Abstract
Background
The picturesque limestone karsts across the Sino-Vietnamese border are renowned biodiversity hotspot, distinguished for extremely high endemism of calciphilous plants restricted to caves and cave-like microhabitats that have functioned as biological refugia on the otherwise harsh habitats. To understand evolutionary mechanisms underlying the splendid limestone flora, dated phylogeny is reconstructed for Asian Begonia, a species-rich genus on limestone substrates represented by no less than 60 species in southern China, using DNA sequences of nrITS and chloroplast rpL16 intron. The sampling includes 94 Begonia species encompassing most major Asian clades with a special emphasized on Chinese species.
Results
Except for two tuberous deciduous species and a species with upright stems, a majority of Sino-Vietnamese limestone Begonia (SVLB), including sect. Coelocentrum (19 species sampled) and five species of sect. Diploclinium, Leprosae, and Petermannia, are rhizomatous and grouped in a strongly supported and yet internally poorly resolved clade (Clade SVLB), suggesting a single evolutionary origin of the adaptation to limestone substrates by rhizomatous species, subsequent species radiation, and a strong tendency to retain their ancestral niche. Divergence-time estimates indicate a late Miocene diversification of Clade SVLB, coinciding with the onset of the East Asian monsoon and the period of extensive karstification in the area.
Conclusions
Based on our phylogenetic study, Begonia sect. Coelocentrum is recircumscribed and expanded to include other members of the Clade SVLB (sect. Diploclinium: B. cavaleriei, B. pulvinifera, and B. wangii; sect. Leprosae: B. cylindrica and B. leprosa; sect. Petermannia: B. sinofloribunda). Because species of Clade SVLB have strong niche conservatism to retain in their ancestral habitats in cave-like microhabitats and Begonia are generally poor dispersers prone to diversify allopatrically, we propose that extensive and continuous karstification of the Sino-Vietnamese limestone region facilitated by the onset of East Asian monsoon since the late Miocene has been the major driving force for species accumulation via geographic isolation in Clade SVLB. Morphologically species of Clade SVLB differ mainly in vegetative traits without apparent adaptive value, suggesting that limestone Begonia radiation is better characterized as non-adaptive, an underappreciated speciation mode crucial for rapid species accumulations in organisms of low vagility and strong niche conservatism.
Similar content being viewed by others
Background
Karsts are distinctive landforms and hydrological systems formed by the dissolution of highly soluble and porous bedrock (e.g., carbonate rocks such as limestone) with geological ages ranging from the Cambrian to the Quaternary (Ford and Williams2007). Around the world, carbonate rocks cover ca. 11% of world’s land surface; however, with the expansion of subterranean hydrological systems, the karst realm makes up more than 14% of the global land areas (Williams2008). With more than 800,000 km2, Southeast Asia and southern China contain the most extensive limestone karsts on earth (Gillieson2005). In particular, the vast karst terrain stretching across the Sino-Vietnamese bordering region (southern China [Guangxi, western Guangdong, southern Guizhou, and southeastern Yunnan] and northern Vietnam; Xu et al.2012a), renowned for spectacular landscapes of fengcongs (cone karst; Waltham2008), fenglins (tower karst; Waltham2008), and caves, is the largest area on earth with pure carbonate bedrock (Xu1995) and has been considered as the model for karst studies (Sweeting1978).
Behind these picturesque landscapes, limestone karsts of SE Asia and southern China also abound in rich and marvelous flora with strikingly morphological variation and exceedingly high endemism (Xu1995; Clements et al.2006; Zhu2007). In China, 61 of the ca. 250 Chinese endemic plant genera occur solely in Guangxi (Qin and Liu2010), a majority of which are associated with the limestone ecosystem. Of the ca. 470 species (58 genera) of Gesneriaceae recorded from China, at least 200 species (41 genera) are found in Guangxi, with most species confined to limestone karsts. Among them, ca. 100 species and 10 genera are endemic to Guangxi (Qin and Liu2010). Of the 18 Begonia L. species from Sabah, Borneo associated with limestone substrates, 12 (67%) are only known from a single locality (Kiew2001). In Kuching (Sarawak, Boreno), all 15 limestone Begonia species are endemic to the local hills only (Kiew2004).
Despite their remarkable biodiversity, however, a great proportion of karsts remain to be explored and very little is known regarding the evolutionary processes that have generated the marvelous floras on these limestone landscapes (Clements et al.2006). Since Begonia is one of the best represented plant groups on tropical limestone karsts (Clements et al.2006), it provides an important model for understanding the role that limestone karsts have played in generating high species diversity and endemism.
With recent estimates ranging from ca. 1500 (e.g., Goodall-Copestake et al.2010; Thomas et al.2011a) to more than 1600 species (Hoover et al.2004), the pantropically distributed Begonia sits firmly as one of the ten most species-rich flowering plant genera (Frodin2004). However, despite their almost ubiquitous presence in the tropical and subtropical forest ecosystems across Africa, America, and Asia, with few exceptions (e.g., B. grandis Dryand., B. longifolia Blume, and B. palmata; Gu et al.2007), most Begonia species have very narrow distribution ranges (Tebbitt et al.2006; Hughes and Hollingsworth2008) and single-site endemic species are common, especially in the limestone karsts (e.g., Kiew2001; Peng et al.2008b; Dewitte et al.2011).
Begonia taxonomy is notoriously challenging because of the genus’ enormous size and often poorly preserved morphological characters in herbarium specimens (Hoover et al.2004; Hughes and Girmansyah2011), generating great obstacles for further studies. To deal with the unwieldy number of species in the genus, sectional classifications have long been adopted (Doorenbos et al.1998; Ku1999; Shui et al.2002; Kiew2005; Gu2007). Traditionally these infrageneric classifications have relied primarily on morphological and anatomical characters of fruits and ovaries, with each of the ca. 70 recognized sections confined to one continent only (Doorenbos et al.1998), with one known exception (B. afromigrata J.J. de Wilde, sect. Tetraphila; de Wilde et al.2011).
With more than 760 species currently known, Asia harbors the greatest species diversity of Begonia (Rajbhandary et al.2011; Thomas et al.2011a). In the past decade renewed interest and ongoing explorations to under-collected regions have resulted in the description of more than 140 new species in Asia (e.g., de Wilde et al.2011; Thomas et al.2011b; Averyanov and Nguyen2012; Peng et al.20122013). Traditionally these Asian species are classified into 22 sections of highly unequal sizes (Doorenbos et al.1998; Shui et al.2002; Gu2007; Hughes and Pullan2007; Kiew2005; Thomas et al.2011a), with eight of the largest sections: sect. Coelocentrum Irmsch., Diploclinium (Lindl.) A. DC., Petermannia (Klotzsch) A. DC., Platycentrum (Klotzsch) A. DC., Parvibegonia A. DC., Reichenheimia (Klotzsch) A. DC., Sphenanthera (Hassk.) Warb., and Symbegonia (Warb.) L.L. Forrest & P.M. Hollingsworth, accounting for 95% of the species diversity (Thomas et al.2011a). However, recent molecular phylogenetic analyses have demonstrated the paraphyly (sect. Platycentrum and Petermannia) or polyphyly (sect. Diploclinium, Leprosae, Reichenheimia and Sphenanthera) of most big sections (Tebbitt et al.2006; Thomas et al.2011a), indicating the homoplasious or plesiomorphic nature of those morphological characters long emphasized in sectional classification (Tebbitt et al.2006; Thomas et al.2011a).
Based on recent treatment of Begoniaceae in the Flora of China (Gu et al.2007) and subsequent studies (Liu et al.2007; Peng et al.20072008a,b20122013; Li et al.2008; Shui2007; Wei et al.2007), ca. 170 species of Begonia assigned to 9 (Shui et al.2002) or 7 (Gu2007) sections are currently known from continental China, with sect. Coelocentrum (47 spp.), Diploclinium (41 spp.), and Platycentrum (62 spp.) accounting for the majority (88.6%) of the species diversity. Among these, ca. 60 species (35% of the Begonia diversity in China) are known primarily from limestone substrates in Guangdong (1 species), Guangxi (44 species), Guizhou (3 species) and Yunnan (19 species) provinces (Gu et al.2007; Liu et al.2007; Peng et al.20072008a,b201020122013). These limestone begonias include one species of sect. Alicida C.B. Clarke (B. peii; Shui et al.2002), four species of sect. Diploclinium (B. cavaleriei H. Lév. [Guangxi, Yunnan, Guizhou], B. grandis Dryand. [widespread across eastern China], B. pulvinifera C.I Peng & Y. Liu [Guangxi], and B. wangii; Gu et al.2007), three species of sect. Platycentrum (B. psilophylla Irmsch. [Yunnan], B. rubropunctata S.H. Huang & Y.M. Shui [Yunnan], and B. subhowii; Shui et al.2002), two species of sect. Leprosae (T.C. Ku) Y.M. Shui (B. cylindrica D.R. Liang & X.X. Chen [Guangxi] and B. leprosa Hance [Guangdong, Guangxi]), one species of sect. Petermannia (B. sinofloribunda; Shui and Chen2004), three species of sect. Reichenheimia (B. chingii Irmsch. [Guangxi], B. lithophila C.Y. Wu [Yunnan], and B. parvula; H. Lév. & Vaniot [Yunnan]; Shui et al.2002), and all 49 species of sect. Coelocentrum (Gu et al.2007; Ku et al.2008; Liu et al.2007; Peng et al.2008a,b20122013).
Morphologically well circumscribed by its parietal placentation and rhizomatous perennation (Shui et al.2002; Gu et al.2007), Begonia sect. Coelocentrum is one of the most characteristic limestone plants confined to cave-like microhabitats (i.e., caves, crevices, and fissures) of the Sino-Vietnamese karst region (Peng et al.2008a; Qin and Liu2010), with most species known from a single or a few localities and differing from one another by leaf shape, pubescence, texture, and variegation (Gu et al.2007). In the past decade, explorations and taxonomic studies in the region have more than tripled the number of species in this section from 15 (Ku1999) to over 50 (Gu et al.2007;Liu et al.2007; Ku et al.2008; Peng et al.20072008a,b20122013; Averyanov and Nguyen2012;), representing a remarkable example of species radiations across the Sino-Vietnamese limestone karsts comparable to those on oceanic islands and tropical high mountains (Rundell and Price2009).
Given the sectional classification schemes, it is tempting to speculate that Begonia should have adapted to the limestone substrates at least seven times in China. However, Chinese Begonia species, especially those found on limestone substrate, were very poorly sampled in recent phylogenetic analyses (Tebbitt et al.2006; Rajbhandary et al.2011; Thomas et al.2011a2012). For example, despite its high diversity, only two species of sect. Coelocentrum were sampled in the studies by Thomas et al. (2011a2012) and its relationships to other limestone Begonia in the region remain to be explored. Additionally, although sect. Diploclinium and Platycentrum, both major representatives of Chinese Begonia, have been proven non-monophyly (Thomas et al.2011a), it remains unclear as to what extent the infrageneric taxonomic framework of Chinese Begonia has to be revised, and what mechanisms could have been responsible for the high Begonia diversity on the Sino-Vietnamese limestone karsts.
This article studies phylogenetic relationships of limestone Begonia of Sino-Vietnamese karsts and concurrently evaluate the monophyly of major Begonia sections present in China. To gain further insights into the history of Begonia diversification, molecular divergence time estimates are also performed. This study focuses on continental Asian Begonia species, complementing recent studies by Thomas et al. (2011a2012) that emphasized the evolutionary patterns of SE Asian Begonia.
Methods
Taxon sampling
Our sampling strategy aimed to increase the number of species of limestone Begonia of continental China not represented in previous molecular phylogenetic analyses, while maximizing representatives of all major clades in Asia (Thomas et al.2011a). In total, 94 Asian species (44 species of mainland China) were sampled, including one species of sect. Alicida C.B. Clarke (ALI), eight species of sect. Baryandra A. DC. (BAR), 19 species of sect. Coelocentrum (COL), 12 species of sect. Diploclinium (DIP), one species of sect. Haagea (Klotzsch) A. DC. (HAA), four species of sect. Leprosae (T.C. Ku) Y.M. Shui (LEP), one species of sect. Parvibegonia A. DC. (PAR), 25 species of sect. Petermannia (PET), 14 species of sect. Platycentrum (PLA), three species of sect. Reichenheimia (REI), one species of sect. Ridleyella Irmsch. (RID), three species of sect. Sphenanthera (SPH), one species of sect. Symbegonia (SYM), and B. boisiana Gagnep., which is assigned to sect. Petermannia by Kiew (2007) but placed as unassignable (UA) to existing section in Doorenbos et al. (1998) and Hughes and Pullan (2007). Two South African species were chosen as outgroups based on previous phylogenetic analyses (Goodall-Copestake et al.20092010; Thomas et al.2011a). Of the 96 species sampled, 49 species (51%) have never been investigated using molecular data. The majority of the sampled species were field-collected plants maintained in the experimental greenhouse of the Academic Sinica, Taipei, Taiwan. Voucher information and GenBank accession numbers are detailed in Additional file1.
Molecular data
DNA of Begonia species is difficult to extract, especially for those collected from limestone substrates. Additionally, silica-gel dried leaves of Begonia tend to yield poor quality DNA. To circumvent these issues, total genomic DNA was extracted from fresh leaves of living collections using a modified CTAB protocol optimized for Begonia (Kopperud and Einset1995).
Two DNA sequence regions were used: the nuclear ribosomal DNA (nrDNA) internal transcribed spacer (ITS) region and the chloroplast DNA rpL16 intron. Previous analyses based on ITS (e.g., Tebbitt et al.2006) suggested its usefulness in resolving interspecific relationships while chloroplast sequences were more suitable for reconstructing the backbone structure of the Asian Begonia phylogeny (Thomas et al.2011a). Although the rpL16 intron has thus far never been used in reconstructing the Begonia phylogeny, this region amplified and sequenced easily and shows adequate phylogenetic resolution in Asian Begonia. For each polymerase chain reaction (PCR), amplification was performed in a total volume of 25 μl, including 12.5 μl of Taq DNA Polymerase Master Mix Red (Ampliqon, Copenhagen, Denmark), 1 μl of each forward and reverse primer (10 μM), 2 μl of template DNA, and 8.5 μl of ddH2O. For ITS, the primers 5P and 26S1Rev primers (Clement et al.2004) were used for both PCR amplification and sequencing. For rpL16 intron, three primers (rpL16-F: GCT ATG CTT AGT GTG TGA CTC G; rpL16-R: CGT CCY GCT TCT ATT TGT CTA G; Beg_rpL16: GTT TCA CAT TAT CTG GAT CG) were designed to optimize PCR amplification (rpL16-F and rpL16-R) and sequencing (Beg_rpL16 and rpL16-R) in Begonia. PCR reactions were carried out by a denaturation-step in 94°C for 5 min, 30 thermo-cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 90 s (60 s for ITS), and a final extension in 72°C for 5 min. PCR products were purified using QIAquick PCR purification Kit (Qiagen, Valencia, California, U.S.A.) and then sequenced using an ABI PRISM dye terminator cycle sequencer, model 3700 (Applied Biosystems, Foster City, California, U.S.A.).
Phylogenetic analyses
DNA sequences were aligned using the program MUSCLE implemented in the software MEGA5 (Tamura et al.2011) with subsequent manual adjustments. The aligned matrix was analyzed using maximum parsimony (MP) and maximum likelihood (ML) optimality criteria and Bayesian Inference (BI). MP analyses were performed using PAUP* v.4.0b10 (Swofford2002). All characters were unordered and equally weighted and gaps were treated as missing. Heuristic searches were conducted with 1000 replicates of random addition, 10 trees held at each step during stepwise addition, tree-bisection-reconnection (TBR) branch-swapping, and deepest descent option in effect. Brach supports (parsimony bootstrap: PB) were assessed by 1000 bootstrap replicates with 10 random taxon additions and heuristic search options. To assess the congruence among the two data sets, the incongruence length difference test (ILD test for partition-homogeneity test in PAUP*) was performed. One thousand homogeneity replicates of heuristic searches were conducted with 100 random sequence additions and MulTrees not in effect. Ten ML analyses with 5000 bootstrap (likelihood bootstrap: LB) resamplings were conducted using RAxML-HPC2 v7.2.7-3 (Stamatakis et al.2008) via the CIPRES Portals (http://www.phylo.org/index.php/portal/). The matrix was partitioned (i.e., ITS vs. rpL16 intron) with a gamma model of rate heterogeneity and proportion of invariable sites estimated by the program. The program MrBayes v3.1.2 (Ronquist and Huelsenbeck2003) was employed for BI analyses. The aligned matrix was partitioned into ITS and rpL16 data sets. Prior to the analysis, DNA substitution models for each partition were selected by MrModeltest (Nylander2004) based on the Akaike information Criterion (AIC). BI analyses were conducted using mixed models specified to each data partition. Four runs of Metropolis-coupled Markov chain Monte Carlo (MCMCMC) analyses were performed, with a random starting tree and four chains for each run (one cold and three heated). The MCMCMC length was ten million generations and the chain was sampled every 1000th generation from the cold chain. To assure that the cold chain swapped successfully with the heated chains and the Metropolis coupling functioned well, the resulting log files were by inspecting the trace plots of parameters using the program Tracer v1.5 (Drummond and Rambaut2007). The function ‘Compare and Cumulative’ of the program AWTY (Nylander et al.2008) was employed to check topological convergence and convergence of posterior probabilities (PP) of splits from different runs. Bayesian clade posterior probabilities and average branch lengths were calculated based on the sampled trees after the first 25% of the sampled trees were discarded as burn-in.
Molecular dating
The software package BEAST v1.6.1 (Drummond and Rambaut2007) was employed to perform divergence-time estimates. The aligned matrix was formatted for BEAST using BEAUti with the data partitioned to allow specification of DNA substitution models for each partition (see BI analysis above). Because suitable fossils in Begoniaceae are not available, the phylogenetic tree was calibrated using two normally distributed priors based on divergence time estimated by Thomas et al. (2012) for Asian Begonia: the mean of estimated divergence ages of the crown node of Asian Begonia (Clade 10 in Figure three of Thomas et al.2012; the node marked with a rhombus sign in Figure 1) at 16.1 (95% Highest Posterior Density range [HPD] 9.1–22.5) mya (million years ago) and Clade 49 in Figure three of Thomas et al.2012 (clade D, marked with a star sign in Figure 1) at 13.0 (7.7–18.9 HPD) mya. Clade D in Figure three of Thomas et al. (2012) includes sect. Coelocentrum and Petermannia, and species of sect. Diploclinium (Clade BAR) centered in the Philippines recently classified under the recircumscribed sect. Baryandara (Rubite et al.2013). For these two clades, the calibration priors were modeled with the mean of 16.1 mya and the standard deviation of 3.265 (95% probability interval: 9.7–22.5 mya) for the Asian Begonia Clade and the mean of 13.0 mya and the standard deviation of 2.7 (95% probability interval: 7.7–18.3 mya) for Clade 49 to account for HPDs of age estimates of the original analysis. The tree prior was set to the Yule process and a single uncorrelated-rates relaxed molecular clock model assuming a lognormal distribution of rates (UCLD) was applied to all partitions. Two independent runs of BEAST analyses were conducted, each with 50 million generations and sampling every 1,000th generation.
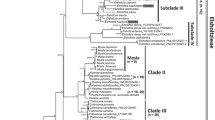
Best-scoring maximum likelihood phylogram. Clade support values (LB: likelihood bootstrap/PB: parsimony bootstrap/PP: posterior probability) larger than 50% are indicated at each node. Dashed branches indicate LB, PB, and PP all smaller than 50%/0.5 while thick branches denote those present in the strict consensus tree of MP analysis and PP ≥0.95. Species in bold denote limestone species distributed in the Sino-Vietnamese limestone karsts (Gu et al.,2007). Arrows point to clades and sections discussed in the text. Taxon names are followed by sectional placement and distribution (in parentheses). The rhombus (◇) and star (☆) signs denote calibration points for molecular dating. Sectional classification and clade names in Thomas et al. (2011a) are marked to the right to allow easier cross-study comparison. Sources of sectional placement for each species are cited in Additional file1. Sectional abbreviations: ALI: Alicida, AUG: Augustia, BAR: Baryandra, COL: Coelocentrum, DIP: Diploclinium, HAA: Haagea, LEP: Leprosae, PAR: Parvibegonia, PET: Petermannia, PLA: Platycentrum, REI: Reichenheimia, RID: Ridleyella, SPH: Sphenanthera, SYM: Symbegonia, UA: unassigned.
Results
Information of sequence variability in each partition is summarized in Table 1. The alignment file is available upon request to the corresponding author (CIP). The combined data matrix included 1974 aligned positions (ITS: 864 bp; rpL16: 1110 bp), of which 29% of the aligned sequences (31.6% in ITS and 26.9% in rpL 16) were excluded because of uncertain alignment. MP analyses yielded 149503 equally parsimonious trees of 3480 steps (CI = 0.4086; RI = 0.5542; RC = 0.2264). For BI analyses, AIC selected the model GTR+G+I for ITS and GTR+G for rpL16.
The result of ILD test (P = 0.001) indicates significant incongruence between ITS and the rpL16 data sets. However, because ILD test has been doubted for generating misleading conclusions under certain circumstances (Yoder et al.2001), topologies of MP analysis of ITS, rpL16, and the concatenated data sets were compared (Wiens1998). While relationships generated from ITS and the combined data sets are highly congruent, topology of rpL16 is largely incongruent with the formers (data not shown). Nevertheless, supports for the conflict relationships between ITS (and the concatenated) and rpL16 topologies are all less than 50% and the combined data set has much higher support values for most clades, suggesting a ‘soft incongruence’ (Wiens1998). Therefore, the two data sets were combined for all subsequent analyses.
For MP and ML analyses, 70–79%, 80–90%, and 90–100% bootstrap supports (LB and PB) are considered as moderate, well, and strongly supported relationships, respectively. For BI analyses, the threshold for a well-supported clade is 95% PP. The best scored ML tree (ML optimization likelihood = -20967.197060), with bootstrap support values (PB and LB) and the posterior clade probabilities (PP), is depicted in Figure 1. Clades designated in Thomas et al. (2011a) and sectional classification are also marked to the right of the ML tree in Figure 1 to allow easier cross-comparisons between studies.
Topology of the best-scoring ML tree is largely compatible with those uncovered in recent phylogenetic analyses of similar scale (Rajbhandary et al.2011; Thomas et al.2011a), though relationships among deeper nodes are very poorly supported (Figure 1). In the best-scoring ML tree, all sampled Asian Begonia species form a moderately supported clade (LB: 74) with strong support in BI analysis (PP: 0.97). Within the Asian Clade, the Indian species B. dipetala Graham (sect. Haagea) is sister to a clade composed of all the remaining Asian species, though lacking support (Figure 1). Within this latter clade, species are grouped with low supports into two clades (Clades C and D; Figure 1) corresponding to Clade C1 + C2 and Clade D in Thomas et al. (2011a).
Within Clade C, eight sampled species of tuberous and deciduous habit form a poorly-supported successive grade (Figure 1) sister to a moderately to strongly supported Clade PLA-SPH (LB: 75, PB: 78, PP: 1). This grade, corresponding well (except for sect. Parvibegonia) to the DIP I grade in Thomas et al. (2011a), includes two Indochinese species (sect. Alicida: B. alicida; sect. Parvibegonia: B. variabilis), five Chinese species (sect. Diploclinium: B. fimbristiupla, B. grnadis, and B. labordei; sect. Reichenheimia: B. chingii and B. parvula), and one species of Taiwan (sect. Diploclinium: B. ravenii C.I Peng & Y.K. Chen). Clade PLA-SPH is mainly composed of species of sect. Platycentrum and Sphenanthera, plus one species of sect. Leprosae (B. longicarpa K.Y. Guan & D.K. Tian) and two species of sect. Diploclinium (B. alveolata T.T. Yu and Begonia ruboides C.M. Hu ex C.Y. Wu & T.C. Ku). Of the Chinese species of the DIP I grade, B. chingii and B. parvula are distributed exclusively on limestone substrates of Guangxi and Yunnan, respectively, while B. grandis is the most widespread Begonia species in China found in a variety of habitats including limestone (Gu et al.2007).
The poorly resolved Clade D (Figure 1) comprises five subclades: two poorly supported (Clades D1 and D2) and three strongly (Clades PET, SVLB, and BAR). Clade D1 consists of three species restricted to limestone outcrops: B. bataiensis Kiew (sect. Leprosae) from southwestern Vietnam (Tam et al.2005), B. kingiana (sect. Ridleyella) from Peninsular Malaysia (Kiew2005), and B. boisiana from central and northern Vietnam (Tebbitt2005) of the southern limit of the Sino-Vietnamese limestone karsts. Clade D2 is composed of two species of sect. Reichenheimia of Sunda Shelf (B. goegoensis N.E. Br. and B. yappii Ridl.) and B. peltatifolia H.L. Li (sect. Diploclinium) endemic to Hainan, China (Gu et al.2007). This latter species is morphologically assignable to the redefined sect. Baryandra (= Clade BAR in Figure 1 or DIP II in Thomas et al.2011a), a strongly supported clade expanding Philippines’ monophyletic sect. Baryandra (B. oxysperma) to include species of sect. Diploclinum centered in the Philippines and adjacent SE Asian islands (Rubite2012; Rubite et al.2013). The strongly supported (LB: 100, PB: 94, PP: 1) Clade PET is composed of all sampled species of the species-rich SE Asian sect. Petermannia (including sect. Symbegonia) except for B. sinofloribunda, which is nested within Clade SVLB with strong supports (LB: 98, PB: 82, PP: 1). With the addition of B. sinofloribunda, a rhizomatous species distributed in southern Guangxi, the clade of Sino-Vietnamese limestone Begonia (Clade SVLB) encompasses all sampled rhizomatous limestone Begonia distributed in southern China and northern Vietnam, including all sampled species of sect. Coelocentrum, two species of sect. Leprosae (B. leprosa Hance and B. cylindrica D.R. Liang & X.X. Chen), and two species of sect. Diploclinium (B. cavaleriei H. Lév. and B. pulvinifera C.I Peng & Yan Liu). Within the strongly supported Clade SVLB, phylogenetic resolutions are low and weakly supported (Figure 1).
The maximum clade credibility chronogram and the summary of the divergence time estimates and clade supports (PP) generated by the BEAST software are shown in Figure 2 and Table 2
Maximum clade credibility chronogram estimated by BEAST. The rhombus (◇) and star (☆) signs denote calibration points for molecular dating. Dashed clades indicate PP < 0.75 and thicken clades indicate PP ≥ 0.95. Node heights indicate mean ages, with 95% highest posterior density (HPD) date ranges shown by the node bars. Clades with PP ≥ 0.75 are numbered and their clade support and divergent ages mean (95% HPD) are shown in Table 2.
The crown age of Clade 38, corresponding to Clade D of Thomas et al. (2011a) and Clade D49 of Thomas et al. (2012) that includes sect. Coelocentrum, Petermannia, and Baryandra (= DIP II), was estimated to be 12.64 mya, with the HPD date ranging from 8.54 to 16.67 mya. For Clade SVLB the mean divergent crown age was estimated to be 8.06 mya, with the HPD date ranging from 5.09 to 11.21 mya (Figure 2; Table 2).
Discussion
Although relationships among deep nodes of Asian Begonia remain poorly supported in our current study, the basic topology and major lineages identified (Figure 1) are largely congruent with previous studies (Tebbitt et al.2006; Rajbhandary et al.2011; Thomas et al.2011a2012) with some exceptions. For instance, our ML tree (Figure 1) indicates a sister relationship between the Indian species B. dipetala (sect. Haagea) and the rest of Asian Begonia, while B. dipetala was placed sister to Clades B + C1 + C2 with weak support in Thomas et al. (2011a). With the exclusion of B. sinofloribunda, sect. Petermannia (Clade PET) of Clade D is monophyletic with strong support (Figure 1); however, in Thomas et al. (2011a) this section is polyphyletic, separated into two strongly clades (Clade PET I and PET II) with Clade PET I basal to the subclade encompassing Clades PET II, BRA (sect. Bracteibegonia A. DC.), DIP II (= Clade BAR in Figure 1), REI, and B. kingiana (sect. Ridleyella). It is worth to mention, however, that Clade BAR is the basal clade of the Platycentrum-Sphanenthera clade in Tebbitt et al. (2006). Additionally while Clade SVLB is placed sister to sect. Petermannia in present study (Figure 1), sect. Coelocentrum is the basal clade sister to the rest of Clade D in Thomas et al. (2011a). However, except for the monophyly of Clade PET (PET I + PET II) in present study, supports for relationships of these deep nodes are low, indicating that they are mostly soft incongruence (Wiens1998). Additionally conflicts between our and previous studies could also be attributable to the limited phylogenetic signals of the molecular markers employed in the current analyses. Alternatively, past hybridization events could have also led to the incongruence between the chloroplast-based relationships in Thomas et al. (2011a) and those unveiled by our study using the combination of ITS and chloroplast markers (Goodall-Copestake et al.2009; Dewitte et al.2011; Thomas et al.2011a), as reports on interspecific hybridizations have become increasingly common in Asian Begonia (e.g., Peng et al.2010). Because a full discussion of phylogenetic relationships of Asian Begonia is beyond the scope of present study, our discussion is limited to well-supported clades pertaining to the evolution of limestone Begonia of China.
Taxonomic implications of the phylogenetic analyses
Based on a very different set of taxonomic sampling, our analyses corroborate results of previous analyses (Tebbitt et al.2006; Thomas et al.2011a) in confirming the non-monophyly of most major Asian sections, including sect. Diploclinium, Leprosae, Petermannia, Platycentrum, Reichenheimia, and Sphenanthera (Figure 1). Additionally, our expanded sampling of Chinese Begonia species further demonstrates the paraphyly of sect. Coelocentrum, dominating in the strongly supported Clade SVLB otherwise also composed of two species of sect. Diploclinium (B. cavaleriei and B. pulvinifera), two species of sect. Leprosae (i.e., B. cylindrica and B. leprosa), and one species of sect. Petermannia (B. sinofloribunda). Despite being one of the biggest (47 species) and morphologically most diverse sections in China, only three rhizomatous species of sect. Diploclinium (B. cavalerieri, B. pulvinifera, and B. wangii) are known from the Sino-Vietnamese limestone karsts (Gu et al.2007; Peng et al.2006), none sampled in previous phylogenetic analyses. Our study thus further strengthens the polyphyly of sect. Diploclinium and the homoplasious nature of its diagnostic characters, i.e., bilamellate, 3-locular axillary placentation, in Asian Begonia (Thomas et al.2011a).
Sect. Leprosae, raised for three species (B. cylindrica, B. leprosa and B. longicarpa) bearing cylindric and berry-like fruits in southern China (Shui et al.2002), has been questioned to be artificial when B. bataiensis Kiew, a tuberous species bearing the characteristic fruit type from limestone karsts of southwestern corner of Vietnam described (Tam et al.2005). Subsequently molecular study sampling two species (B. leprosa and B. longicarpa) showed that sect. Leprosae is polyphyletic with its type species B. leprosa (limestone species) placed sister to sect. Coelocentrum and B. longicarpa (not limestone species) nested within clade dominated by sect. Platycentrum and Sphenanthera, suggesting that the cylindric and berry-like fruits evolved independently (Tebbitt et al.2006). With all four recognized species sampled, our analyses attested further the polyphyly of sect. Leprosae, with two limestone species (B. cylindrica and B. leprosa) in the Sino-Vietnamese karsts nested within Clade SVLB, B. longicarpa in Clade PLA-SPH, and B. bataienssi sister to B. kingiana (Figure 1).
Known only from hills of limestone karsts of the southern Guangxi adjacent to the Vietnamese border (Gu et al.2007), B. sinofloribunda, a rare species uniquely characterized by branched aerial, subwoody stems and pendulous inflorescences with small flowers and ovaries showing axillary placentas, has been variously classified in sect. Platycentrum (Gu2007; Ku1999), Diploclinium (Shui et al.2002 and Petermannia (Shui and Chen2004). Our analysis nevertheless indicates strongly that B. sinofloribunda is derived from within Clade SVLB (Figure 1), greatly increase the morphological diversity of Clade SVLB.
Based on our study, although species of Clade SVLB vary greatly in their ovary anatomy and fruit type that are crucial for sectional classification in Begonia, they are all rhizomatous distributed exclusively in cave-like microhabitats of the limestone karsts of the Sino-Vietnamese bordering areas. Additionally all available data, including an unpublished chromosome count of B. sinofloribunda (Peng, unpublished data), indicate that members of Clade SVLB are uniformly characterized by the chromosomal number 2n = 30 Gu et al. (2007; Peng et al.2010; Thomas et al.2011a) or its triploid number 2n = 45 (Peng et al.2013). Given the obvious cohesiveness in their perennation organs as well as ecology, biogeography and chromosome cytology, we thus propose to expand the definition of sect. Coelocentrum (see Taxonomic Treatment) to include B. sinofloribunda, three species of sect. Diploclinium (B. cavaleriei, B. pulvinifera, and B. wangii; see Taxonomic Treatment) and two species of sect. Leprosae (B. cylindrica and B. leprosa).
Phylogenetics and molecular dating of Clade SVLB
Except for species of Clade SVLB, the only Begonia species found on the Sino-Vietnamese limestone karsts are the tuberous B. chingii Irmsch. and B. grandis (Gu et al.2007) of the DIP I grade (Figure 1) and B. boisiana that possesses upright stems (Tebbitt2005). The phylogenetic relationships therefore indicate that, in the Sino-Vietnamese limestone karsts, Begonia had evolved to adapt to the harsh limestone habitats four times, twice evolved with the tuberous and deciduous habit (or once and secondary invading the none-limestone habitat by B. labordei, Figure 1), once by the species with upright stem (B. boisiana), and once by the rhizomatous ancestor of Clade SVLB (Figure 1). Interestingly, although tubers and a deciduous habit appear to be suitable for surviving in the desiccation-prone limestone habitats such as most karst Begonia in Madagascar (Grubb2003; Keraudren-Aymonin1983), our data strongly suggest that it is the rhizomatous Clade SVLB that succeeded and proliferated in the Sino-Vietnamese limestone karst terrains.
Within the strongly supported Clade SVLB, phylogenetic resolution is poor (Figure 1), possibly indicating a rapid species radiation (Hughes and Eastwood2006; Kozak et al.2006). Based on the divergence times calculated by BEAST, the crown age of Clade SVLB (Clade 35 in Figure 2; Table 2) was estimated to be ca. 8.06 (HPD 5.09–11.21) mya during the middle to late Miocene, with the stem age extending back to ca. 11 mya (Figure 2). Although these age estimates are likely problematic because of the employment of secondary calibration, even within the lowest end (ca. 5 mya), the crown age of the Sino-Vietnamese limestone Begonia is much older than those estimated in recent phylogenetic literature such as species radiations triggered by the Pleistocene uplift of the South American Andes (e.g., Hughes and Eastwood2006; Hoorn et al.2010).
Interestingly, the estimated crown age of Clade SVLB at 8.06 (HPD 5.09–11.21) mya (Table 2; Figure 2) appears to coincide well with the most extensive stage of karstifications in Guangxi during the Miocene (Liu19972003) and the onset and intensification of the East Asian monsoon at ca. 7.2 (An2000) to 11 mya (Zheng et al.2004). In Asia, the climate has been affected strongly by the altitude of the Himalayas and the Tibetan Plateau. By the late Miocene, the coeval uplift of Himalaya-Tibetan region had attained significant heights that greatly altered Asian atmospheric circulation, resulting in the onset of the warm and humid East Asian summer monsoon (An et al.2001). Because high temperature and precipitation brought by the summer monsoon are favorable conditions for the development of karsts (Zhang1989), the late Miocene onset of the East Asian monsoon would have greatly facilitated the development of limestone karsts across the Sino-Vietnamese limestone regions (Liu19972003), resulting in the remarkable landscapes and providing ample habitats for the spreading and diversification of limestone karst plants such as Begonia. Recently the uplift of Himalaya-Tibetan region and intensification of East Asian monsoon have also been suggested to be responsible for triggering the rapid diversification of both seasonally and wet adapted Begonia in continental Asia (Rajbhandary et al.2011) and the fern genus Lepisorus in China and Japan (Wang et al.2012).
Diversity pattern of Clade SVLB
Species of the Clade SVLB, comprising ca. 60 species (see Taxonomic Treatment) mainly distributed in Guangxi, inhabit the ‘twilight zone’ (Poulson and White1969) of limestone caves and cave-like microhabitats such as shaded fissures and crevices abundantly occurring throughout the Sino-Vietnamese limestone karst region (Sweeting1978; Schindler1982; Xu1995; Zhu2007). Such cave-like microhabitats, characterized by constant temperature, high humidity, and indirect and low light (Liang et al.2010), function as shelters on the limestone bedrock characterized by highly alkaline conditions, thin soil layers, and severe desiccation due to high porosity (Clements et al.2006). In addition to Begonia, these cave-like microhabitats are also inhabited by several species-rich, calciphilous herbaceous plant groups such as Aspidistra Ker Gawl. (e.g., Liu et al.2011), a number of genera of gesnerids (e.g., Weber et al.2011; Xu et al.2012a2012b), Elatostema L. (e.g., Wei et al.2011), Impatiens (e.g., Yu et al.2009), and the fern genus Polystichum (e.g., Zhang and He2011), etc. Many of these limestone plants are often highly ornamental and have long been commercially exploited (Vermeulen and Whitten1999). Biogeographically most of these calciphilous cave plant species have a very limited distribution ranges and site-endemics are very common (Liu et al.2007; Peng et al.2008a2012; Xu et al.2012a; Zhang and He2011). In individual caves or crevices, species diversity is often low; however, across the landscapes, plant composition varies greatly from one cave to another, resulting in low α diversity and yet extremely high β diversity across the landscape (Hughes and Hollingsworth2008).
Phylogenetic niche conservatism of Clade SVLB
The monophyly of the rhizomatous Clade SVLB suggests a single evolutionary origin of the adaptation to the cave-like limestone microhabitats, and shows that ecology is good indicator of taxonomic relationships than traditional morphological characters used to defined sections. Inspecting the chronogram (Figure 2) reveals a 3 MY-time lag between the estimated stem and crown age of Clade SVLB, which might signify a long period needed for their ancestor to gradually adapt to the calcium-rich and cave-like microhabitats on the limestone karsts before the commencement of species radiation. Its monophyly and prolonged existence since the late Miocene also suggest the strong tendency in the evolution of this clade to retain the ancestral ecological niche, presenting an apparent case of strong ‘phylogenetic niche conservatism’ (Kozak et al.2006; Wiens2004).
Cave-like microhabitat as paleorefugia
Limestone caves and crevices/fissures are formed through the development of underground hydrological systems with maximum discharge and dissolutional aggressiveness (Palmer1991). The development and ‘collapse’ of caves are essential processes shaping the karst topography (Ford and Williams2007; Palmer1991). In more recent histories, anthropogenic factors have also greatly accelerated the destruction of caves and cave-like microhabitats in the karsts (Clements et al.2006). Together with the observations of the unique ecology of limestone Begonia ,limestone caves and crevices currently inhabited by rich plant communities can be viewed as “biological refugia, habitats that support populations not able to live elsewhere in a landscape (Nekola1999”. More precisely, these cave-like microhabitats in the Sino-Vietnamese limestone karsts qualify as “paleorefugia, now-fragmented relicts of a formerly widespread matrix community (Nekola1999)”. On the contrary, “neorefugia represent habitats that are younger than the surrounding biological matrix (Nekola1999)”.
Clade SVLB as an example of a non-adaptive radiation on limestone karsts
The onset of Asian summer monsoon in the late Miocene (An et al.2001) provided ideal conditions for the karst development (Zhang1989) across the limestone terrains of the Sino-Vietnamese bordering region (Liu1997), resulting in numerous crevices, fissures, and caves and the splendid fenglins and fengcongs across the landscapes. Although limestone karsts are an unsuitable growth substrate for most plants, over time, some plant groups (e.g., Aspidistra, Begonia, Impatiens, various gesnerid genera, Elatostema, etc.) adapted to and flourishing in the cave-like microhabitats. However, cave-like microhabitats are fragile and collapsed constantly due to the processes of karstification (Palmer1991) associated with the persistence of East Asian monsoon (An et al.2001; Liu1997), resulting in fragmentation and isolation of these microhabitats. Moreover, because of the strong niche conservatism (Wiens2004) to the cave-like microhabitats, populations of the calciphilous plants had been forced to track the paleorefugia (Wiens2004; Kozak et al.2006; Comes et al.2008). Moreover, because Begonia species are poor dispersers (Hughes and Hollingsworth2008), gene flow among populations would be retarded, greatly augmenting effects of random genetic drift (Comes et al.2008; Hughes and Hollingsworth2008). As geological weathering processes have occurred continuously throughout the Sino-Vietnamese limestone karsts, population diversification likely would have resulted in steady accumulation of species (Kozak et al.2006; Comes et al.2008; Hughes and Hollingsworth2008). However, because the major driving force of this evolutionary process is geographic isolation, the diversity pattern is better characterized as ‘non-adaptive radiation’, an underappreciated speciation mode crucial for rapid species accumulations in organisms of low vagility and strong niche conservatism (Gittenberger1991; Kozak et al.2006; Comes et al.2008; Rundell and Price2009). Morphologically, species of Clade SVLB all have very similar flowers, distinguishing from one another mainly by vegetative characters such as leaf morphology in shape, pubescence, texture, and variegation (Gu et al.2007). Recent studies, however, show that different variegations in Begonia leaves have no apparent adaptive differences (Sheue et al.2012; Zhang et al.2009), suggesting that the species accumulation in Begonia was mainly a non-adaptive process (Gittenberger1991; Kozak et al.2006; Comes et al.2008; Rundell and Price2009), though further studies are definitely needed to test the non-adaptive nature of leaf morphology in Clade SVLB.
Conclusions
The proposed scenario of species diversification in Clade SVLB driven by geological and climatic conditions in the Sino-Vietnamese limestone karst should have also affected other calciphilous cave plants in the region, resulting in concordant phylogenetic patterns across lineages. It is also worth noting that the biology of those calciphilous limestone karst plants appears to be highly similar to some amphibians (e.g., salamanders and frogs) whose populations mainly differentiate via isolation-by-distance (IBD) and allopatric speciation has been the primary mode responsible for species diversification because of their low vagility and strong niche conservatism in fragmented landscapes (Kozak et al.2006; Fouquet et al.2007). As such, a positive correlation between genetic and geographic distances is expected among populations of a species and IBD might still be observed among closely related and recently diversified species (Fouquet et al.2007), resulting in geographically constrained monophyly in species (Fouquet et al.2007; Hughes and Hollingsworth2008; Dewitte et al.2011). The predicted phylogenetic and population genetic patterns thus form testable hypotheses for further population genetic studies and better sampled Begonia phylogenetic analyses as well as other limestone cave plants in the Sino-Vietnamese limestone karst region and other major karst ecosystems worldwide.
Taxonomic Treatment
Synopsis of Begonia sect. Coelocentrum Irmsch.
Begonia sect. Coelocentrum Irmsch., Mitt. Inst. Allg. Bot. Hamburg 10: 533. 1939; Doorenbos et al., The section of Begonia 84. 1998; Ku, Fl. Reipubl. Popularis Sin. 52(1): 127, 129. 1999; Shui et al., Bot. Bull. Acad. Sin. 43: 314. 2002; Gu, Fl. China 13: 205. 2007. LECTOTYPE SPECIES: B. porteri H. Lév. & Vaniot (designated by Barkley and Baranov1972).—Begonia sect. Leprosae (T.C. Ku) Y.M. Shui, Bot. Bull. Acad. Sin. 43: 321. 2002, syn. nov.
Herbs monoecious, terrestrial, perennial, rhizomatous without erect stems and tubers, acaulescent, rhizome herbaceous or slightly woody. Leaves alternate, variable, mostly broadly ovate, oblique at base, rarely palmate, palmately compound, or occasionally peltate, glabrous to tomentose, often variegated. Inflorescence axillary, cymose (dichasial or dichasial at base and monochasial at apex), rarely thyrsoid, staminate flowers basal and carpellate flowers distal, protandrous. Perianth segments free, usually white or pink, rarely greenish or yellowish. Staminate flower with 4, or rarely 2 tepals. Carpellate flowers usually with 3 tepals, rarely 2 or 5 (–6); ovary 3-winged, or rarely unwinged, wings equal to unequal in fruit, mostly 1-locular, parietal placentation, rarely 3-locular with axile placentation. Fruit a 3-winged capsule, nodding, or rarely berry-like, cylindric.
Cytology. 2n = 30 or rarely 45 (Peng et al.200620072008b201020122013;Gu et al. 2007; Liu et al.2007).
Distribution and Habitat. Currently 61 species, two varieties, and one natural hybrid distributed in the caves, fissures and forest floors of limestone karsts in southern China (Guangxi, southern Guizhou, and southeastern Yunnan provinces) and northern Vietnam, at elevations between ca. 100 and 1,300 m.
Species Included and Distribution—B. arachnoidea C.I Peng, S.M. Ku & Yan Liu (China: Guangxi), B. asteropyrifolia Y.M. Shui & W.H. Chen (China: Guangxi), B. aurantiflora C.I Peng, Yan Liu & S.M. Ku (China: Guangxi), B. auritistipula Y.M. Shui & W.H. Chen (China: Guangxi), B. austroguangxiensis Y.M. Shui & W.H. Chen (China: Guangxi), B. babeana Aver. & H.Q. Nguyen (Vietnam), B. bamaensis Yan Liu & C.I Peng (China: Guangxi), B. biflora T.C. Ku (China: Yunnan; Vietnam), B. ×breviscapa C.I Peng, Yan Liu & S.M. Ku (China: Guangxi), B. cavaleriei H. Lév. (China: Guangxi, Yunnan, Guizhou; Vietnam), B. chongzuoensis Yan Liu, S.M. Ku & C.I Peng (China: Guangxi), B. cirrosa L.B. Sm. & Wassh. (China: Guangxi, Yunnan), B. crystallina Y.M. Shui & W.H. Chen (China: Yunnan), B. curvicarpa S.M. Ku, C.I Peng & Yan Liu (China: Guangxi), B. cylindrica D.R. Liang & X.X. Chen (China: Guangxi), B. daxinensis T.C. Ku (China: Guangxi), B. debaoensis C.I Peng, Yan Liu & S.M. Ku (China: Guangxi), B. fangii Y.M. Shui & C.I Peng (China: Guangxi), B. ferox C.I Peng & Yan Liu (China: Guangxi), B. filiformis Irmsch. (China: Guangxi), B. fimbribracteata Y.M. Shui & W.H. Chen (China: Guangxi), B. guangxiensis C.Y. Wu (China: Guangxi), B. huangii Y.M. Shui & W.H. Chen (China: Yunnan), B. jingxiensis D. Fang & Y.G. Wei (China: Guangxi), B. kui C.I Peng (Vietname), B. lanternaria Irmsch. (China: Guangxi; Vietnam), B. leprosa Hance (China: Guangxi, Guangdong), B. liuyanii C.I Peng, S.M. Ku & W.C. Leong (China: Guangxi), B. longgangensis C.I Peng & Yan Liu (China: Guangxi), B. longistyla Y.M. Shui & W.H. Chen (China: Yunnan), B. luochengensis S.M. Ku, C.I Peng & Yan Liu (China: Guangxi), B. luzhaiensis T.C. Ku (China: Guangxi), B. mashanica D. Fang & D.H. Qin (China: Guangxi), B. masoniana Irmsch. (China: Guangxi; Vietnam), B. morsei Irmsch. (China: Guangxi), B. nahangensis Aver. & H.Q. Nguyen (Vietnam), B. ningmingensis D. Fang, Y.G. Wei & C.I Peng (China: Guangxi), B. ningmingensis var. bella D. Fang, Y.G. Wei & C.I Peng (China: Guangxi), B. obliquifolia S.H. Huang & Y.M. Shui (China: Yunnan), B. ornithophylla Irmsch. (China: Guangxi), B. pengii S.M. Ku & Yan Liu (China: Guangxi), B. phuthoensis H.Q. Nguyen (Vietnam), B. picturata Yan Liu, S.M. Ku & C.I Peng (China: Guangxi), B. platycarpa Y.M. Shui & W.H. Chen (China: Yunnan), B. porteri H. Lév. & Vaniot (China: Guangxi, Guizhou), B. porteri var. macrorhiza Gagnep. (Vietnam), B. pseudodaxinensis S.M. Ku, Yan Liu & C.I Peng (China: Guangxi), B. pseudodryadis C.Y. Wu (China: Yunnan), B. pseudoleprosa C.I Peng, Yan Liu & S.M. Ku (China: Guangxi), B. pulvinifera C.I Peng & Yan Liu (China: Guangxi), B. retinervia D. Fang, D.H. Qin & C.I Peng (China: Guangxi), B. rhynchocarpa Y.M. Shui & W.H. Chen (China: Yunnan), B. rugosula Aver. (Vietnam), B. semiparietalis Yan Liu, S.M. Ku & C.I Peng (China: Guangxi), B. setulosopeltata C.Y. Wu (China: Guangxi), B. sinofloribunda Dorr (China: Guangxi), B. sonlaensis Aver. (Vietnam), B. subcoriacea C.I Peng, Yan Liu & S.M. Ku (China: Guangxi), B. suboblata D. Fang & D.H. Qin (China: Guangxi), B. umbraculifolia Y. Wan & B.N. Chang (China: Gaungxi), B. variifolia Y.M. Shui & W.H. Chen (China: Guangxi), B. wangii T.T. Yu (China: Guangxi, Yunnan), B. zhengyiana Y.M. Shui (China: Yunnan).
Notes—Unpublished data in our ongoing phylogenetic study indicates that B. wangii T.T. Yu, previously assigned to sect. Diploclinium (Shui et al.2002), is nested firmly within Clade SVLB and hereby transferred to sect. Coelocentrum.
References
An Z-S: The history and variability of the East Asian paleomonsoon climate. Quaternary Sci Rev 2000, 19: 171–187. doi: 10.1016/S0277–3791(99)00060–8 doi: 10.1016/S0277-3791(99)00060-8
An Z-S, Kutzbach JE, Prell WL, Porter SC: Evolution of Asian monsoons and phased uplift of the Himalayan-Tibetan plateau since Late Miocene times. Nature 2001, 411: 62–66. doi: 10.1038/35075035 doi: 10.1038/35075035
Averyanov LV, Nguyen HQ: Eleven new species of Begonia L. (Begoniaceae) from Laos and Vietnam. Turczaninowia 2012, 15: 5–32.
Barkley FA, Baranov AI: The sections of Begoniaceae. Buxtonia 1972, 1(supplement):1–8.
Clement WL, Tebbitt MC, Forrest LL, Blair JE, Brouillet L, Eriksson T, Swensen SM: Phylogenetic position and biogeography of Hillebrandia sandwicensis (Begoniaceae): a rare Hawaiian relict. Amer J Bot 2004, 91: 905–917. doi: 10.3732/ajb.91.6.905 doi: 10.3732/ajb.91.6.905
Clements R, Sodhi NS, Schilthuizen M, Ng PKL: Limestone karsts of Southeast Asia: imperiled arks of biodiversity. BiosCience 2006, 56: 733–742. doi:10.1641/0006–3568(2006)56[733:LKOSAI]2.0.CO;2 doi:10.1641/0006-3568(2006)56[733:LKOSAI]2.0.CO;2
Comes HP, Tribsch A, Bittkau C: Plant speciation in continental island floras as exemplified by Nigella in the Aegean Archipelago. Philos T Roy Soc B 2008, 363: 3083–3096. doi: 10.1098/rstb.2008.0063 doi: 10.1098/rstb.2008.0063
de Wilde JJFE, Hughes M, Rodda M, Thomas DC: Pliocene intercontinental dispersal from Africa to Southeast Asia highlighted by the new species Begonia afromigrata (Begoniaceae). Taxon 2011, 60: 1685–1692.
Dewitte A, Twyford AD, Thomas DC, Kidner CA, Huylenbroeck JV: The origin of diversity in Begonia : genome dynamism, population processes and phylogenetic patterns. In The dynamical processes of biodiversity—Case studies of evolution and spatial distribution. Edited by: Grillo O, Venora G. Tech Open Access; 2011:27–52. doi:10.5772/23789 doi:10.5772/23789
Doorenbos J, Sosef MSM, Wilde JJFE: The sections of Begonia. Wageningen: Wageningen Agriculture University; 1998.
Drummond AJ, Rambaut A: BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 2007, 7: 214. doi:10.1186/1471–2148–7-214 doi:10.1186/1471-2148-7-214
Ford D, Williams P: Karst hydrogeology and geomorphology. Chichester, England: John Wiley & Sons; 2007.
Fouquet A, Gilles A, Vences M, Marty C, Blanc M, Gemmell NJ: Underestimation of species richness in neotropical frogs revealed by mtDNA analyses. PLoS ONE 2007, 2: e1109. doi:10.1371/journal.pone.0001109 doi:10.1371/journal.pone.0001109
Frodin DG: History and concepts of big plant genera. Taxon 2004, 53: 753–776.
Gillieson D: Karst in Southeast Asia. In The physical geography of Southeast Asia. Edited by: Gupta A. Oxford: Oxford University Press; 2005:157–176.
Gittenberger E: What about non-adaptive radiation? Biol J Linn Soc 1991, 43: 263–272. doi:10.1111/j.1095–8312.1991.tb00598.x doi:10.1111/j.1095-8312.1991.tb00598.x
Goodall-Copestake WP, Harris DJ, Hollingsworth PM: The origin of a mega-diverse genus: dating Begonia (Begoniaceae) using alternative datasets, calibrations and relaxed clock methods. Bot J Linn Soc 2009, 159: 363–380. doi:10.1111/j.1095–8339.2009.00948.x doi:10.1111/j.1095-8339.2009.00948.x
Goodall-Copestake WP, Perez-Espona S, Harris DJ, Hollingsworth PM: The early evolution of the mega-diverse genus Begonia (Begoniaceae) inferred from organelle DNA phylogenies. Biol J Linn Soc 2010, 101: 243–250. doi:10.1111/j.1095–8312.2010.01489.x doi:10.1111/j.1095-8312.2010.01489.x
Grubb PJ: Interpreting some outstanding features of the flora and vegetation of Madagascar. Perspect Plant Ecol 2003, 6: 125–146. doi:10.1078/1433–8319–00046 doi:10.1078/1433-8319-00046
Gu C-Z: Infrageneric classification of Begonia . In Flora of China, vol 13. Edited by: Wu Z-Y, Raven PH, Hong D-Y. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press; 2007:205–207.
Gu C-Z, Peng C-I, Turland NJ: Begoniaceae. In Flora of China, vol 13. Edited by: Wu Z-Y, Raven PH, Hong D-Y. Beijing and St. Louis: Science Press and Missouri Botanical Garden; 2007:153–207.
Hoorn C, Wesselingh FP, ter Steege H, Bermudez MA, Mora A, Sevink J, Sanmartin I, Sanchez-Meseguer A, Anderson CL, Figueiredo JP, Jaramillo C, Riff D, Negri FR, Hooghiemstra H, Lundberg J, Stadler T, Sarkinen T, Antonelli A: Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 2010, 330: 927–931. doi:10.1126/science.1194585 doi:10.1126/science.1194585
Hoover WS, Karegeannes C, Wiriadinata H, Hunter JM: Notes on the geography of South-East Asian Begonia and species diversity in montane forests. Telopea 2004, 10: 749–764.
Hughes C, Eastwood R: Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA 2006, 103: 10334–10339. doi:10.1073/pnas.0601928103 doi:10.1073/pnas.0601928103
Hughes M, Girmansyah D: A revision of Begonia sect. Sphenanthera (Hassk.) Warb. from Sumatra. Gard Bull Singapore 2011, 62: 27–39.
Hughes M, Hollingsworth PM: Population genetic divergence corresponds with species-level biodiversity patterns in the large genus Begonia . Mol Ecol 2008, 17: 2643–2651. doi:10.1111/j.1365–294X.2008.03788.x doi:10.1111/j.1365-294X.2008.03788.x
Hughes M, Pullan M: Southeast Asian Begonia Database. 2007. . Accessed 19 Aug 2013 http://www.rbge.org.uk . Accessed 19 Aug 2013
Keraudren-Aymonin M: Begoniacées. In Flore de Madagascar et des Comores, vol Famille 144. Edited by: Leroy J-F. Paris: Muséum national d’histoire naturelle; 1983:7–108.
Kiew R: The limestone begonias of Sabah, Borneo—Flagship species for conservation. Gard Bull Singapore 2001, 53: 241–286.
Kiew R: Begonia sabahensis Kiew & J.H.Tan (Begoniaceae), a new yellow-flowered Begonia from Borneo. Gard Bull Singapore 2004, 56: 73–77.
Kiew R: Begonias of Peninsular Malaysia. Kota Kinabalu, Borneo: Natural History Publications; 2005.
Kiew R: Notes on Vietnamese Begonia (Begoniaceae), including three new species. Adansonia 2007, 29(3):229–238.
Kopperud C, Einset JW: DNA isolation from Begonia leaves. Plant Mol Biol Rep 1995, 13: 129–130. doi:10.1007/BF02668783 doi:10.1007/BF02668783
Kozak KH, Weisrock DW, Larson A: Rapid lineage accumulation in a non-adaptive radiation: phylogenetic analysis of diversification rates in eastern North American woodland salamanders (Plethodontidae: Plethodon ). P Roy Soc B-Biol Sci 2006, 273: 539–546. doi:10.1098/rspb.2005.3326 doi:10.1098/rspb.2005.3326
Ku T-C: Begoniaceae. In Flora Reipublicae Popularis Sinicae, vol. 52(1). Edited by: Ku T-C. Beijing: Science Press; 1999:126–269.
Ku S-M, Kono Y, Liu Y: Begonia pengii (sect. Coelocentrum , Begoniaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2008, 49: 167–175.
Li H-Z, Ma H, Zhou Z-K, Guan K-Y: A new species of Begonia (Begoniaceae) from Guangxi, China. Bot J Linn Soc 2008, 157: 83–90. doi:10.1111/j.1095–8339.2008.00771.x doi:10.1111/j.1095-8339.2008.00771.x
Liang K-M, Lin Z-F, Liu N, Zhang Q-M, Wang J, Wang Z-F, Guan L-L: Characteristics of sun- and shade-adapted populations of an endangered plant Primulina tabacum Hance. Photosynthetica 2010, 48: 494–506. doi:10.1007/s11099–010–0066–8 doi:10.1007/s11099-010-0066-8
Liu J-R: The development history of the Guangxi tropical karst geomorphology and its sequences. Carsologica Sin 1997, 16: 332–345.
Liu J-R: A re-discussion on the developing history of the high cluster peak in the west and northwest Guangxi. Carsologica Sin 2003, 22: 191–196.
Liu Y, Ku S-M, Peng C-I: Begonia bamaensis (sect. Coelocentrum , Begoniaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2007, 48: 465–473.
Liu Y, Kono Y, Lin C-R, Xu W-B, Peng C-I: Aspidistra erecta (Asparagaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2011, 52: 367–373.
Nekola JC: Paleorefugia and neorefugia: the influence of colonization history on community pattern and process. Ecology 1999, 80: 2459–2473. doi:10.1890/0012–9658(1999)080[2459:PANTIO]2.0.CO;2 doi:10.1890/0012-9658(1999)080[2459:PANTIO]2.0.CO;2
Nylander JAA: MrModeltest, version 2. Evotionary Biology Centre. Uppsala University; 2004. . Accessed 16 Oct 2012 https://github.com/nylander/MrModeltest2 . Accessed 16 Oct 2012
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL: AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 2008, 24: 581–583. doi:10.1093/bioinformatics/btm388 doi:10.1093/bioinformatics/btm388
Palmer AN: Origin and morphology of limestone caves. Geol Soc Am Bull 1991, 103: 1–21. doi:10.1130/0016–7606(1991)103?<?0001:OAMOLC?>?2.3.CO;2 doi:10.1130/0016-7606(1991)103?<?0001:OAMOLC?>?2.3.CO;2
Peng C-I, Leong W-C, Ku S-M, Liu Y: Begonia pulvinifera (sect. Diploclinium , Begoniaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2006, 47: 319–327.
Peng C-I, Hsieh T-Y, Ngyuen QH: Begonia kui (sect. Coelocentrum , Begoniaceae), a new species from Vietnam. Bot Stud 2007, 48: 127–132.
Peng C-I, Ku S-M, Kono Y, Chung K-F, Liu Y: Two new species of Begonia (sect. Coelocentrum , Begoniaceae) from limestone areas in Guangxi, China: B. arachnoidea and B. subcoriacea . Bot Stud 2008, 49: 405–418.
Peng C-I, Liu Y, Ku S-M: Begonia aurantiflora (sect. Coelocentrum , Begoniaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2008, 49: 83–92.
Peng C-I, Liu Y, Ku S-M, Kono Y, Chung K-F: Begonia × breviscapa (Begoniaceae), a new intersectional natural hybrid from limestone areas in Guangxi, China. Bot Stud 2010, 51: 107–117.
Peng C-I, Ku S-M, Kono Y, Liu Y: Begonia chongzuoensis (sect. Coelocentrum , Begoniaceae), a new calciphile from Guangxi, China. Bot Stud 2012, 53: 285–292.
Peng C-I, Yang H-A, Kono Y, Chung K-F, Huang Y-S, Wu W-H, Liu Y: Novelties in Begonia sect. Coelocentrum: B. longgangensis and B. ferox from limestone areas in Guangxi, China. Bot Stud 2013, 54: 44. doi: 10.1186/1999–3110–54–44 doi: 10.1186/1999-3110-54-44
Poulson TL, White WB: The cave environment. Science 1969, 165: 971–981. doi:10.1126/science.165.3897.971 doi:10.1126/science.165.3897.971
Qin H-N, Liu Y (Eds): A checklist of vascular plants of Guangxi. Beijing: Science Press; 2010.
Rajbhandary S, Hughes M, Phuttai T, Thomas DC, Shrestha KK: Asian Begonia : out of Africa via the Himalayas? Gard Bull Singapore 2011, 63: 277–286.
Ronquist F, Huelsenbeck JP: MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19: 1572–1574. doi:10.1093/bioinformatics/btg180 doi:10.1093/bioinformatics/btg180
Rubite RR: Delimitation of Begonia L. sections Diploclinium and Baryandra (Begoniaceae) in the Philippines. Asia Life Sci 2012, 21: 363–373.
Rubite RR, Hughes M, Alejandro G, Peng C-I: Recircumscription of Begonia sect. Baryandra (Begoniaceae): evidence from molecular data. Bot Stud 2013, 54: 38. doi:10.1186/1999–3110–54–38 doi:10.1186/1999-3110-54-38
Rundell RJ, Price TD: Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol Evol 2009, 24: 394–399. doi:10.1016/j.tree.2009.02.007 doi:10.1016/j.tree.2009.02.007
Schindler JS: Karst of China. Ground Water 1982, 20: 226–230. doi:10.1111/j.1745–6584.1982.tb02754.x doi:10.1111/j.1745-6584.1982.tb02754.x
Sheue C-R, Pao S-H, Chien L-F, Chesson P, Peng C-I: Natural foliar variegation without costs? The case of Begonia . Ann Bot 2012, 109: 1065–1074. doi:10.1093/aob/mcs025 doi:10.1093/aob/mcs025
Shui Y-M: Begonia tetralobata (Begoniaceae), a new species from China. Ann Bot Fenn 2007, 44: 76–79.
Shui Y-M, Chen W-H: Revision to sect. Petermannia of Begonia (Begoniaceae) in China. Acta Bot Yunnanica 2004, 26: 482–486.
Shui Y-M, Peng C-I, Wu C-Y: Synopsis of the Chinese species of Begonia (Begoniaceae), with a reappraisal of sectional delimitation. Bot Bull Acad Sin 2002, 43: 313–327.
Stamatakis A, Hoover P, Rougemont J: A rapid bootstrap algorithm for the RAxML web servers. Syst Biol 2008, 57: 758–771. doi:10.1080/10635150802429642 doi:10.1080/10635150802429642
Sweeting MM: Landscape of one-seventh of China. Geogr Mag (London) 1978, 5: 393–400.
Swofford DL: PAUP* 4.0: Phylogenetic analysis using parsimony (* and other methods), v. 4.0 Beta 10. Sunderland, Massachusetts: Sinauer Associates; 2002.
Tam TQ, Kiew R, Vermeulen JJ: Begonia bataiensis Kiew, a new species in Section Leprosae (Begoniaceae) from Vietnam. Gard Bull Singapore 2005, 57: 19–23.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011, 28: 2731–2739. doi:10.1093/molbev/msr121 doi:10.1093/molbev/msr121
Tebbitt MC: Begonias: cultivation, identification, and natural history. Portland: Timber Press; 2005.
Tebbitt MC, Lowe-Forrest L, Santoriello A, Clement WL, Swensen SM: Phylogenetic relationships of Asian Begonia , with an emphasis on the evolution of rain-ballist and animal dispersal mechanisms in sections Platycentrum , Sphenanthera and Leprosae . Syst Bot 2006, 31: 327–336. doi:10.1600/036364406777585784 doi:10.1600/036364406777585784
Thomas DC, Hughes M, Phutthai T, Rajbhandary S, Rubite RR, Ardi WH, Richardson JE: A non-coding plastid DNA phylogeny of Asian Begonia (Begoniaceae): Evidence for morphological homoplasy and sectional polyphyly. Mol Phylogenet Evol 2011, 60: 428–444. doi:10.1016/j.ympev.2011.05.006 doi:10.1016/j.ympev.2011.05.006
Thomas DC, Ardi WH, Hughes M: Nine new species of Begonia (Begoniaceae) from south and west Sulawesi, Indonesia. Edinburgh J Bot 2011, 68: 225–255. doi:10.1017/S0960428611000072 doi:10.1017/S0960428611000072
Thomas DC, Hughes M, Phutthai T, Ardi WH, Rajbhandary S, Rubite RR, Richardson JE: West to east dispersal and subsequent rapid diversification of the mega-diverse genus Begonia (Begoniaceae) in the Malesian archipelago. J Biogeogr 2012, 39: 98–113. doi:10.1111/j.1365–2699.2011.02596.x doi:10.1111/j.1365-2699.2011.02596.x
Vermeulen J, Whitten T: Biodiversity and cultural property in the management of limestone resources. Lessons from East Asia. Washington, DC: The World Bank; 1999.
Waltham T: Fengcong, fenglin, cone karst and tower karst. Cave Karst Sci 2008, 35: 77–88.
Wang L, Schneider H, Zhang X-C, Xiang Q-P: The rise of the Himalaya enforced the diversification of SE Asian ferns by altering the monsoon regimes. BMC Plant Biol 2012, 12: 210. doi:10.1186/1471–2229–12–210 doi:10.1186/1471-2229-12-210
Weber A, Wei Y-G, Puglisi C, Wen F, Mayer V, Möller M: A new definition of the genus Petrocodon (Gesneriaceae). Phytotaxa 2011, 23: 49–67.
Wei Z-D, Shui Y-M, Zhang M-D, Zhang R-M: Begonia coelocentroides Y. M. Shui & Z. D. Wei, a new species of Begoniaceae from Yunnan, China. Acta Phytotax Sin 2007, 45: 86–89.
Wei Y-G, Monro AK, Wang W-T: Addition to the Flora of China: seven new species of Elatostema (Urticaceae) from the karst landscapes of Guangxi and Yunnan. Phytotaxa 2011, 29: 1–27.
Wiens JJ: Combining data sets with different phylogenetic histories. Syst Biol 1998, 47: 568–581. doi:10.1080/106351598260581 doi:10.1080/106351598260581
Wiens JJ: Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 2004, 58: 193–197. doi:10.1111/j.0014–3820.2004.tb01586.x doi:10.1111/j.0014-3820.2004.tb01586.x
Williams P: World heritage caves and karst. Gland: IUCN (International Union for Conservation of Nature); 2008.
Xu Z-R: A study of the vegetation and floristic affinity of the limestone forests in southern and southwestern China. Ann MO Bot Gard 1995, 82: 570–580.
Xu W-B, Pan B, Liu Y, Peng C-I, Chung K-F: Two new species, Primulina multifida and P. pseudomollifolia (Gesneriaceae), from karst caves in Guangxi, China. Bot Stud 2012, 53: 165–175.
Xu W-B, Zhang Q, Wen F, Liao W-B, Pan B, Chang H, Chung K-F: Nine new combinations and one new name of Primulina (Gesneriaceae) from South China. Phytotaxa 2012, 64: 1–8.
Yoder AD, Irwin JA, Payseur BA: Failure of the ILD to determine data combinability for slow loris phylogeny. Syst Biol 2001, 50: 408–424. doi:10.1080/106351501300318003 doi:10.1080/106351501300318003
Yu S-X, Hou Y-T, Chen Y-L, Qin H-N: Impatiens lobulifera (Balsaminaceae), a new species from limestone areas in Guangxi, China. Bot Stud 2009, 50: 365–370.
Zhang L-Y: Some special geomorphic processes of the monsoon area in East China. Catena 1989, 16: 121–134. doi:10.1016/0341–8162(89)90036–2 doi:10.1016/0341-8162(89)90036-2
Zhang L-B, He H: Polystichum fengshanense , sp. nov. (sect. Haplopolystichum , Dryopteridaceae) from Karst Caves in Guangxi, China based on morphological, palynological, and molecular evidence. Syst Bot 2011, 36: 854–861. doi:10.1600/036364411x604877 doi:10.1600/036364411x604877
Zhang Y, Hayashi T, Hosokawa M, Yazawa S, Li Y-H: Metallic lustre and the optical mechanism generated from the leaf surface of Begonia rex Putz. Sci Hortic-Amsterdam 2009, 121: 213–217. doi:10.1016/j.scienta.2009.01.030 doi:10.1016/j.scienta.2009.01.030
Zheng H-B, Powell CM, Rea D-K, Wang J-L, Wang P-X: Late Miocene and mid-Pliocene enhancement of the East Asian monsoon as viewed from the land and sea. Global Planet Change 2004, 41: 147–155. doi:10.1016/j.gloplacha.2004.01.003 doi:10.1016/j.gloplacha.2004.01.003
Zhu H: The karst ecoystem of southern China and its biodiversity. Trop Forestry 2007, 35: 44–47.
Acknowledgements
The authors are grateful to W.-B. Xu (IBK) and Y.-M. Shui (KUN) in China, H. T. Nguyen (CPC Herbarium) in Vietnam, and FRIM staff (KEP) in Peninsula Malaysia, for their supports. Assistances over the years by HAST research assistants H.-Y. Chen, S.-M. Jeng, C.-I Huang, S.-M. Ku, T.-Y. Liu, C.-K. Yang, and M.-C. Yu are deeply appreciated. We thank M. Hughes, M. C. Tebbitt, and D. C. Thomas for critical reviews of the manuscript. This work was supported in part by National Science Council Grants (Taiwan) NSC 98-2621-B-001-002-MY3 and NSC 101-2621-B-001-003-MY3 to CIP and KFC, National Geographic Society (USA) Grant (8358-07) to CIP, YL and KFC, and grants from Academia Sinica to CIP.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KFC, CIP, and YL designed the project and secured funding; all authors contributed to the collecting of the plant materials; WCL collected the molecular data; KFC and WCL performed the analyses. KFC wrote the first draft of the manuscript and all authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chung, KF., Leong, WC., Rubite, R.R. et al. Phylogenetic analyses of Begonia sect. Coelocentrum and allied limestone species of China shed light on the evolution of Sino-Vietnamese karst flora. Bot Stud 55, 1 (2014). https://doi.org/10.1186/1999-3110-55-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1999-3110-55-1