs) genes [27]. The plastome is highly conserved in terms of its gene structure, sequence, content, order compared to nuclear and mitochondrial genomes [28, 29]. Moreover, the plastome is uniparental inheritance and possesses highly variable sites [30]. For these obvious advantages, the plastome has become a powerful tool to generate highly supported and resolved phylogenies and to explore more efficient specific DNA barcodes [25, 27, 28, 31,32,33]. In recent years, with the rapid development of next-generation sequencing technology and bioinformatics technology, a large number of sequence data of plastomes has become more accessible at a much lower cost [34], and it has been extensively and successfully applied to solve the plants phylogenies, especially for those taxonomically difficult taxa within the family Apiaceae [30,31,32, 35,36,37,38,39,40].
Plastomes also opened a promising window for disclosure of the genus Sanicula. Currently, although there were related studies on the plastomes of genus Sanicula, all of them only reported the Sanicula plastome structure or conducted a simple analyses, which failed to address the phylogenetic relationships of the genus [16, 41,42,43]. In the current study, we obtained seventeen plastomes of Sanicula species (fifteen newly sequenced and two previously reported) and performed comprehensively plastid phylogenomics analyses of this taxonomically difficult genus. Since the plastid genome is maternally inherited, it does not fully reveal the evolutionary history of the taxon, while ITS sequence is biparentally inherited and represents another different genetic pattern [30]. Therefore, to obtain a comprehensive understanding of phylogenetic relationships, we also used ITS sequence to construct the phylogenetic tree. Our major aims were to: (1) characterize the plastomes of Sanicula plants and select highly variable hotspot regions as candidate DNA barcodes for species authentication of Sanicula; (2) evaluate the potential of plastome for resolving the phylogeny of genus Sanicula and re-evaluate the section-level classification system and interspecific relationships; (3) investigate the evolutionary history of the genus.
Results
Basic characteristics of Sanicula plastomes
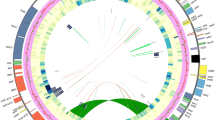
The complete plastome size of the seventeen Sanicula taxa ranged from 154,500 bp (S. odorata (Raf.) Pryer & Phillippe.) to 155,847 bp (S. lamelligera) (Table 1). All of them exhibited a typical quadripartite structure, including a large single copy region (LSC: 85,074–86,197 bp), a small single copy region (SSC: 17,047–17,132 bp) separated by a pair of inverted repeat regions (IRs: 26,176–26,334 bp) (Fig. 1; Table 1). The total GC content was account for 38.20%, except for S. astrantiifolia (38.10%); the IR regions had the highest GC content (42.9% – 43.0%) and the SSC region had the lowest GC content (32.4%– 32.6%). Four rRNAs (rrn16, rrn23, rrn4.5 and rrn5) genes possessed the same GC content (55.3%) among seventeen plastomes. All platomes encoded 113 unique genes, including 79 PCGs, 30 tRNA genes and 4 rRNA genes (Table 1) and these unique genes had four categories: Self-replication, Genes for photosynthesis, Other genes and Genes of unknown function. Among them, 46 coding genes were related to photosynthesis, which were divided into six group: Subunits of photosystem I, Subunits of photosystem II, Subunits of cytochrome, Subunits of ATP synthase, Large subunit of Rubisco and Subunits of NADH dehydrogenase. Seventeen genes contained intron and fourteen of them (ndhB, trnA, ndhA, trnI, rpl2, rpl16, petB, trnV, trnL, rpoC1, atpF, trnG, rps16 and trnK) harbored one intron and three of them (clpP, rps12 and ycf3) harbored two introns (Table S1).
Whole plastome maps of seventeen Sanicula species. Genes showed outside of outward layer circle are transcribed clockwise, while those insides are transcribed counterclockwise. The genes belonging to different functional groups are color-coded. The pale gray area of the inner circle denotes the GC content of plastome
Codon usage bias and relative synonymous codon usage (RSCU) analysis
In order to analyze the codon usage of Sanicula plastomes, we screened the CDSs with the length more than 300 bp and finally attained 53 CDSs. We identified 21,162–21,202 codons in these seventeen plastomes, which have similar codon usage bias. The Leu had the highest number of codons (2,210–2,240) and it was encoded by UUA, UUG, CUU, CUC, CUA and CUG. Ser was the second most abundant amino and it was encoded by AUU, AUC and AUA, while the Cys had the least codons (217–265) with UGU and UGC encoded except for TER (Table S2). Among these 64 codons, the codon AUU occurred the most and the frequency was reached to 850–874, while UGC was the least (51–82 occurrences) except three terminator codons (Fig. 2, Table S2). The most frequent terminator codons was UAA with 29–52 occurrences, while the other two were UAG (15–17) and UGA (14–49), respectively. The RSCU values of the 64 codons unchanged significantly and showed similar codon preferences with values ranging from 0.34 to 1.91 (Fig. 2). Notably, the RSCU values for thirty codons was greater than 1.00 and six codons (UUA, UCU, ACU, GCU, UAU and AGA) were regarded as “overrepresented” codons with RSCU values greater than 1.6 except for GAU. The remainging 32 codons had RSCU values less than 1, and fifteen of them (CUC, CUG, GUC, AGC, ACG, GCG, UAC, CAG, AAC, GAC, GAG, UGC, UGC, CGG and GGC) were considered to be “underrepresented” codons with RSCU values less than 0.6. Two codons (GCU and GUC) displayed no preferences (RSCU = 1). All codons with RSCU > 1.00 ended with an A or a U, except for UUG codon (Fig. 2).
Repeat sequences and simple sequence repeats (SSRs) analysis
In this study, we detected 782 repeat sequences in seventeen plastomes and classified them into four types: forward repeats, palindromic repeats, reverse repeats, and complementary repeats. Among them, the most frequent repeats was the palindromic repeats (390), compared to other three types of repeats: forward repeats (376), reverse repeats (13) and complementary repeats (3) (Fig. 3A, Table S3). The species – S. rugulosa Diels. – had the least repeats (42), while three species (S. lamelligera, S. serrata H. Wolff., S. tienmuensis Shan & Constance.) had the most repeats (49). Forward and palindromic repeats were present in seventeen plastomes, and the complementary repeats only occurred in S. lamelligera and S. odorata. Nine Sanicula plastomes had reverse repeats. (Fig. 3A, Table S3).
In addition, we identified 979 SSRs in seventeen plastomes (Table S3). Among these SSRs, the mono-repeats were the most abundant (460, 46.99%), followed by di-repeats 365 (37.28%), tetra-repeats 111 (11.34%), tri-repeats 38 (3.88%), hexanucleotide repeats 3 (0.31%) and penta-repeatswere 2 (0.2%). All plastomes had the mono-repeats, di-repeats, tri-repeats and tetra-repeats (Fig. 3B). The penta-repeats and hexanucleotides were very rare, with penta-repeats (AAAAT/ATTTT and ACTAT/AGTAT) appearing only in S. flavovirens Z.H.Chen, D.D. Ma et W. Y. Xie. and hexanucleotide (ACATAT/ATATGT) occurring only in S. hacquetioides Franch., S. rugulosa and S. serrata. In the mono-repeats, A/T motifs were more abundant (24–32) than G/C motif (0–2). Likewise, AT sequences were also particularly rich in di-repeats and tri-repeats (Fig. 3B, Table S3).
Comparison of plastome analysis
We examined the borders between inverted repeat and single-copy (IR/SC) among seventeen Sanicula plastomes.The results indicated that these Sanicula species had similar IR boundaries and adjacent genes were identical (Fig. 4). The rps19 gene was located at the junction of the LSC/IRa, and it had the same length of 221 bp in the LSC region and 58 bp in the IRa region in sixteen plastomes, but in S. flavovirens, the entire rps19 gene was located in the LSC region. The IRa/SSC junction region was located between the trnN gene and ndhF gene. The trnN and ndhF genes were 2,146–2,164 bp and 5–11 bp away from the IRa/LSC borders. The ycf1 genes, crossing the SSC/IRb borders, were located at the SSC and IRb regions with 3,447–3,479 bp and 1,819–1,837 bp. The IRb/LSC boundary was located between rpl2 gene and trnH gene. In these seventeen plastomes (except S. flavovirens), the rpl2 gene was 115–118 bp away from the IRb/LSC borders and the trnH gene was 2 bp away from the IRb/LSC borders, whereas in S. flavovirens, the rpl2 gene was only 32 bp away from the LSC region, and the trnH gene was 87 bp away from the IRb region (Fig. 4). Thus, these findings indicated that seventeen Sanicula plastomes were highly conservative.
The Mauve result revealed that gene arrangement of the seventeen plastomes was highly conserved (Fig. 5). The result performed by the mVISTA program illustrated that the plastomes of seventeen taxa were also highly conserved (Fig. 6). In addition, the mVISTA result found that the matrix contained 2472 variable sites (1.56%) and 1021 informative sites (0.65%) within the 15,8067 alignment positions. As a result, the whole plastomes sequence exhibited high similarity and no significant divergence was observed.
Mutation hotspots in genus Sanicula
High nucleotide diversity (Pi) value means that certain regions have large variation and can be used to design the potential DNA barcodes. In this study, we calculated Pi values of four regions among the Sanicula plastomes. The results showed that there were 146 sites, including 107 sites in the LSC region, 19 sites in the SSC region and 20 sites in the IRs regions (Table S4). The findings also indicated that the Pi value of non-coding region was remarkably higher than that of the coding region (Fig. 7). Based on the sequence divergence, twelve mutation hotspot regions were selected as candidate DNA barcodes, containing seven protein coding genes (atpF, cemA, accD, rpl22, rbcL, matK and ycf1) and five non-coding protein coding genes (trnH-psbA, ycf4-cemA, rbcL-accD, trnE-trnT and trnG-trnR). The seven protein coding genes with the highest sequence variation had obviously higher values of 0.00411 (0.79%), 0.00571 (1.16%), 0.00424 (0.7%), 0.00592 (1.02%), 0.00413 (0.85%), 0.00510 (0.73%) and 0.00691 (1.47%), respectively (Fig. 7A, Table S4). Among them, six genes were located in the LSC region and one gene (ycf1 gene) was located at the SSC/IR junction. Five non-coding genes were considered as highly divergent hotspots with values of 0.03175 (7.07%), 0.01956 (0.99%), 0.02345 (2.81%), 0.03031 (1.99%) and 0.01852 (3%) (Fig. 7B, Table S4), all of which were located in the LSC region.
Phylogeny analyses for genus Sanicula
The 79 shared CDSs of 67 complete plastomes and 80 ITS sequences were performed to reconstruct the phylogeny of Sanicula (Table S5). Although the tree topologies of the plastome data and ITS sequences were incongruent, both strongly suggested that the monophyly of Saniculoideae and Apioideae. In addition, sister groups between Saniculoideae and Apioideae were robustly supported. The Sanicula species involved in this study clustered together and the Eryngium species also clustered together (Fig. 8).
Phylogenetic trees constructed by maximum likelihood (ML) and Bayesian inference (BI). The bootstrap values (BS) of ML and posterior probabilities (PP) of BI are listed at each node. (*) represents the node with PP = 1.00/ BS = 100. – means the values < 0.50/50. Red words indicates the newly sequenced species. A CDS tree; B: ITS tree
In the CDS-based phylogenetic tree, the results of the maximum likelihood (ML) and Bayesian inference (BI) analyses yielded a well-resolved topologies and the topologies were highly identical as expected (Fig. 8A). It was clearly observed that the sections of Sanicula involved in the current study were not respectively recovered as monophyletic group. Instead, seventeen members of Sanicula were obviously divided into three clades. S. rubriflora F. Schmidt ex Maxim. clustered with S. flavovirens and S. chinensis, belonging to Clade I with robust support (PP = 1.00, BS = 100). Clade II included four species (S. serrata, S. rugulosa, S. hacquetioides, and S. giraldii H. Wolff.). The remaining ten species (S. odorata, S. astrantiifolia, S. tienmuensis, S. orthacantha, S. oviformis X.T. Liu & Z.Y. Liu., S. caerulescens, S. elongata K.T. Fu., S. pengshuiensis, S. lamelligera, and S. orthacantha var. stolonifera) gathered together in Clade III, which could be divided into four subclades. It was noticed that Subclade A contained only one species: S. odorata. In Subclade B, S. astrantiifolia was sister to S. tienmuensis with weak supports (BS < 50, PP = 1.00). S. orthacantha, S. oviformis and S. caerulescens formed a strong Subclade C (PP = 1.00, BS = 100), of which S. oviformis and S. caerulescens clustered together and then resolved as sister to S. orthacantha. S. elongata, S. pengshuiensis, S. lamelligera and S. orthacantha var. stolonifera belonged to Subclade D, of which S. elongata clustered with S. pengshuiensis with more closely relationship (Fig. 8A).
The topologies of ITS tree obtained from ML and BI analyses were also highly identical (Fig. 8B). Although weakly supported in the ITS analyses, both analyses also suggested that the sections of Sanicula were not monophyletic group. The phylogenetic position of most species were consistent with the CDS-based phylogenetic trees, but there were a little conflicts. For example: (1) S. elongata clustered with S. pengshuiensis in the CDS-based phylogenetic trees (BS = 98, PP = 1.00), while the former formed a clade with S. tienmuensis in the ITS phylogenetic trees (BS = 94, PP = 1.00); (2) S. rugulosa was sister to S. hacquetioides in the CDS-based phylogenetic trees (BS = 100, PP = 1.00), whereas the former resolved sister to S. giraldii with depressed support (BS < 50, PP = 0.65) in the ITS phylogenetic trees; (3) S. orthacantha formed a clade with S. oviformis and S. caerulescens in the CDS-based phylogenetic trees (BS = 80, PP = 0.97), while S. orthacantha gathered together with S. lamelligera in the ITS phylogenetic trees with robust support (BS = 100, PP = 1.00); (4) S. tienmuensis clustered with S. astrantiifolia in the CDS-based phylogenetic trees (BS < 50, PP < 0.5), whereas the former was sister to S. elongata with strong support (BS = 94, PP = 1.00) in the ITS analyses (Fig. 8B).
Divergence time estimation
Based on four calibration points (one leaf macrofossil of Araliaceae and three calibration points constrained to a lognormal distribution), we estimated the divergence time of the genus Sanicula. Molecular dating results indicated that the family Apiaceae diverged from the family Araliaceae at 81.06 Ma (95% HPD: 53.97–87.24 Ma) in the Upper-Cretaceous. For the family Apiaceae, the divergence time between subfamily Apioideae and subfamily Saniculoideae was estimated to be 64.19 Ma (95% HPD: 58.28–68.27) when was from the Upper-Cretaceous to the late Paleocene period. Subsequently, the subfamily Apioideae diverged at around 55.54 Ma (95% HPD: 47.77–63.39 Ma) in the early Eocene period. Within the subfamily Saniculoideae, the divergence time between the genus Sanicula and Eryngium occurred at 37.84 Ma (95% HPD: 20.33–52.21 Ma) in the late Eocene period. In addition, the diversification of Sanicula were happened at 18.38 Ma (95% HPD: 10.68–25.28 Ma) in the early Miocene period (Fig. 9).
The divergence time estimation based on 79 shared genes. The maximum credibility tree from the divergence times estimated with BEAST. The 95% highest posterior density (HPD) estimates for each well-supported clade are represented by bars. Orange nails indicate the calibration points for the molecular dating
Discussion
Comparative analysis of Sanicula plastomes
Comparative analyses of plastome can provide valuable insights into understanding patterns of molecular evolution [44, 45]. In this study, a comprehensive comparison of seventeen Sanicula plastomes were carried out. All Sanicula plastomes had a typical quadripartite structure, consisting of one LSC region, one SSC region and a pair of IRs region (Fig. 1). Similarly, this structure was also detected in other plastomes of angiosperm plants [46,47,48]. Although gene loss is a common evolutionary event that frequently occurs in other genera of Apiaceae, such as the ycf15 gene was lost in genus Peucedanum [31] and the trnT-GGU gene was lost in genus Peucedanum and Kitagawia [31, 35], it was not discovered in genus Sanicula. In addition, the gene arrangement and GC content of the seventeen plastomes displayed high similarity. These findings demonstrated that the Sanicula plastomes were quite conserved. It is noteworthy that the GC content in the IR regions was relatively higher than that in the LSC and SSC regions (Table 1). The uneven distribution of GC content may be attributed to the higher GC content of the four rRNA genes (rrn16, rrn23, rrn4.5, and rrn5) in the IR regions, which is a common feature observed in other plants [49,50,51], as well as in Apiaceae [30,31,32].
IR contraction and expansion is a common phenomenon in the Apiaceae plastomes [20, 30,31,32, 35,36,37,38,39,40]. This phenomenon was also observed in Sanicula plastomes. Our study displayed that the rps19 gene had the length of 221 bp in the LSC region and 58 bp in the IRa region in sixteen plastomes, but in S. flavovirens, the entire rps19 gene was located in the LSC region. In these seventeen plastomes (except S. flavovirens), the rpl2 gene was 115–118 bp away from the IRb/LSC borders and the trnH gene was 2 bp away from the IRb/LSC borders, whereas in S. flavovirens, the rpl2 gene was only 32 bp away from the IRb/LSC borders, and the trnH gene was 87 bp away from the IRb/LSC borders, which resulted in shorter plastome length of S. flavovirens than in the other sixteen Sanicula species. Therefore, we supported the hypothesis that the genome size variation was caused by IR contraction and expansion. Furthermore, we also examined the inverted repeats types described in the Apiaceae [20, 30,31,32, 35,36,37,38,39,40] and found that the situation observed in the genus Sanicula was also detected in the other genera of Apiaceae, such as in Hansenia Turcz., Haplosphaera Hand.-Mazz., Sinodielsia H. Wolff [37].
Plastome evolution of Sanicula
Codons provide a link between nucleic acids and proteins, conveying genetic information [39]. The patterns of codon usage show basic characteristics of molecular evolution [52]. In species, codon usage is not random, as some synonymous codons are used more frequently than others, a phenomenon known as codon usage bias (CUB) [53]. CUB is considered to be a driver of gene evolution and it provides a great deal of information for understanding molecular evolution [54]. It exists in all species’s genomes and is influenced by many factors, such as mutation pressure, natural selection, gene composition and gene length [55]. In the present study, all codons with RSCU > 1.00 were strongly biased towards A/U at the third codon position in seventeen Sanicula plastomes, which was consistent with the observation in most angiosperms [56], as well as in Apiaceae [30,31,32, 35,36,37,38,39]. Perhaps the high AT content in plastome is the major reason for the bias towards A/U for codons ending in A/U [39]. Leucine was the most abundant amino acid and was encoded by the highest number of codons, while cysteine was the rarest amino acid, as in most Apiaceae species [30,31,32].
Repeat sequences can cause gene insertion, deletion, substitution and duplication, which can lead to the generation of divergent regions in genome rearrangements. Therefore, these repeats can provide crucial information for phylogenetic and population studies [57]. Among the seventeen Sanicula plastomes, palindromic repeats were the majority of the four types of repeats, which have also been reported in the genus Ligusticum [30] and Cnidium [39].
SSRs are shorter tandem or microsatellite repeat sequences with repeat units of 1–6 bp, which are widely distribute in different regions of the plastome [58]. Due to a large amount of variation within the species, SSRs have been extensively used as valuable molecular markers for species authentication, population evolutionary analyses, phylogenetic relationship analyses, plant taxonomy, geography of species, genetic diversity and population structure studies [59]. Our research identified 979 SSRs in seventeen Sanicula plastomes. Mononucleotide were the most abundant SSRs, followed by dinucleotide, tetranucleotide, trinucleotide, hexanucleotide and pentanucleotide repeats. In addition, most of the SSRs contained A/T motifs, resulting in the AT richness of the seventeen Sanicula plastome. The similar pattern has also been observed in other Apiaceae plants [32]. In particular, the longest SSR was hexanucleotide (ACATAT/ATATGT) and it can serve as genetic markers in population genetics and phylogeography studies of Sanicula in the future.
Candidate DNA barcodes
With the rapid development of sequencing technologies and DNA barcoding, variable loci (e.g., matK, rbcL and trnH-psbA) have been recognized as universal DNA barcodes that were widely used for species identification, resource management, phylogenetic analyses and species evolution research in land plants [31, 36, 60, 61]. Although universal DNA barcoding have successfully distinguished many species, they have failed for some taxonomically notorious taxa and have encountered weak solutions [62]. Therefore, it is urgently needed to develop more effective variable loci as candidate DNA barcodes to solve the dilemma. Since plastome contain large-scale differentiation hotspots, thus it has the strong potential for screening DNA barcodes [63, 64]. In the current study, we investigated that the sequence variability in the non-coding region was higher than in the coding region, which was also detected in other genera of Apiaceae [31, 32]. Moreover, we identified five non-coding protein coding genes (trnH-psbA, ycf4-cemA, rbcL-accD, trnE-trnT and trnG-trnR) and seven protein coding genes (atpF, cemA, accD, rpl22, rbcL, matK and ycf1) that could be used as candidate DNA barcodes for Sanicula species identification. Of these twelve DNA barcodes, except for three universal DNA barcodes (rbcL, matK and trnH-psbA) and two promising DNA barcodes (accD and ycf1) in some plants [65], the remaining seven high variable regions (atpF, cemA, rpl22, ycf4-cemA, rbcL-accD, trnE-trnT and trnG-trnR) were different from those of other species [31, 66]. Therefore, they could be regarded as specific DNA barcodes to distinguish Sancilua species. These findings offered a valuable reference for further developing DNA barcodes for Sanicula.
Phylogeny inference and taxonomic implication of Sanicula
The plastome is uniparental inheritance, lacks recombination, and has highly variable loci; thus, it has been widely used in phylogenetic studies of angiosperms, especially at the low taxonomic levels of Apiaceae [20, 30,31,32, 35,36,37,38,39,40, 66, 67]. In the present study, the plastome data generated a well-resolved phylogenetic tree for Sanicula. The supports and resolutions of the plastome-based tree were significantly improved compared to those of the ITS-based tree constructed in the current and previous studies [5, 13, 15, 17].
The incongruences between ITS-based and plastome-based phylogenies are a common phenomenon in Apiaceae [31, 33, 35, 36, 67], and our results are no exception. These incongruences are mainly caused by hybridization/introgression, incomplete lineage sorting (ILS) and chloroplast capture events [20], as well as the maternal inheritance of plastid and biparental inheritance of ITS, which may also be an important factor [30].
Although topological incongruence existed between the plastome data and the ITS sequences, both successfully recognized all Sanicula species involved in the current study clustered together, which was consistent with several previous molecular phylogenetic analyses [5, 7, 12, 15, 17,18,19, 42]. Meanwhile, both topologies supported the sections within the genus were not being monophyletic group and the current classification may be inappropriate. Therefore, we considered that the current section-level classification system of Sanicula needs to be improved and revised. Although the current study did not provide a new taxonomic treatment of Sanicula taxa due to lack of insufficient sampling or the low supports and resolutions, our study laid the foundations and served as a framework for future taxonomic studies of Sanicula.
In addition, we clarified the taxonomic relationships of the four species. In the present study, both analyses indicated that S. orthacantha var. stolonifera was relatively far from S. orthacantha. Their notably different morphological characters of the two taxa further supported the phylogenetic results. For example, the rhizomes features (short, tuberlike, woody, oblique rootstock bearing elongated, fibrous roots, occasionally having fleshy stoloniferous) and narrow-linear, acute calyx teeth were observed in S. orthacantha, while slender, elongate and lignified nodes stoloniferous rhizomes and ovate calyx teeth were found in S. orthacantha var. stolonifera [13]. Thereby, S. orthacantha var. stolonifera should be regarded as an independent species rather than a variety of S. orthacantha based on the molecular and morphological evidence. A published study has treated S. pengshuiensis as a synonymy of S. lamelligera [14]. Although the relationship between the two species was unsolved in ITS-based tree, the plastome-based tree robustly supported that S. pengshuiensis clustered with S. elongata. The morphological characteristics of them also supported the above phylogenetic results, such as inflorescence cymose branched, peduncles elongate was detected in S. pengshuiensis, while inflorescence 2–4-dichotomously or trichotomously branched, sometimes subcorymbose was found in S. lamelligera [13]. Hence, we suggested that S. pengshuiensis should be regarded as an independent species rather than a synonymy of S. lamelligera.
Estimation divergence time
Our analysis based on date estimation revealed that the divergence between the family Araliaceae and Apiaceae occurred during the Upper-Cretaceous period, with an approximate median age of 81.06 Ma (95% HPD: 53.97–87.24 Ma). This time was roughly consistent with the findings of Xie et al., who also used plastomes to estimate the divergence time of Apiales [40], but earlier than some studies (between 60 and 72 Ma [20, 68]. As for the family Apiaceae, the subfamilies Apioideae and Saniculoideae diverged at the beginning of the Paleogene, approximately 64.19 Ma (95% HPD: 58.28–68.27 Ma). The time was slightly earlier than other previous studies [20, 68, 69], but aligned closely with findings inferred from trnD-trnT + rpl16 intron, with a deviation of 65.78 Ma (95% HPD: 58.21–74.31 Ma) [70]. The diversification of the subfamily Apioideae was estimated to have occurred during the early Eocene period 55.54 Ma. This time was earlier than that suggested by previous studies conducted by Wen et al. (43.45 Ma and 49.78 Ma) [20, 68]. On the other hand, in subfamily Saniculoideae, the genus Sanicula and Eryngium genera took place during the late Eocene period, precisely 37.84 Ma years ago (95% HPD: 20.33–52.21 Ma). This estimation was comparatively earlier than the previous records [20]. The potential discrepancy between our study and earlier previous researches may be caused by the differences in sampling and fossil calibrations used [40, 71]. Our results were considered more accurate and credible because the study contained a larger sample of Sanicula taxa and a broader range of calibration points, which were frequently employed in other evolutionary analyses [18, 40, 71, 72]. Therefore, we obtained a more precise divergence times.
Within subfamily Saniculoideae, the origin of genus Sanicula was estimated to have occurred during the late Eocene (37.84 Ma). Previous studies have reported that the initial uplift of the Qinghai-Tibetan Plateau (QTP) occurred at 45–35 Ma [71]. The QTP, which extends to the Hengduan Mountains (HDM) located on the southeastern edge of the current QTP [73], encompasses the most extensive range. Thus, the monolithic uplift of QTP led to the uplift of HDM, and some studies have suggested that the early uplift of the southern part of the HDM can be traced back to the period of late Paleocene or even the Eocene [74]. The uplift of the HDM resulted in the formation of a highly complex topography, giving rise to the creation of distinct and isolated “sky islands” [75, 76]. The “sky islands” have played a vital role in plant origin, speciation and evolution throughout the HDM and neighbouring regions, which has resulted in the emergence and diversification of various lineages, including Plethodon ouachitae, Saussurea and Cupressus L [77,78,79,80]. Therefore, we hypothesized that the uplift of the HDM may also have contributed to the origin of Sanicula species. The diversification of Sanicula occurred at 18.38 Ma, during the early Miocene. This period coincided with significant changes in East Asian monsoon intensity due to global temperature fluctuations [81], which led to substantial transformations in numerous biological communities in East Asia. This environmental shifts potentially facilitated the diversification of many species, such as Primulina, Lepisorus and Begonia [82,83,84]. Therefore, we assumed that the intensification of the East Asian monsoon might also acted as an indispensable role in promoting the diversification of Sanicula. Moreover, the uplift of HDM also likely influenced the diversification of this genus. The HDM experienced extensive uplift caused by the collision between the Indian plate and Eurasia, occurring after the Miocene and reaching its peak elevation before the Late Pliocene [85, 86]. This geological event triggered a series of topographical and climatic changes. The altered topography provided diverse habitats that facilitated species differentiation, as observed in the cases of Caragana and Lilium [87, 88]. Therefore, we supposed that the diversification of Sanicula species was closely related to the prevalence of the East Asian monsoon and the uplift of HDM events.
Conclusion
In this study, we successfully sequenced and assembled the complete plastomes of fifteen Sanicula species. Together with two previously reported plastomes, we performed a comprehensively comparative analyses. The results revealed that the genome structure, gene number, GC content, gene rearrangement and codon usage of seventeen Sanicula plastomes were highly conserved. Nevertheless, twelve highly variable regions were still selected as potentially strong DNA barcodes, which exhibited superior efficacy in species identification and phylogenetic relationship construction. Phylogenetic analyses indicated that the Sanicula species involved in this study clustered together. However, the existing section classification system was deemed non-natural. The incongruences between ITS-based and plastome-based phylogenies were also observed in the genus. These discrepancies could largely be attributed to the events of hybridization/introgression, incomplete lineage sorting (ILS) and chloroplast capture, and the maternal inheritance of plastids, while the biparental inheritance of ITS. Molecular clock analysis indicated that the origin of genus Sanicula occurred during the late Eocene, which was significantly correlated with the uplift of the HDM. And the diversification of the genus occurred in the early Miocene, which was largely influenced by the prevalence of the East Asian monsoon and the uplift of the HDM. In conclusion, our study holds substantial significance and value for the investigation of the taxonomy, phylogeny, and evolution of the Sanicula genus.
Methods
Plant material and DNA extraction
The samples of fifteen Sanicula taxa were collected from the wild and then the fresh basal young leaves were immediately dried and stored with silica gel for further DNA extraction. The formal identification of these taxa was carried out by Professor Xingjin He (Sichuan University). Vouchers were stored in the herbarium of Sichuan University (Chengdu, China) under the deposition number listed in Table S6. Total DNA of newly collected samples was extracted from silica gel-dried fresh leaf tissues using the modified CTAB method [89]. We also newly sequenced fifteen Sanicula ITS sequences and submitted them to NCBI (accession numbers: OQ651137-OQ651152) ( Table S5). To re-evaluate the taxonomic system of the genus more objectively, we also downloaded the plastomes and ITS sequences of this genus that were currently available on the NCBI.
Genome sequencing, assembly and annotation
The quality and quantity of DNA were tested using 1% agarose gel electrophoresis, and high-quality DNA was sequenced on an Illumina HiSeq2500 platform from Novogene (Beijing, China) according to the standard Illumina sequencing protocols [90]. Paired-end 150 reads were generated from libraries with an insert size of 300 bp. The raw data were filtered by software fastP v0.15.0 to obtain high-quality reads, with -n 10 and -q 15 [91]. For plastome assembly, we employed two strategies. First, we used NOVOPlasty v2.7.2 [92] to assemble complete plastomes, with the default parameters and rbcL sequence extracted from the plastome of S. lamelligera (MT561031) as the seed. To validate the accuracy of plastome assembly, GetOrganelle pipeline [93] was also used to assemble the plastomes, using the plastome sequence of S. lamelligera (GenBank accession: MT561031) as reference. And the same assembled results were obtained by both methods. The assembled plastomes were initially annotated with the program DOGMA [94]. Then, the start and stop codons and intron positions were checked and manually corrected when necessary in Geneious v9.0.2 [95] and the plastomes of a given species recovered by different assembly strategies were also compared using Geneious v9.0.2 [95]. The circular plastome map was depicted by online program CHLOROPLOT (https://irscope.shinyapps.io/Chloroplot/) [96] and the newly sequenced and assembled seventeen complete plastomes were submitted into NCBI (accession numbers: OQ612639-OQ612643 and OQ626817-OQ626828) (Table S5).
Codon usage bias
The protein-coding genes (PCGs) were extracted from seventeen Sanicula plastomes for codon analysis. All overlapping genes were removed and the remaining 79 PCGs for each species. Since shorter genes may bias the codon usage estimation [97] and to avoid sampling bias [98], 79 PCGs with CDSs length shorter than 300 bp were excluded from this study, and 53 CDSs were finally screened. Relative synonymous codon usage (RSCU) is the value of the observed and expected frequency of a codon encoding a specific amino acid [99]. The RSCU value is divided into three ranges by the value 1.00. Among them, the RSCU value > 1.00, = 1.00, and < 1.00 represents positive codon usage bias, no bias, and negative codon usage bias, respectively. Especially, codons with RSCU values greater than 1.6 and less than 0.6 are regarded as “over-represented” and “under-represented” codons, respectively [100]. The RSCU values were calculated using the CodonW v1.4.2 program [101] and the RSCU heatmap of the seventeen plastomes was emerged by GraphPad Prism 7 [102].
Repeat sequence and simple sequence repeats (SSRs) analysis
The four repetitive sequences: Forward (F), Reverse (R), Palindromic (P) and Complementary (C) repeats of the seventeen Sanicula plastomes were detected by the online REPuter program [103]. The parameters were set with maximum computed repeats > 90%, minimal repeat size ≥ 30 bp, and a hamming distance = 3. All overlapping repeat sequences were removed. Simple sequence repeats (SSRs) were then checked using the MISA Perl script [104]. The minimum number of repeat units parameter was set to 10, 5, 4, 3, 3, and 3 for mono-, di-, tri-, tetra-, penta-, and hexanucleotides, respectively.
Comparative plastome of Sanicula
We compared the boundaries between inverted repeat and single-copy (IR/SC) among the seventeen plastomes in Geneious v9.0.2 [95]. The junction regions of LSC/IRb/SSC/IRa were visualized by IRscope [105] after manually adjusted. Then, the DNA rearrangements among seventeen Sanicula plastomes were detected by using Mauve Alignment [106] implemented in Geneious v9.0.2 [95], with other parameters set as the default values. Furthermore, we also investigated the sequence divergence of Sanicula plastomes using mVISTA viewer [107], setting S. astrantiifolia as the reference.
Identification of high divergence hotspots
The protein coding regions, non-coding regions and intron regions of the seventeen plastomes were extracted in Geneious v9.0.2 [95] to identify mutation hotspot regions. We then aligned the sequences using MAFFT v7.221 [108] and the sequences were manually corrected with BioEdit software [109] and Geneious v9.0.2 [95]. And the alignment with less than 200 bp in length were discarded, mainly because the relatively short and insufficient sequence can yield the variation [110]. Finally, we evaluated nucleotide diversity (Pi) [111] using DnaSP version 5.0 software [112] to further investigate the molecular evolution of the Sanicula plastome.
Phylogenetic analysis
To illustrate the phylogenetic relationship of genus Sanicula, 67 plastomes and 80 ITS sequences were used to reconstruct the phylogenetic tree (Table S5). Among them, fifteen species of family Araliaceae were chosen as outgroup referred to the phylogenetic result of previous study [40]. The 80 ITS sequences were straightway aligned with MAFFT v7.221 [107] to gain the matrix. For plastome data, 79 commonly shared CDSs of 67 species were manually extracted in Geneious v9.0.2 [95] and aligned with MAFFT v7.221 [107]. Then the alignments were concatenated as a super matrix with PhyloSuite v1.2.2 [113]. Two matrixes were subjected to Maximum-Likelihood analysis (ML) and Bayesian Inference (BI). In detail, for the ML analyses, RAxML v8.2.8 [114] with GTRGAMMA model was performed to reconstruct the phylogenetic tree, and 1000 replicates was suggested to estimate the support value (BS) for each node according to the RAxML manual. Bayesian inference was performed by MrBayes v3.2.7 [115] and the best-fit nucleotide substitution model (GTR + I + G) for matrix of concatenated plastid protein-coding and (SYM + I + G) for ITS dataset were determine by Modeltest v3.7 [116] under the Akaike Information Criteria (AIC) [117]. The Markov chain Monte Carlo (MCMC) algorithm was run for 5,000,000 generations (sampling every 100 generations) with two runs and four chains (three heated chains and one cold chain). The running finished until the average standard deviation of split frequencies was below 0.01. An initial 25% of sampled trees were discarded, and the remainder were retained to generate the consensus tree and calculate posterior probabilities (PP). The results of ML and BI phylogenetic analyses were visualized in FigTree v1.4.2 [118].
Divergence time estimation
Bayesian relaxed clock analysis in program Bayesian Evolutionary Analysis Sampling Trees (BEAST v1.10.4) [119] was performed to estimate the divergence time of genus Sanicula. In the preliminary experiment, we analyzed random trees because the starting tree could not converge after two runs, but the ML phylogenetic tree was almost fully supported. Thus, the ML tree inferred from matrix of concatenated plastid protein-coding was used to fix topology. One leaf macrofossil of Araliaceae and three calibration points constrained to a lognormal distribution were used to constrain the phylogenetic tree. (i) the stem node of Hedera L. was constrained to a minimum age of 23.0 Ma based on leaf macrofossil of Hedera sp [120]. ; (ii) based on the fossilized leaves of Araliaceae, the stem node of Harmsiopanax ingens Philipson. was set as 49.28–72.9 Ma age [121]; (iii) according to the study of Wen et al., the root point was set set to 68.88 Ma (95% HPD: 53.97–87.24 Ma) [20]; (iv) referring to the previous research of the evolutionary timescale, 54.17 Ma (95% HPD: 45.55–66.44) was implemented as a minimum age of subfamily Apioideae and Saniculoideae [20]. BEAUti was used to set criteria for analysis under the uncorrelated relaxed molecular clock model, with a Yule tree prior and the best-fit nucleotide substitution model (GTR + I + G) detected by Modeltest v3.7. Two independent replications of MCMC simulations were run for 2 × 109 generations with sampling every 2000 generations, with the first 20% trees being discarded as burn-in and the convergence of remaining runs was assessed through Tracer v1.7.1 [122] to examine the effective sample size (ESS) of all parameters not less than 200. The maximum clade credibility tree with median ages and 95% highest posterior density (HPD) intervals were produced with TreeAnotator v2.1.2 [123] and the final result was exhibited in FigTree v1.4.2 [118].
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the NCBI repository, https://www.ncbi.nlm.nih.gov/. Accession numbers: OQ612639-OQ612643, OQ626817- OQ626828 and OQ651137-OQ651152.
Abbreviations
- CTAB:
-
Cetyl trimethylammonium bromide
- PCR:
-
Polymerase chain reaction
- ITS:
-
Internal transcribed spacer
- CDS:
-
Protein-coding sequences
- bp:
-
Base pair
- BI:
-
Bayesian inference
- PP:
-
Posterior probability
- BS:
-
Branch support
- AIC:
-
Akaike information criterion
- ML:
-
Maximum Likelihood
- MCMC:
-
Markov chain Monte Carlo
- IR:
-
Inverted repeat
- LSC:
-
Large single copy
- SSC:
-
Single copy
- SSR:
-
Simple sequence repeat
- RSCU:
-
Relative synonymous codon usage
- Pi:
-
Nucleotide diversity
- rRNA:
-
Ribosomal RNA
- tRNA:
-
Transfer RNA
References
Pryer KM, Phillippe LR. A synopsis of the genus Sanicula (Apiaceae) in eastern Canada. Can J Bot. 1989;67(3):694–707.
Pimenov MG, Leonov MV. The genera of the Umbelliferae: a nomenclator. Kew: Royal Botanic Gardens; 1993.
Van Wyk B-E, Tilney PM, Magee AR. African Apiaceae: a synopsis of the Apiaceae/Umbelliferae of Sub-Saharan Africa and Madagascar/Ben-Erik Van Wyk, Patricia M Tilney & Anthony R Magee. Pretoria: Briza Academic Books; 2013.
Koch WDJ. Generum tribriumque plantarum umbelliferarum nova dispositis. Nova Acta Acad Caes Leop-Carol. 1824;12:55–156.
Vargas P, Baldwin BG, Constance L. A phylogenetic study of Sanicula sect. Sanicoria and S. sect. Sandwicenses (Apiaceae) based on nuclear rDNA and morphological data. Syst Bot. 1999;24(2):228.
Linnaeus C. Species Plantarum. Stockholm: Laurentii Salvii; Oxford University Press; 1753.
Calviño CI, Downie SR. Circumscription and phylogeny of apiaceae subfamily saniculoideae based on chloroplast DNA sequences. Mol Phylogenet Evol. 2007;44:175–91.
Shan RH, Constance L. The genus Sanicula (Umbelliferae) in the old world and the new. Univ Calif Press. 1951;25:1–7.
De Candolle AP. Umbelliferae. In: De Candolle AP, editor. Prodromus systematis naturalis regni vegetabilis, Vol. 4. Paris: Treüttel and Würtz; 1830. p. 55–220.
De Candolle A. Monographie des Campanulées. Paris, Chez Mme. Veuve Desray, 1830.
Drude Oscar. Umbelliferae. In: Engler, A. die natü rlichen Pflanzenfamilien. Leipzig: Wilhelm Engelmann. 1898;37:63–128.
Wolf H. Umbelliferae-Saniculoideae. In: Engler A, editor. Das Pfanzenreich. Volume IV. Leipzig & Berlin: Wilhelm Engelmann; 1913. pp. 1–305.
Sheh ML, Phillippe LR. Sanicula L. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. Volume 14. Beijing Press. St. Louis: Missouri Botanical Garden Press;: Science Press; 2005. pp. 19–24.
Li HM, Song CF. Taxonomic studies on the genus Sanicula (Apiaceae) from China (I): the identity of S. orthacantha var. Pumila and S. Pengshuiensis. Phytotaxa. 2022;532(2):114–38.
Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG. Relationships among genera in Saniculoideae and selected Apioideae (Umbelliferae) inferred from nrITS sequences. Taxon. 2002;51(1):91–101.
Yang C, Yao XY, Chen ZX, Downie SR, Wang QZ. The chloroplast genomes of Sanicula (Apiaceae): plastome structure, comparative analyses and phylogenetic relationships. Nord J Bot. 2022;2022(8):e03549.
Calviño CI, Martínez SG, Downie SR. Morphology and biogeography of Apiaceae subfamily Saniculoideae as inferred by phylogenetic analysis of molecular data. Am J Bot. 2008;95(2):196–214.
Vargas P, Baldwin BG, Constance L. Nuclear ribosomal DNA evidence for a western north American origin of hawaiian and south American species of Sanicula (Apiaceae). PNAS. 1998;95(1):235–40.
Kadereit JW, Repplinger M, Schmalz N, Uhink CH, Wörz A. The phylogeny and biogeography of Apiaceae subf. Saniculoideae tribe Saniculeae: from south to north and south again. Taxon. 2008;57(2):365–82.
Wen J, Xie DF, Price M, Ren T, Deng YQ, Gui LJ, Guo XL, He XJ. Backbone phylogeny and evolution of Apioideae (Apiaceae): new insights from phylogenomic analyses of plastome data. Mol Phylogenet Evol. 2021;161: 107183.
Standard of Chinese herbal medicines in Sichuan Province (Trial draft). Sichuan: Sichuan Health Bureau; 1979. p. 10.
Li XS. Studies on the chemical constitutes and biological activities of Feijing Cao. Master's thesis. Hunan University of Traditional Chinese Medicine: Major of Chinese Medicine; 2011.
Chen ZX. Taxonomic study on the genus Sanicula (Apiaceae) from China. Master's thesis. Huaqiao University: Major of Biology; 2019.
Jansen RK, Ruhlman TA. Plastid genomes of seed plants. In: Bock R, Knoop V, editors. Genomics of chloroplasts and mitochondria. Dordrecht: Springer Netherlands; 2012. p 103–126.
Ruhlman TA, Jansen RK. The plastid genomes of flowering plants. Chloroplast Biotechnol. 2014;1132:3–38.
Mower JP, Vickrey TL. Structural diversity among plastid genomes of land plants. Adv Bot Res. 2018;85:263–92.
Ravi V, Khurana JP, Tyagi AK, Khurana PJ. An update on chloroplast genomes. Plant Syst Evo. 2008;271:101–22.
Palmer JD. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325e354.
Daniell H, Lin C, Yu M, Chang W. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17(1):134.
Ren T, Li ZX, Xie DF, Gui LJ, Peng C, Wen J, He XJ. Plastomes of eight Ligusticum species: characterization, genome evolution, and phylogenetic relationships. BMC Plant Biol. 2020;20(1):1–14.
Liu CK, Lei JQ, Jiang QP, Zhou SD, He XJ. The complete plastomes of seven Peucedanum plants: comparative and phylogenetic analyses for the Peucedanum Genus. BMC Plant Biol. 2022;22(1):101.
Song BN, Liu CK, Xie DF, Xiao YL, Tian RM, Li ZX, Zhou SD, He XJ. Plastid phylogenomic analyses reveal the taxonomic position of Peucedanum franchetii. Plants. 2023;12(1):97.
Li J, Cai J, Qin HH, Megan P, Zhang Z, Yu Y, Xie DF, Zhou SD, He XJ, Gao XF. Phylogeny, Age, and evolution of Tribe Lilieae (Liliaceae) based on whole plastid genomes. Front Plant Sci. 2021;12:3158.
Metzker ML. Sequencing technologies – the next generation. Nat Rev Genet. 2010;11(1):31–46.
Lei JQ, Liu CK, Cai J, Price M, Zhou SD, He XJ. Evidence from Phylogenomics and morphology provide insights into the phylogeny, Plastome Evolution, and taxonomy of Kitagawia. Plants. 2022;11(23): 3275.
Cai J, Qin HH, Lei JQ, Liu CK, He XJ, Zhou SD. The phylogeny of Seseli (Apiaceae, Apioideae): insights from molecular and morphological data. BMC Plant Biol. 2022;22(1):534.
Gou W, Jia SB, Price M, Guo XL, Zhou SD, He XJ. Complete plastid genome sequencing of eight species from Hansenia, Haplosphaera and Sinodielsia (Apiaceae): Comparative analyses and phylogenetic implications. Plants. 2020;9(11):1523.
Tian RM, Aou XYM, Song BN, Li ZX, He XJ, Zhou SD. Plastid phylogenomic analyses reveal a cryptic species of Ligusticopsis (Apiaceae, Angiosperms). Int J Mol Sci. 2023;24:7419.
Ren T, Aou X, Tian RM, Li ZB, Peng C, He XJ. Complete chloroplast genome of Cnidium monnieri (Apiaceae) and comparisons with other Tribe Selineae Species. Diversity. 2022;14(5):323.
Xie DF, Xie C, Ren T, Song BN, Zhou SD, He XJ. Plastid phylogenomic insights into relationships, divergence, and evolution of Apiales. Planta. 2022;256(6):117.
Chen ZX, Yao XY, Wang QZ. The complete chloroplast genome of Sanicula chinensis. Mitochondrial DNA Part B. 2019;4(1):734–5.
Chen ZX, Yao XY, Downie SR, Wang QZ. Assembling and analysis of Sanicula orthacantha chloroplast genome. Biodivers Sci. 2019;27(4):366.
Wang Z, Ren WC, Yan S, Zhang MQ, Liu YW, Ma W. Characterization of the complete chloroplast genome of Sanicula rubriflora F. Schmidt ex Maxim. Mitochondrial DNA Part B. 2021;67:1999–2000.
Gitzendanner MA, Soltis PS, Yi TS, Li DZ, Soltis DE. Plastome phylogenetics: 30 years of inferences into plant evolution. In: Adv Bot Res. Academic Press; 2018. p. 293–313.
Yao G, Jin JJ, Li HT, Yang JB, Mandala VS, Croley M, Mostow R, Douglas AN, Chase WM, Christenhusz JM, Soltis DE, Soltis SP, Smith AS, Brockington SF, Moore MJ, Yi TS, Li DZ. Plastid phylogenomic insights into the evolution of Caryophyllales. Mol Phylogenet Evol. 2019;134:74–86.
Tian XY, Guo J, Zhou XJ, Ma K, Ma YG, Shi TS, Shi YH. Comparative and evolutionary analyses on the complete plastomes of five Kalanchoe horticultural plants. Front Plant Sci. 2021;12:705874.
Lee HO, Joh HJ, Kim K, Lee SC, Kim NH, Park JY, Park SH, Park MS, Kim S, Kwak M, Kim K, Lee WK, Yang TJ. Dynamic chloroplast genome rearrangement and DNA barcoding for three Apiaceae species known as the medicinal herb “Bang-Poong.” Int J Mol Sci. 2019;20(9):2196.
Wang Y, Zhang CF, Odago WO, Jiang H, Yang JX, Hu GW, Wang QF. Evolution of 101 Apocynaceae plastomes and phylogenetic implications. Mol Phylogenet Evol. 2023;180:107688.
Mardanov AV, Ravin NV, Kuznetsov BB, Samigullin TH, Antonov AS, Kolganova TV, Skyabin KG. Complete sequence of the duckweed (Lemna minor) chloroplast genome: structural organization and phylogenetic relationships to other angiosperms. J Mol Evol. 2008;66:555–64.
Jiang DZ, Cai XD, Gong M, Xia MQ, Xing HT, Dong SS, Tian SM, Li JL, L In JY, Liu YQ, Li HL. Complete chloroplast genomes provide insights into evolution and phylogeny of Zingiber (Zingiberaceae). BMC Genom. 2023;24(1):1–18.
Niu ZT, Xue QY, Wang H, Xie XZ, Zhu SY, Liu W, Ding XY. Mutational biases and GC-biased gene conversion affect GC content in the plastomes of Dendrobium Genus. Int J Mol Sci. 2017;18(11):2307.
Yan Q, Philmus B, Hesse C, Kohen M, Chang JH, Loper JE. The rare codon AGA is involved in regulation of pyoluteorin biosynthesis in Pseudomonas protegens Pf-5. Front Microbiol. 2016;7:497.
Sharp PM, Li WH. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24:28–38.
Mitreva M, Wendl MC, Martin J, Wylie T, Yin Y, Larson A, Parkinson J, Waterston RH, McCarter JP. Codon usage patterns in Nematoda: analysis based on over 25 million codons in thirty-two species. Genome Bio. 2006;7(8):1–19.
Salim HM, Cavalcanti AR. Factors influencing codon usage bias in genomes. J Braz Chem Soc. 2008;19:257–62.
Maheswari P, Kunhikannan C, Yasodha R. Chloroplast genome analysis of angiosperms and phylogenetic relationships among Lamiaceae members with particular reference to teak (Tectona grandis Lf). J Biosci. 2021;46:1–9.
Wang X, Xu YF, Fan HY, Cui N, Meng XN, He JJ, Ran NN, Yu Y. Research progress of plant nucleotide-binding leucine-rich repeat protein. Horticulturae. 2023;9(1):122.
Chen C, Zhou P, Choi YA, Huang S, Gmitter FG. Mining and characterizing microsatellites from citrus ESTs. Theor Appl Genet. 2006;112:1248–57.
Echt CS, DeVerno LL, Anzidei M, Vendramin GG. Chloroplast microsatellites reveal population genetic diversity in red pine, Pinus resinosa Ait. Mol Ecol. 1998;7(3):307–16.
Gregory TR. DNA barcoding does not compete with taxonomy. Nature. 2005;434(7037):1067–1067.
Li X, Li YF, Zang MY, Li MZ, Fang YM. Complete chloroplast genome sequence and phylogenetic analysis of Quercus acutissima. Int J Mol Sci. 2018;19(8):2443.
Cao Q, Xu LH, Wang JL, Zhang FQ, Chen SL. Molecular phylogeny of subtribe Swertiinae. BBR. 2021;41(03):408–18.
Chen XL, Zhou JG, Cui YX, Wang Y, Duan BZ, Yao H. Identification of Ligularia herbs using the complete chloroplast genome as a super-barcode. Front Pharmacol. 2018;9:695.
Li XW, Yang Y, Henry RJ, Rossetto M, Wang YT, Chen SL. Plant DNA barcoding: from gene to genome. Biol Rev. 2015;90(1):157–66.
Duchene D, Bromham L. Rates of molecular evolution and diversification in plants: chloroplast substitution rates correlate with species-richness in the Proteaceae. BMC Evol Biol. 2013;13(1):1–11.
Li ZX, Guo XL, Price M, Zhou SD, He XJ. Phylogenetic position of Ligusticopsis (Apiaceae, Apioideae): evidence from molecular data and carpological characters. AoB Plants. 2022;14(2): plac008.
Qin HH, Cai J, Liu CK, Zhou RX, Price M, Zhou SD, He XJ. The plastid genome of twenty-two species from Ferula, Talassia, and Soranthus: comparative analysis, phylogenetic implications, and adaptive evolution. BMC Plant Biol. 2023;23(1):1–18.
Wen J, Yu Y, Xie DF, Peng C, Liu Q, Zhou SD, He XJ. A transcriptome based study on the phylogeny and evolution of the taxonomically controversial subfamily Apioideae (Apiaceae). Ann Bot. 2020;125:937–53.
Calviño CI, Teruel FE, Downie SR. The role of the Southern Hemisphere in the evolutionary history of Apiaceae, a mostly north temperate plant family. J Biogeogr. 2016;43:398–409.
Nicolas AN, Plunkett GM. Diversification times and biogeographic patterns in Apiales. Bot Rev. 2014;80:30–58.
Barba-Montoya J, Reis MD, Schneider H, Donoghue PCJ, Yang Z. Constraining uncertainty in the timescale of angiosperm evolution and the veracity of a cretaceous Terrestrial Revolution. New Phytol. 2018;218(2):819–34.
Valcárcel V, Wen J. Chloroplast phylogenomic data support Eocene amphi-Pacific early radiation for the Asian Palmate core Araliaceae. J Syst Evol. 2019;57(6):547–60.
Zhang Y, Li B, Zheng D. Datasets of the boundary and area of the Tibetan Plateau. Acta Geogr Sin. 2014;69(S):164–8.
Wu SD, Wang Y, Wang ZF, Shrestha N, Liu JQ. Species divergence with gene flow and hybrid speciation on the Qinghai – Tibet Plateau. New Phytol. 2022;234(2):392–404.
Lopez-Pujol J, Zhang FM, Sun HQ, Ying TS, Ge S. Centres of plant endemism in China: places for survival or for speciation? J Biogeogr. 2011;38:1267–80.
Sklenář P, Hedberg I, Cleef AM. Island biogeography of tropical alpine floras. J Biogeogr. 2014;41(2):287–97.
Shepard DB, Burbrink FT. Lineage diversification and historical demography of a sky island salamander, Plethodon ouachitae, from the Interior Highlands. Mol Ecol. 2008;17(24):5315–35.
Wang YJ, Susanna A, Von Raab-Straube E, Milne R, Liu JQ. Island-like radiation of Saussurea (Asteraceae: Cardueae) triggered by uplifts of the Qinghai–Tibetan Plateau. Biol J Linn Soc. 2009;97(4):893–903.
Xu TT, Abbott RJ, Milne RI, Mao KS, Du FK, Wu GL, Ciren ZX, Miehe G, Liu JQ. Phylogeography and allopatric divergence of cypress species (Cupressus L.) in the Qinghai-Tibetan Plateau and adjacent regions. BMC Evol Biol. 2010;10:1–10.
Qiu YX, Fu CX, Comes HP. Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of quaternary climate and environmental change in the world’s most diverse temperate flora. Mol Phylogenet Evol. 2011;59:225–44.
Li SF, Valdes PJ, Farnsworth A, Davies-Barnard T, Su T, Lunt DJ, Spicer RA, Liu J, Deng WYD, Huang J, Tang H, Ridgwell A, Chen LL, Zhou ZK. Orographic evolution of northern Tibet shaped vegetation and plant diversity in eastern Asia. Sci Adv. 2021;7(5): eabc7741.
Kong H, Condamine FL, Harris AJ, Chen J, Pan B, Möller M, Hoang VS, Kang M. Both temperature fluctuations and east Asian monsoons have driven plant diversification in the karst ecosystems from southern China. Mol Ecol. 2017;26(22):6414–29.
Wang L, Schneider H, Zhang XC, Xiang QP. The rise of the Himalaya enforced the diversification of SE Asian ferns by altering the monsoon regimes. BMC Plant Biol. 2012;12:1–9.
Chung KF, Leong WC, Rubite RR, Repin R, Kiew R, Liu Y, Peng CI. Phylogenetic analyses of Begonia sect. Coelocentrum and allied limestone species of China shed light on the evolution of sino-vietnamese karst flora. Bot Stud. 2014;55:1–15.
Zhao YJ, Yin GS, Pan YZ, Tian B, Gong X. Climatic refugia and geographical isolation contribute to the speciation and genetic divergence in Himalayan-Hengduan tree peonies (Paeonia delavayi and Paeonia ludlowii). Front Genet. 2021;11: 595334.
Dupont-Nivet G, Lippert PC, Van Hinsbergen DJJ, Meijers MJM, Kapp P. Palaeolatitude and age of the indo–Asia collision: palaeomagnetic constraints. Geophys J Int. 2010;182:1189–98.
Zhang ML, Fritsch PW. Evolutionary response of Caragana (Fabaceae) to Qinghai–Tibetan Plateau uplift and Asian interior aridification. Plant Syst Evol. 2010;288:191–9.
Gao YD, Harris AJ, Zhou SD, He XJ. Evolutionary events in Lilium (including Nomocharis, Liliaceae) are temporally correlated with orogenies of the QT plateau and the Hengduan Mountains. Mol Phylogenet Evol. 2013;68(3):443–60.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bull. 1987;19:11–5.
Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–45.
Chen SF, Zhou YQ, Chen YR, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884-890.
Dierckxsens N, Mardulyn P, Smits G. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. NAR. 2017;45(4):e18–e18.
Jin JJ, Yu WB, Yang JB, Song Y, dePamphilis CW, Yi TS, Li DZ. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21(1):1–31.
Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20:3252–5.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–9.
Zheng S, Poczai P, Hyvönen J, Tang J, Amiryousefi A. Chloroplot: an online program for the versatile plotting of organelle genomes. Front Genet. 2020;11: 576124.
Kessler MD, Dean MD. Effective population size does not predict codon usage bias in mammals. Ecol Evol. 2014;4:3887–900.
Yang Y, Zhu J, Feng L, Tao Z, Bai G, Yang J, Zhao G. Plastid genome comparative and phylogenetic analyses of the key genera in Fagaceae: highlighting the effect of codon composition bias in phylogenetic inference. Front Plant Sci. 2018;9:82.
Wu HG, Bao ZY, Mou CX, Chen ZH, Zhao JW. Comprehensive analysis of codon usage on porcine Astrovirus. Viruses. 2020;12:991.
Wong EH, Smith DK, Rabadan R, Peiris M, Poon LL. Codon usage bias and the evolution of influenza A viruses. Codon usage biases of influenza virus. BMC Evol Biol. 2010;10: 253.
Peden JF. Analysis of codon usage. PhD thesis. Nottingham University: Department of Genetics; 1999.
Mitteer DR, Greer BD, Fisher WW, Cohrs VL. Teaching behavior technicians to create publication-quality, single‐case design graphs in graphpad prism 7. J Appl Behav Anal. 2018;51(4):998–1010.
Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. NAR. 2001;29(22):4633–42.
Thiel T, Michalek W, Varshney R, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L). Theor Appl Genet. 2003;106:411–22.
Amiryousefi A, Hyvonen J, Poczai P. IRscope: an online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2018;34:3030–1.
Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–403.
Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32(suppl2):W273-279.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Hall AT. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic Acids Symp Ser. 1999; p. 95–98.
Hong SY, Cheon KS, Yoo KO, Lee HO, Cho KS, Suh JT, Kim SJ, Nam JH, Sohn GB, Kim YH. Complete chloroplast genome sequences and comparative analysis of Chenopodium quinoa and C. album. Front Plant Sci. 2017;8:1696.
Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci. 1979;76:5269–73.
Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–2.
Zhang D, Gao F, Jakovlić I, Zou H, Zhang J, Li WX, Wang GT. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour. 2020;20(1):348–55.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3.
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard M, Huelsenbeck JP. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–42.
Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–8.
Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53(5):793–808.
Rambaut A, Drummond A. FigTree, version 1.4.2. 2015. http://tree.bio.ed.ac.uk/software/fgtree/. Accessed 4 Jun 2023.
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018;4:vey016.
Kong WS. Vegetational history of the Korean Peninsula. Global Eco Biogeogr. 2000;9:391–402.
Manchester SR. Fruits and seeds of the middle Eocene nut beds Flora, Clarno formation, Oregon. Palaeontogr Am. 1994;58:38–9.
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in bayesian phylogenetics using Tracer 1.7. Syst biol. 2018;67(5):901–4.
Rambaut A, Drummond AJ. TreeAnnotator v2.1.2. Edinburgh: University of Edinburgh, Institute of Evolutionary Biology; 2014.
Acknowledgements
We are grateful to Ting Ren, Jiaqing Lei and Huan-huan Qin for their valuable discussions. We thank Herbarium PE, KUN, SZ, NAS, CDBI for providing specimens consulting.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32070221, 32170209), and Survey on the Background Resources of Chengdu Area of Giant Panda National Park (Project No.: 510101202200376). The funders were not involved in the design of the research, collection, analysis and interpretation of data, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
X-JH and S-DZ designed the work. B-NS, C-KL, A-QZ, Y-LX, and R-MT collected the materials; B-NS, C-KL, D-FX and H-C analyzed the data; B-NS wrote the manuscript; X-JH, S-DZ, C-KL, H-C and D-FX revised the manuscript. All authors gave final approval of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Collection of the fifteen samples completely complies with national and local legislation permission. Plant samples used in the study were not included in the list of national key protected plants and not collected from national park or natural reserve. According to national and local legislation, no specific permission was required for collecting these plants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
List of unique genes identified in plastomes of Sanicula.
Additional file 2: Table S2.
Codon usage and relative synonymous codon usage (RSCU) values of 53 protein-coding genes of seventeen Sanicula plastomes.
Additional file 3: Table S3.
Repeat sequence and simple sequence repeats in seventeen Sanicula plastomes.
Additional file 4: Table S4.
Nucleotide diversity (Pi) of coding and non-coding regions.
Additional file 5: Table S5.
The plastomes data included in phylogenetic analyses with GenBank accession.
Additional file 6: Table S6.
The sample information of seventeen Sanicula in this study
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, BN., Liu, CK., Zhao, AQ. et al. Phylogeny and diversification of genus Sanicula L. (Apiaceae): novel insights from plastid phylogenomic analyses. BMC Plant Biol 24, 70 (2024). https://doi.org/10.1186/s12870-024-04750-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04750-0