Abstract
Background
Rice (Oryza sativa) is one of the most important food crops in the world. Rice blast, caused by the fungal pathogen Magnaporthe oryzae, is one of the most destructive rice diseases worldwide. To effectively cope with this problem, the use of rice blast resistance varieties through innovative breeding programs is the best strategy to date. The Thai rice variety Jao Hom Nin (JHN) showed broad-spectrum resistance against Thai rice blast isolates. Two QTLs for blast resistance in JHN were reported on chromosome 1 (QTL1) and 11 (QTL11).
Results
Monogenic lines of QTL1 (QTL1-C) and QTL11 (QTL11-C) in the CO39 genetic background were generated. Cluster analysis based on the disease reaction pattern of QTL1-C and QTL11-C, together with IRBLs, showed that those two monogenic lines were clustered with IRBLsh-S (Pish) and IRBL7-M (Pi7), respectively. Moreover, sequence analysis revealed that Pish and Pi7 were embedded within the QTL1 and QTL11 delimited genomic intervals, respectively. This study thus concluded that QTL1 and QTL11 could encode alleles of Pish and Pi7, designated as Pish-J and Pi7-J, respectively. To validate this hypothesis, the genomic regions of Pish-J and Pi7-J were cloned and sequenced. Protein sequence comparison revealed that Pish-J and Pi7-J were identical to Pish and Pi7, respectively. The holistic disease spectrum of JHN was found to be exactly attributed to the additive ones of both QTL1-C and QTL11-C.
Conclusion
JHN showed broad spectrum resistance against Thai and Philippine rice blast isolates. As this study demonstrated, the combination of two resistance genes, Pish-J and Pi7-J, in JHN, with each controlling broad-spectrum resistance to rice blast disease, explains the high level of resistance. Thus, the combination of Pish and Pi7 can provide a practical scheme for breeding durable resistance in rice against rice blast disease.
Similar content being viewed by others
Background
Rice is one of the most important staple food crops in the world. The increasing world population leads to increased demand for rice production. In order to feed the growing population, rice varieties that are resistant to known diseases and that can produce high yields should be used (Khush and Jena 2009). Rice blast, caused by an ascomycete Magnaporthe oryzae, is one of the most destructive diseases of rice worldwide (Ahn et al. 2000), which leads to economic losses of more than 70 billion dollars (Scheuermann et al. 2012). In many Asian countries, the outbreak of rice blast which led to complete losses in rice production has been reported (Sobrizal and Anggiani 2007; Variar 2007; Lei et al. 2016; Hairmansis et al. 2016; Khan et al. 2016; Nguyet et al. 2016).
The most powerful tool to control rice blast is to use resistant varieties. These varieties protect rice plants against the pathogens, upon infection, via the induction of hypersensitive response (HR), which occurs via gene-for-gene recognition of a pathogen effector (Avr) and a host-encoded resistance (R) protein (Gururani et al. 2012). The majority of plant R genes encode proteins that have putative central nucleotide-binding-site (NBS) and carboxy-terminal leucine-rich repeats (LRRs). These NBS-LRR proteins are divided into two major classes: the first class has an N-terminal domain which shares homology with the mammalian toll-interleukin-1-receptor (TIR) domain while the second class encodes an amino-terminal coiled-coil motif (CC-NBS-LRR) (DeYoung and Innes 2006; Meyers et al. 2003). To date, there are four types of coding proteins of R genes against rice blast: (1) NBS-LRR protein; (2) CC-NBS-LRR protein; (3) B-lectin binding and intracellular serine–threonine kinase domain protein; and (4) proline-rich protein (Chen et al. 2006; Zhou et al. 2006; Ashikawa et al. 2008; Fukuoka et al. 2009). The first rice blast resistance gene was cloned by Wang et al. 1999 and more than 20 R genes were cloned since then. These blast resistance genes are not randomly positioned on rice chromosomes (Sharma et al. 2012). Many R genes were cloned from chromosomes 6, 11, and 12. Some are clustered on a gene family (Sharma et al. 2012). Many allelic genes have been reported, for example Pish/Pi35 on chromosome 1 (Takahashi et al. 2010; Fukuoka et al. 2014), Pi9/Pi2/Pizt/Pi50 on chromosome 6 (Qu et al. 2006; Zhou et al. 2006; Su et al. 2015), Pi5/Pii on chromosome 9 (Lee et al. 2009; Takagi et al. 2013), and Pikh/Pikm/Pik/Pikp/Pi1 on chromosome 11 (Ashikawa et al. 2008; Xu et al. 2008; Yuan et al. 2011; Zhai et al. 2011; Hua et al. 2012). To date, 11 avirulence (Avr) genes in rice blast fungus have been cloned. Nine out of 11 genes, namely; AvrPita (Orbach et al. 2000), ACE1 (Bohnert et al. 2004), AvrPia, AvrPii, AvrPik (Yoshida et al. 2009), AvrPiz-t (Li et al. 2009), Avr1-CO39 (Zheng et al. 2011), AvrPib (Zhang et al. 2015), and AvrPi9 (Wu et al. 2015), have their corresponding R genes in rice. However, there are no known R genes in rice for PWL1 (Kang et al. 1995) and PWL2 (Sweigard et al. 1995). There are many scenarios that describe the specificity of recognition between resistance proteins and avirulence proteins. For example, one R gene can recognize more than one Avr gene, an example of which is Pia that can recognize both AvrPia and Avr1-CO39 (Cesari et al. 2013), or it could also be that many R genes can recognize the same Avr gene, as in the case of AvrPik-D that can be recognized by Pik, Pikm, and Pikp (Yoshida et al. 2009). In another case, the presence of two R genes is required for the mediated resistance to rice blast: Pi-km1 and Pi-km2 from Tsuyuake (Ashikawa et al. 2008) and Pi5-1 and Pi5-2 from RIL260 (Lee et al. 2009).
Genes conferring broad-spectrum resistance are necessary in breeding programs. The presence of even only one R gene can induce resistance to many isolates of M. oryzae (Qu et al. 2006). Many broad-spectrum R genes have been validated such as Pi9 (Qu et al. 2006), Pi2, Piz-t (Zhou et al. 2006), Pi40 (t) (Jeung et al. 2007), Pi20 (t) (Li et al. 2008), Pi5 (Lee et al. 2009), Pi1 (Hua et al. 2012), Pi54rh (Das et al. 2012), Pi56 (t) (Liu et al. 2013), and Pi50 (Su et al. 2015). Unfortunately, the resistance controlled by most race-specific R genes is prone to collapse often in a few years after introduction to the rice field due to the quick adaptation of rice blast pathogen (Kiyosawa 1982; Fukuoka et al. 2009).
Many strategies to keep the durability of resistance have been suggested, including multi-varietal planting, pyramiding of major R genes, and using partial resistance genes (Khush and Jena 2009). The use of partial resistance genes is one way to maintain the resistance of R gene(s) in the field. Partial resistance genes such as Pi34 (Zenbayashi-Sawata et al. 2005), pi21 (Fukuoka et al. 2009), and Pi35 (Fukuoka et al. 2014) have been reported. The pyramiding of partial resistance genes for enhancing the durability of resistance has been reportedly used in several breeding programs (Yasuda et al. 2015).
Jao Hom Nin (JHN) is a Thai rice variety which shows broad-spectrum resistance against rice blast in Thailand. Only two out of 346 blast isolates collected from all the rice growing areas in Thailand can overcome the resistance of JHN (Sreewongchai 2008). Moreover, seven out of 124 blast isolates collected worldwide can overcome the resistance of JHN, two of the isolates are from Colombia while five are from China (Sreewongchai 2008). Two Quantitative Trait Loci (QTLs) which are associated with rice blast resistance were mapped on chromosomes 1 and 11 of JHN (Noenplab et al. 2006). The major QTL (QTL11) that controls complete resistance was mapped on chromosome 11 by the SSR markers RM144 and RM224. The minor QTL (QTL1) which confers partial resistance was mapped on chromosome 1 by the flanking markers RM319 and RM 212 (Noenplab et al. 2006). QTL11 is located on the Pik locus which is a gene family locus. Interestingly, QTL1 is located on the Pish locus which is composed of Pi37 (Lin et al. 2007), Pish (Takahashi et al. 2010), Pi35 (Fukuoka et al. 2014), and Pi64 (Ma et al. 2015). In Thailand, JHN is used as a rice blast resistant donor in breeding programs. Introgressions of QTL1 and QTL11 from JHN via marker-assisted selection (MAS) into susceptible cultivars such as KDML105 (Noenplab et al. 2006) and RD6 (Wongsaprom et al. 2010) were successfully accomplished. In this study, the resistance gene/s in both QTL1 and QTL11 of JHN, which confer broad-spectrum resistance against rice blast populations from Thailand and the Philippines, were cloned and characterized and their disease spectrum was validated. Revealing the JHN rice blast resistance genes discloses the powerful resistance gene combination for resistance and durability to rice blast fungus populations in Thailand, the Philippines, and worldwide.
Results
JHN controls broad-spectrum resistance through the combination of QTL1 and QTL11
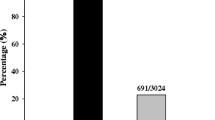
To evaluate the resistance spectrum of JHN, 132 diverse blast isolates collected from the Philippines were used for disease assessment. It was found that JHN showed resistance to 120 out of the 132 isolates, representing 90.9% resistance frequency (Fig. 1 & Additional file 1: Table S1). The reactions of different IRRI-bred blast resistant lines (IRBLs) to these isolates were also assessed, revealing that each line had a varied resistance frequency (Fig. 1). Compared to the majority of IRBLs, JHN controlled a quite high resistance frequency to the Philippine isolates. The data indicated that JHN was a promising rice line controlling broad-spectrum resistance to the Philippine isolates as to the Thai isolates (Sreewongchai 2008). Given the fact that JHN contained at least two resistance loci, i.e., QTL1 on chromosome 1 and QTL11 on chromosome 11 (Noenplab et al. 2006), we generated two monogenic lines which were designated as QTL1-C and QTL11-C containing individual QTL1 and QTL11, respectively, in the CO39 genetic background via marker aided backcrossing (MABC) (Additional file 2: Figure S1). The derived homozygous BC3F3 monogenic lines were used for the spectrum analysis against 42 representative isolates selected from the set of 132 isolates as described above. Eight isolates showed virulence to QTL1-C, QTL11-C, and JHN, indicating that neither QTL1 nor QTL11 controlled resistance to these eight isolates (Table 1). Out of 34 avirulent isolates to JHN, three were virulent to QTL1-C but avirulent to QTL11-C, suggesting that QTL11 rather than QTL1 contributed to the resistance of JHN to these three isolates (Table 1). On the contrary, 12 isolates were avirulent to QTL1-C but virulent to QTL11-C, suggesting that QTL1 rather than QTL11 contributed to the resistance of JHN to these 12 isolates (Table 1). The remaining 19 isolates were avirulent to QTL1-C, QTL11-C, and JHN, suggesting that both QTL1 and QTL11 controlled the resistance to these 19 isolates (Table 1). We thus concluded that the resistance of JHN to these 34 isolates was additively controlled by QTL1 and QTL11.
The resistance frequency of IRBLs and JHN against Philippine rice blast isolates. The resistance frequency of each rice variety was calculated based on the resistance reactions against 132 Philippine isolates as listed in Additional file 1: Table S1. The resistance frequency of JHN was indicated on the top of the bar
Genetic validation of genomic location of QTL1 and QTL11 in JHN
To validate the genetic delimitation of QTL1 and QTL11 in JHN, two F3 populations were generated as illustrated in (Additional file 2: Figure S1), each derived from a single heterozygous F2 plant containing an individual QTLs determined by the flanking simple sequence repeat (SSR) markers of QTL1 (RM212 and RM11744) and of QTL11 (RM224 and RM144) as described previously (Noenplab et al. 2006). An F3 population consisting of 1177 plants for the genetic analysis of QTL1 was inoculated with a JHN-avirulent isolate BN111. A ratio of 907 resistant versus 270 susceptible progenies was observed, fitting an expected 3:1 ratio (X 2: 2.665 and P value: 0.103) (Additional file 3: Table S2). On the other hand, an F3 population comprising of 1346 F3 progenies was inoculated with another JHN-avirulent isolate PO6-6 for the genetic analysis of QTL11. A ratio of 1024 resistant versus 322 susceptible plants was observed, which fitted an expected 3:1 ratio (X 2: 0.833, P: 0.361) (Additional file 3: Table S2). These data indicated that the resistance of the F3 plants of each population was controlled by a single R gene to the isolate BN111 and PO6-6, respectively. Linkage analysis was further conducted to validate the association of QTL1 and QTL11 with respective linked markers described previously (Noenplab et al. 2006). Due to no polymorphism resolved between JHN and CO39 at RM319, another polymorphic SSR marker, RM11744 was used instead together with RM212 as flanking markers for the genetic analysis of QTL1 (Fig. 2a & Additional file 4: Figure S2). All 270 susceptible F3 progenies showed CO39-pattern in PCR amplicon size at both flanking markers, suggesting no segregation of QTL1 from both markers (Fig. 2a). For the linkage analysis of QTL11, RM224 and RM144 were used as flanking markers (Fig. 2b & Additional file 4: Figure S2). All 322 susceptible plants showed CO39-pattern in PCR amplicon size at both flanking markers, suggesting that QTL11 was co-segregated both markers (Fig. 2b). Our data verified that QTL1 and QTL11 were delimited into genomic intervals on chromosome 1 and chromosome 11, respectively, as characterized previously (Noenplab et al. 2006).
An integrated genetic and physical map of QTL1 (a) and QTL2 (b). The chromosomal position of SSR markers delimiting the genomic interval of QTLs by referring to the one in the reference genome of Nipponbare was indicated below the line. The number of recombinants/toal F3 progeny at each SSR marker was indicated above the line. Four and 9 NBS-LRR type R genes predicted in the genomic intervals in Nipponbare for QTL1 and QTL11, respectively, were indicated in boxes with gene IDs. The figure was not draw in scale
QTL1-C and QTL11-C controlled identical resistance spectra as IRBLsh-S and IRBL7-M, respectively
By aligning the positions of flanking SSR markers along respective chromosomes using the Nipponbare reference sequence (MSU Rice Genome Annotation Project, http://rice.plantbiology. msu.edu/), the genomic intervals of QTL1 and QTL11 were determined. The genomic interval of QTL1 corresponded to the region from 33,054,867 bp (RM212) to 33,678,804 bp (RM319) on chromosome 1 in Nipponbare, which is approximately 624 kb in size. Sequence analysis identified 5 predicted NBS-LRR genes in this region, 3 of which corresponded to cloned R genes or their alleles, namely Pi64, Pi37, and Pish/Pi35 (Table 2). On the other hand, the genomic interval of QTL11 was conformed to the region from 27,673,251 bp (RM224) to 28,281,693 bp (RM144) on chromosome 11 in Nipponbare, which is approximately 608 kb in size. Nine NBS-LRR genes were predicted in this region, two of which corresponded to the alleles of Pik-1 and Pik-2 (Table 2).
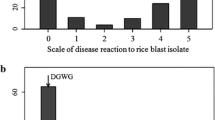
To validate whether QTL1 and QTL11 correspond to any of known R genes in respective delimited genomic intervals, we conducted cluster analysis of the resistance spectrum controlled by QTL1-C, QTL11-C, and other IRBLs. Intriguingly, QTL1-C and IRBLsh-S was clustered together while QTL11-C was clustered with IRBL7-M based on their reactions against 42 blast isolates (Fig. 3). This result promoted us to postulate that QTL1 and QTL11 in JHN are most likely alleles of Pish and Pi7, respectively. We thus named them as Pish-J and Pi7-J.
Pish-J and Pi7-J were alleles of known Pish and Pi7, respectively
To determine the sequence of Pish-J and Pi7-J in JHN, their genomic regions were cloned and completely sequenced. The sequence of Pish-J (GenBank accession number: KY225901) is 4356-bp in length. It contains a predicted 3873-bp coding sequence (CDS), which resulted in a polypeptide product composed of 1290-amino-acids. Sequence comparison revealed that Pish-J is identical in nucleotide sequence to Pish in Nipponbare (GenBank accession number: AP014957.1). We conclude that Pish-J in JHN is an identical allele of Pish. Given that Pik’s functionality is fulfilled by two adjacent NBS-LRR genes, Pik-1 and Pik-2 (Ashikawa et al. 2012), we determined the sequences of their alleles in JHN, designated as Pi7-J-1 and Pi7-J-2, respectively. Pi7-J-1 is 6489-bp in length (GenBank accession number: KY225902). By referring to sequence annotation of Pik-1 (GenBank accession number: AB616658), Pi7-J-1 was predicted to contain three exons interrupted by two introns in the coding region (Additional file 5: Figure S3). It encoded a 1142-amino-acid protein product. Pi7-J-2 is 3263-bp in length (GenBank accession number: KY225903). It contains two exons and encodes a 1021-amino-acid protein product (Additional file 5: Figure S3).
Sequence comparison revealed that Pi7-J-1 showed 99% identity in nucleotide sequence with Pi7-1 (GenBank accession number: HQ660231). It contains two sequence differences along the whole genomic region. One is composed of a single nucleotide “T” deletion at position 1369-bp in the intron and another is a nucleotide transition of G to A at position 5470-bp in the exon 2 of Pi7-J-1 from Pi7-1. The sequence change in the exon belongs to a synonymous substitution and thus does not alter the protein sequence of Pi7-J-1 from Pi7-1. No difference in nucleotide sequences was observed between Pi7-J-2 and Pi7-2 (GenBank accession number: HQ660231). Taken together, we concluded that Pi7-J represented a new allele of Pi7 containing two silent mutations in genomic sequence.
The sequence similarity of Pi7-J-1 and Pi7-J-2 with other known Pi7-1 and Pi7-2 alleles was further investigated. It was found that Pi7-J-1 shared 99% identity in amino acid to Pikp-1 and Pikh-1 while it shared 95% identity to Pik-1, Pikm-1, and Pi1-5 (Additional file 6: Table S3). Pi7-J-2 shared 100% identity in amino acid sequence to Pikp-2 and Pikh-2 while it shared 99% sequence identity to Pik-2, Pikm-2, and Pi1-6 (Additional file 6: Table S3)
The resistance of QTL1-C was controlled by Pish-J rather than Pi64-J and Pi37-J
To exclude the possibility of the contribution of Pi64 and Pi37 to the resistance spectrum conferred by QTL1, their homologues in JHN, named Pi64-J and Pi37-J, respectively, were cloned and sequenced. Pi64-J was 4406-bp in length covering a predicted 3867-bp CDS while Pi37-J was 4474-bp in length covering a predicted 3873-bp CDS. Sequence analysis revealed that Pi64-J and Pi37-J shared 90 and 99% identity in amino acid sequence to Pi64 (Ma et al. 2015) and Pi37 (GenBank accession number: DQ923494.1), respectively, indicating that they were different from known genes. Instead, they were identical in sequence to LOC_Os01g57280 and LOC_Os01g57310, respectively in Nipponbare. Since Nipponbare was used as a reference susceptible variety for both Pi64 and Pi37 (Lin et al. 2007; Ma et al. 2015), we thus concluded that Pish-J rather than Pi64-J and Pi37-J was responsible for the resistance of QTL1-C.
Discussion
The most efficient way to control rice blast disease is the utilization of resistant varieties. It is also low-cost and environment-friendly (Manandhar et al. 1998). The JHN rice variety showed broad-spectrum resistance against Thai and worldwide blast isolates (Sreewongchai 2008). The resistance of JHN was documented in this study by testing it against 132 Philippine blast isolates and found to be effective to most isolates, indicating that it is also quite promising in resistance against rice blast in the Philippines. The dissection of mechanisms underlying the broad-spectrum resistance of JHN against diverse sets of rice blast isolates worldwide is vital for the utilization of JHN-embedded R genes in varietal improvement against rice blast via marker aided selection approach. Noenplab et al. (2006)) documented that two QTLs, namely QTL1 on chromosome 1 and QTL11 on chromosome 11, were responsible for the broad-spectrum resistance of JHN. In this study, we employed a multifaceted approach to further dissect the molecular mechanisms of both QTL1 and QTL11. The genomic interval spanning QTL1 and QTL11 were first delimited within respective regions by flanking markers. Sequence analyses revealed that the Pish and Pik loci were present in the delimited genomic intervals of QTL1 and QTL11, respectively. Cluster analysis of the resistance reactions of derived QTL1 and QTL11 monogenic lines and IRBLs led to the finding of their association with Pish and Pi7, respectively. Finally, gene cloning and sequence analysis revealed that QTL1 and QTL11 encoded alleles of Pish and Pi7, respectively.
The Pish gene was mapped on chromosome 1 by an analysis of QTL due to its moderate resistance and isolated by extensive characterization of retrotransposon-tagged suppressive mutants (Araki et al. 2003; Takahashi et al. 2010). It is located in a gene cluster with a tandem array of four NBS-LRR genes (RGA1 through RGA4) in Nipponbare. It was found that RGA4 rather than RGA1 through RGA3 was responsible for Pish (Takahashi et al. 2010). Significant sequence similarity among different homologues suggests that they were likely derived from successive rounds of duplication from a common progenitor (Takahashi et al. 2010). In addition to Pish, three other functional R genes have been molecularly characterized, namely Pi64, Pi37, and Pi35 corresponding to alleles of RGA2, RGA3, and RGA4 in respective resistant rice varieties (Lin et al. 2007; Ma et al. 2015; Fukuoka et al. 2014). Sequence comparison further revealed that multiple functional polymorphisms cumulatively resulted in the conversion from Pish-mediated race specific resistance to Pi35-mediated non-race specific resistance (Fukuoka et al. 2014). Despite the nature of race-specific resistance, Pish was reported to be effective in many Southeast Asian countries including Cambodia, Vietnam, and the Philippines (Fukuta et al. 2014; Nguyen et al. 2015; Selisana et al. 2017).
A total of six R genes at the Pik locus were characterized at the molecular level including Pikm from Tsuyuake (Ashikawa et al. 2008), Pik from Kusabue (Zhai et al. 2011), Pikp from K60 (Yuan et al. 2011), Pik from Kanto51 (Ashikawa et al. 2012), Pi1 from C101LAC (Hua et al. 2012), and Pikh from K3 (Zhai et al. 2014). The functionality of these Pik alleles is required by the co-existence of two adjacent NBS-LRR genes at the locus. Different Pik alleles activate immunity to rice blast isolates by recognizing different haplotypes of AvrPik allele, demonstrating a classical arms race coevolution model (Kanzaki et al. 2012). Biochemical and structural biology assays revealed that the delicate binding between a heavy-metal associated domain (HMA) in Pikp-1 and AvrPik-D initiated the host immune response to rice blast pathogen (Maqbool et al. 2015). In this study, we demonstrated that JHN contained an allele of Pi7 in IRBL7-M sharing limited sequence differences in nucleotide but not in protein sequence. Resistance spectrum analysis of IRBLs containing different Pik alleles against isolates from Bohol clearly indicated that Pi7, Pikp, and Pik controlled resistance to the isolates containing AvrPik-D but not other AvrPik haplotypes (Selisana et al. 2017). Interestingly, a pathotype test of 2476 rice blast isolates collected from the north, northeast, and south of Thailand in 2007 against IRBLs revealed that IRBLk-ka (Pik) and other Pik-allele containing IRBLs were resistant to most isolates (Mekwatanakarn et al. 2007). A similar result was obtained in other pathotype tests using 2293 and 1375 Thai rice blast isolates collected in 2011 and 2013, respectively (Mekwatanakarn et al. 2011; Mekwatanakarn et al. 2013). Moreover, Pi7 was reported to show high frequency of resistance against rice blast isolates in Cambodia (Fukuta et al. 2014) and Vietnam (Nguyen et al. 2015).
In this study, we demonstrated that the resistance spectrum of JHN is attributable to the additive contribution from both Pi7-J and Pish-J, which underpinned the mechanism of broad-spectrum of resistance to rice blast in JHN. It is indeed that JHN has been widely used as an elite resistant donor in conventional and molecular breeding programs against rice blast disease in Thailand. These breeding programs aim to introgress QTL1 and QTL11 of JHN into commercial Thai rice varieties which are susceptible to rice blast. Examples of these varieties are RD6 (Wongsaprom et al. 2010), Khoa Dok Mali 105 (Nalampangnoenplab 2011), IR77955-24-75-284 (Kotchasatit 2013), Ban Tang (Kaewcheenchai et al. 2014), Jao Hawm Phitsanulok51 (Noenplab et al. 2015), and San Par Tong1 (Yajai and Ketsuwan 2015).
Conclusions
In this study, the R genes which control the resistance of QTL1 and QTL11 in the JHN rice variety were identified. The Pish-J in QTL1 showed partial resistance while the Pi7-J gene in QTL11 showed complete resistance against Thai and Philippine rice blast isolates. The combination of the two broad spectrum blast resistance genes explains why JHN can still show a high level of resistance for a long period of time in Thailand. The results indicate that Pish-J and Pi7-J in JHN are broad-spectrum resistance genes which are excellent candidate genes to be included in Thailand and the Philippines's rice breeding pipeline to maintain rice blast resistance.
Methods
Plant materials
Jao Hom Nin (JHN), a Thai glutinous rice variety, was used as source of resistance genes for cloning and characterization. CO39, a susceptible rice variety, was used as a recipient variety for genetic linkage analysis. LTH and IRBLs from the Gene Bank of the International Rice Research Institute (IRRI) were used for disease spectrum analysis.
Generation of monogenic lines of QTL1 and QTL11 of JHN in the CO39 genomic background using marker assisted backcrossing (MABC)
The monogenic lines of QTL1 and QTL11 of JHN in the CO39 genomic background were generated as illustrated in Additional file 2: Figure S1. In brief, JHN was first crossed with the susceptible rice variety, CO39. The SSR marker RM144 was used to check the real cross of F1 plants (Additional file 7: Table S4). Genomic DNA was extracted from frozen rice leaves by CTAB method as described by Doyle and Doyle (1987). To separate QTL1 and QTL11, the resistant F2 plants selected by the inoculation of the isolate PO6-6 were screened using SSR markers, RM212 and RM11744 for QTL1 and RM224 and RM144 for QTL11 (Additional file 7: Table S4). The homozygous F2 plants for individual QTL1 and QTL11 were further used for the generation of monogenic lines at the generation of BC3F1 by MABC. The derived homozygous BC3F3 plants of QTL1, designated as QTL1-C, and of QTL11, designated as QTL11-C were used for spectrum analysis of QTL1 and QTL11, respectively.
Genetic analysis of QTL1 and QTL11 of JHN
During the generation of monogenic lines of QTL1 and QTL11, the heterozygous F2 plants which harbored either QTL1 or QTL11 were self-pollinated to produce F3 lines for the genetic linkage analysis (Additional file 2: Figure S1). They were inoculated with the differential blast isolates for Pish and Pik, BN111 and PO6-6, respectively (Telebanco-Yanoria et al. 2008). The DNA of susceptible plants was extracted and used for segregation analysis for QTL1 with SSR markers of RM212 and RM11744 and for QTL11 with RM144 and RM224.
Pathotype analysis
JHN was inoculated with 132 Philippine blast isolates (Additional file 8: Table S5) using 1 × 105 conidia/ml suspension. Pathogen inoculation and disease evaluation were performed as previously described by Zhu et al. (2012). The BC3F3 homozygous lines of QTL1-C and QTL11-C were inoculated with 42 representative Philippine blast isolates. These 42 representative isolates can be classified in four categories based on the reactions to Pish and Pik including (I) avirulent to both Pish and Pik, (II) virulent to both Pish and Pik, (III) avirulent to Pish but virulent to Pik, (IV) virulent to Pish but avirulent to Pik. The disease reactions of QTL1-C and QTL11-C were clustered with those of different IRBLs using NTSYS program version 2.21q.
Cloning and comparative sequence analysis of known R genes in the regions of QTL1 and QTL11 in JHN
The genomic DNA of JHN was used as the DNA template for cloning the homologues of Pi64/Pi37/Pish and Pik in the regions of QTL1 and QTL11 in JHN with the primer pairs listed in Additional file 7: Table S4. PCR amplicons were sequenced (Macrogen Inc, South-Korea) and the online software MAFFT version7 (http://mafft.cbrc.jp/alignment/server/) was used sequence analysis and comparison. Gene and protein sequence similarity was determined via BLASTN and BLASTP, respectively (https://www.ncbi.nlm.nih.gov/). The encoded protein sequences were deduced using the translation program at ExPASy (http://web.expasy.org/translate/).
References
Ahn SN, Kim YK, Hong HC, Han SS, Kwon SJ, Choi HC, Moon HP, McCouch S (2000) Molecular mapping of a new gene for resistance to rice blast. Euphytica 116:17–22
Araki E, Yanoria MJT, Ebron LA, Mercado-Escueta D, Takai T, Fukuta Y (2003) Mapping of a rice blast resistance gene Pish. Breed Res 5(2):333
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M (2008) Two adjacent nucleotide-binding site-leucine-rich repeat class genes are required to confer Pikm-specific rice blast resistance. Genetics 180(4):2267–2276
Ashikawa I, Hayashi N, Abe F, Wu J, Matsumoto T (2012) Characterization of the rice blast resistance gene Pik cloned from Kanto51. Mol Breed 30:485–494
Bohnert HU, Fudal I, Dioh W, Tharreau D, Notteghem JL, Lebrun MH (2004) A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell 16:2499–2513
Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, Rivas S, Alaux L, Kanzaki H, Okuyama Y, Morel J, Fournier E, Tharreau D, Terauchi R, Kroj T (2013) The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell 25(4):1463–1481
Chen X, Shang J, Chen D, Lei C, Zou Y, Zhai W, Liu G, Xu J, Ling Z, Cao G, Ma B, Wang Y, Zhao X, Li S, Zhu L (2006) A B-lectin receptor kinase gene conferring rice blast resistance. Plant J 46(5):794–804
Das A, Soubam D, Singh PK, Thakur S, Singh NK, Sharma TR (2012) A novel blast resistance gene, Pi54rh cloned from wild species of rice, Oryza rhizomatis confers broad spectrum resistance to Magnaporthe oryzae. Funct Integr Genomics 12(2):215–228
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7:1243–1249
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15.
Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, Hayashi N, Takahashi A, Hirochika H, Okuno K, Yano M (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325(5943):998–1001
Fukuoka S, Yamamoto SI, Mizobuchi R, Yamanouchi U, Ono K, Kitazawa N, Yasuda N, Fujita Y, Nguyen TTT, Koizumi S, Sugimoto K, Matsumoto T, Yano M (2014) Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Sci Rep 4:1–7
Fukuta Y, Koga I, Ung T, Sathya K, Kawasaki-Tanaka A, Koide Y, Kobayashi N, Obara M, Yadana H, Hayashi N (2014) Pathogenicity of rice blast (Pyricularia oryzae Cavara) isolates from Cambodia. Japan Agric Res Q 48(2):155–166
Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW (2012) Plant disease resistance genes: Current status and future directions. Physiol Mol Plant Pathol 78:51–65
Hairmansis A, Santoso S, Nasution A, Utami D, Fukuta Y (2016) Improvement of differential system for rice blast disease management in Indonesia. In: JIRCAS (Japan International Research Center for Agricultural Sciences, Tsukuba, Japan) workshop The 7th International Rice Blast Conference. pp48
Hua L, Wu J, Chen C, Wu W, He X, Lin F, Wang L, Ashikawa I, Matsumoto T, Wang L, Pan Q (2012) The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theor Appl Genet 125(5):1047–1055
Jeung JU, Kim BR, Cho YC, Han SS, Moon HP, Lee YT, Jena KK (2007) A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet 115(8):1163–1177
Kaewcheenchai R, Sriwisut S, Noenplab A, Nalampangnoenplab A, Ruksopa K, Dungsoongnern P, Promnart U, Rasidee N (2014) Improving blast resistance in a rice variety’Bahng Taen’ by using marker-assisted backcrossing. Proceeding of the 32nd Rice and Temperate Cereal Crop Annual Conference, Petchaburi, Thailand. pp158–170
Kang S, Sweigard JA, Valent B (1995) The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact 8:939–948
Kanzaki H, Yoshida K, Saitoh H, Fujisaki K, Hirabuchi A, Alaux L, Fournier E, Tharreau D, Terauchi R (2012) Arms race co-evolution of Magnaporthe oryzae AVR-Pik and rice Pik genes driven by their physical interactions. Plant J 72(6):894–907
Khan MAI, Bhuiyan MR, Kader MA, Latif MA, Ali MA, Fukuta Y (2016) Research strategies for rice blast disease management using differential systems in Bangladesh. In: JIRCAS (Japan International Research Center for Agricultural Sciences, Tsukuba, Japan) workshop The 7th International Rice Blast Conference. pp47
Khush GS, Jena K (2009) Current Status and Future Prospects for Research on Blast Resistance in Rice (Oryza sativa L.). In: Wang GL, Valent B (eds) Advances in Genetics, Genomics and Control of Rice Blast Disease. Springer, Dordrecht
Kiyosawa S (1982) Genetic and epidemiological modeling of breakdown of plant disease resistance. Annu Rev Phytopathol 20:93–117
Kotchasatit A (2013) UBN03007-47-7-7-26-35-19: An early maturing, non-glutinous promising rice line resistant to blast. Proceedings of the 30th Rice and Temperate Cereal Crops Annual Conference 2013, Bangkok, Thailand. pp56–71
Lee SK, Song MY, Seo YS, Kim HK, Ko S, Cao PJ, Suh JP, Yi G, Roh JH, Lee S, An G, Hahn TR, Wang GL, Ronald P, Jeon JS (2009) Rice Pi5-mediated resistance to Magnaporthe oryzae requires the presence of two coiled-coil-nucleotide-binding-leucine-rich repeat genes. Genetics 181(4):1627–1638
Lei C, Wang J, Zhao Z, Cheng Z, Wang J, Wan J (2016) Rice blast disease and resistance breeding in China. In: JIRCAS (Japan International Research Center for Agricultural Sciences, Tsukuba, Japan) workshop The 7th International Rice Blast Conference. pp52
Li W, Lei C, Cheng Z, Jia Y, Huang D, Wang J, Wang J, Zhang X, Su N, Guo X, Zhai H, Wan J (2008) Identification of SSR markers for a broad-spectrum blast resistance gene Pi20 (t) for marker-assisted breeding. Mol Breed 22(1):141–149
Li W, Wang B, Wu J, Lu G, Hu Y, Zhang X, Zhang Z, Zhao Q, Feng Q, Zhang H, Wang Z, Wang G, Han B, Wang Z, Zhou B (2009) The Magnaporthe oryzae avirulence gene AvrPiz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol Plant-Microbe Interact 22(4):411–20
Lin F, Chen S, Que Z, Wang L, Liu X, Pan Q (2007) The blast resistance gene Pi37 encodes a nucleotide binding site-leucine-rich repeat protein and is a member of a resistance gene cluster on rice chromosome 1. Genetics 177(3):1871–1880
Liu Y, Liu B, Zhu X, Yang J, Bordeos A, Wang G, Leung H (2013) Fine-mapping and molecular marker development for Pi56 (t), a NBS-LRR gene conferring broad-spectrum resistance to Magnaporthe oryzae in rice. Theor Appl Genet 126(4):985–998
Ma J, Lei C, Xu X, Hao K, Wang J, Cheng Z, Ma X, Ma J, Zhou K, Zhang X, Guo X, Wu F, Lin Q, Wang C, Zhai H, Wang H, Wan J (2015) Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol Plant-Microbe Interact 28(5):558–568
Manandhar HK, Jørgensen HJL, Mathur SB, Petersen VS (1998) Suppression of rice blast by preinoculation with avirulent Pyricularia oryzae and the nonrice pathogen Bipolaris sorokiniana. Phytopathology 88(7):735–739
Maqbool A, Saitoh H, Franceschetti M, Stevenson CEM, Uemura A, Kanzaki H, Kamoun S, Terauchi R, Banfield MJ (2015) Structural basis of pathogen recognition by an integrated HMA domain in a plant NLR immune receptor. Elife 4:e08709
Mekwatanakarn P, Cobelli P, Nalumpangnernplub A, Rithmontree T, Ketsuwan K, Klinmanee C, Deerith ST (2007) Determination of pathotype diversity of rice blast fungal population in Thailand. Thai Rice Res J 1(1):52–64
Mekwatanakarn P, Chamarerk V, Jairin J, Kotchasatit U, Kotchasatit A, Jongdee B, Saleetoe S, Homsombat W, Nalumpangnernplub A, Yajai P (2011) Diversity of rice blast population and its implementation to breeding program for disease resistance. Proceeding of 2nd Rice annual conference year 2011. Rice and national farmers’ day, Bangkok, pp 249–266
Mekwatanakarn P, Chamarerk V, Jairin J, Kotchasatit A, Lhachantuek S, Saleetoe S, Phengrat K, Kantajun A (2013) Population diversity of blast fungus using international standard differential monogenic lines. Bureau of Rice Research and Development, Thailand, http://eproofing.springer.com/journals/index.php?token=Y2bqrZvbPwcSO1T3gDML2IYF_kCDRPjJpTi3NoAnuw. Accessed 4 Oct 2016.
Meyers BC, Kozik A, Griego A, Kuang HH, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15:809–834
Nalampangnoenplab A (2011) Minimization of rice blast severity by means of multilines in the lower north. Proceeding of rice research symposium 2011: Rice research center groups in upper and lower northern region, Phrae, Thailand. pp225–241
Nguyen TTT, Truong HTH, Nguyen LT, Nguyen LHK (2015) Identification of rice blast resistance genes in south central coast of Vietnam using monogenic lines under field condition and pathogenicity assays. J Agric Sci Technol A B & Hue Univ J Sci 5:491–500
Nguyet TMN, Long HH, Ngoc BN, Nhai TN, Thuy TTN, Koga I, Hayashi N, Fukuta Y (2016) Development of differential system and genetic variation in resistance to blast disease in Vietnamese rice. In: JIRCAS (Japan International Research Center for Agricultural Sciences, Tsukuba, Japan) workshop The 7th International Rice Blast Conference. pp49
Noenplab A, Vanavichit A, Toojinda T, Sirithunya P, Tragoonrung S, Sriprakhon S, Vongsaprom C (2006) QTL mapping for leaf and neck blast resistance in Khao Dawk Mali105 and Jao Hom Nin recombinant inbred lines. Sci Asia 32(2):133–142
Noenplab A, Nalumpangnernplub A, Palawisut S (2015) Increasing blast resistance in HPSL1 using marker assisted selection. In: Proceeding of The 8th rice researchconference 2015. Rice research center groups in upper and lower northern region, Chiang Rai, pp 3–16
Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B (2000) A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 12:2019–2032
Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL (2006) The broad spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172(3):1901–1914
Scheuermann KK, Raimondi JV, Marschalek R, De-Andrade A, Wickert E (2012) Magnaporthe oryzae genetic diversity and its outcomes on the search for durable resistance. The Molecular Basis of Plant Genetic Diversity., pp 331–356
Selisana SM, Yanoria MJ, Quime B, Chaipanya C, Lu G, Opulencia R, Wang GL, Mitchell T, Correll J, Talbot N, Leung H, Zhou B (2017) Avirulence (AVR) gene-based diagnosis complements existing pathogen surveillance tools for effective deployment of resistance (R) genes against rice blast disease. Phytopathology First Look site http://dx.doi.org/10.1094/PHYTO-12-16-0451-R. posted 02/07/2017
Sharma TR, Rai AK, Gupta SK, Vijayan J, Devanna BN, Ray S (2012) Rice blast management through host-plant resistance: retrospect and prospects. Agric Res 1(1):37–52
Sobrizal S, Anggiani S (2007) Rice blast disease in Indonesia. In: JIRCAS (Japan Inter national Research Center for Agricultural Sciences, Tsukuba, Japan) Working Report. 53:71–79
Sreewongchai T (2008) Identification of Magnaporthe grisea avirulence genes specific to rice blast resistance genes. PhD thesis. Kasetsart University, Thailand, p 110
Su J, Wang W, Han J, Chen S, Wang C, Zeng L, Feng A, Yang J, Zhou B, Zhu X (2015) Functional divergence of duplicated genes results in a novel blast resistance gene Pi50 at the Pi2/9 locus. Theor Appl Genet 128(11):2213–2225
Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B (1995) Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 7:1221–1233
Takagi H, Uemura A, Yaegashi H, Tamiru M, Abe A, Mitsuoka C, Utsushi H, Natsume S, Kanzaki H, Matsumura H, Saitoh H, Yoshida K, Cano LM, Kamoun S, Terauchi R (2013) MutMap-Gap: Whole-genome resequencing of mutant F2 progeny bulk combined with de novo assembly of gap regions identifies the rice blast resistance gene Pii. New Phytol 200(1):276–283
Takahashi A, Hayashi N, Miyao A, Hirochika H (2010) Unique features of the rice blast resistance Pish locus revealed by large scale retro transposon-tagging. BMC Plant Biol 10(1):175
Telebanco-Yanoria MJ, Imbe T, Kato H, Tsunematsu H, Ebron LA, Vera-Cruz CM, Kobayashi N, Fukuta Y (2008) A set of standard differential blast isolates (Magnaporthe grisea (Hebert) Barr.) from the Philippines for rice (Oryza sativa L.) resistance. Japan Agric Res Q 42:23–34
Variar M (2007) Pathogenic variation in Magnaporthe grisea and breeding for blast resistance in India. In: JIRCAS (Japan International Research Center for Agricultural Sciences, Tsukuba, Japan) Working Report. 53:87–95
Wang ZX, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19(1):55–64
Wongsaprom C, Sirithunya P, Vanavichit A, Pantuwan G, Jongdee B, Sidhiwong N, Lanceras-Siangliw J, Toojinda T (2010) Two introgressed quantitative trait loci confer a broad-spectrum resistance to blast disease in the genetic background of the cultivar RD6 a Thai glutinous jasmine rice. Field Crop Res 119:245–251
Wu J, Kou Y, Bao J, Li Y, Tang M, Zhu X, Ponaya A, Xiao G, Li J, Li C, Song MY, Cumagun CJR, Deng Q, Lu G, Jeon JS, Naqvi NI, Zhou B (2015) Comparative genomics identifies the Magnaporthe oryzae avirulence effector AvrPi9 that triggers Pi9-mediated blast resistance in rice. New Phytol 206(4):1463–1475
Xu X, Hayashi N, Wang CT, Kato H, Fujimura T, Kawasaki S (2008) Efficient authentic fine mapping of the rice blast resistance gene Pik-h in the Pik cluster, using new Pik-h differentiating isolates. Mol Breed 22(2):289–299
Yajai P, Ketsuwan K (2015) The use of anther culture for developing rice blast resistant true breeding lines. Proceeding of The 8th rice research conference 2015: Rice research center groups in upper and lower northern region, Chiang Rai, Thailand. pp47–52
Yasuda N, Mitsunaga T, Hayashi K, Koizumi S, Fujita Y (2015) Effects of pyramiding quantitative resistance genes pi21, Pi34, and Pi35 on rice leaf blast disease. Plant Dis 99(7):904–909
Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, Tosa Y, Chuma I, Takano Y, Win J, Kamoun S, Terauchi R (2009) Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell Online 21(5):1573–1591
Yuan B, Zhai C, Wang W, Zeng X, Xu X, Hu H, Lin F, Wang L, Pan Q (2011) The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor Appl Genet 122(5):1017–1028
Zenbayashi-Sawata K, Ashizawa T, Koizumi S (2005) Pi34-AVRPi34: A new gene-for-gene interaction for partial resistance in rice to blast caused by Magnaporthe grisea. J Gen Plant Pathol 71(6):395–401
Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, Wang L, Pan Q (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol 189(1):321–334
Zhai C, Zhang Y, Yao N, Lin F, Liu Z, Dong Z, Wang L, Pan Q (2014) Function and interaction of the coupled genes responsible for Pik-h encoded rice blast resistance. PLoS One 9(6):e98067
Zhang S, Wang L, Wu W, He L, Yang X, Pan Q (2015) Function and evolution of Magnaporthe oryzae avirulence gene AvrPib responding to the rice blast resistance gene Pib. Sci Rep 5:11642
Zheng Y, Zheng W, Lin F, Zhang Y, Yi Y, Wang B, Lu G, Wang Z, Wu W (2011) AVR1-CO39 is a predominant locus governing the broad avirulence of Magnaporthe oryzae 2539 on cultivated rice (Oryza sativa L.). Mol Plant-Microbe Interact 24(1):13–17
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Maria B, Wang GL (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant-Microbe Interact 19:1216–1228
Zhu X, Chen S, Yang J, Zhou S, Zeng L, Han J, Su J, Wang L, Pan Q (2012) The identification of Pi50 (t), a new member of the rice blast resistance Pi2/Pi9 multigene family. Theor Appl Genet 124:1295–1304
Acknowledgements
We appreciated Dr. Cailin Lei at Chinses Academy of Agricultural Sciences for providing the genomic sequence of Pi64. We would also like to thank Mr. Robert Harman II for grammatically reviewing the manuscript. This work was supported by Funding from LEE Foundation, International Rice Research Institute (IRRI), the National Center for Genetic Engineering and Biotechnology (BIOTEC) and National Science and Technology Development Agency (NSTDA) Thailand NSTDA Research Chair Grant (No. P12-01898) and Kasetsart University Research and Development Institute (KURDI).
Authors’ contributions
Project conception (CJ, BZ). Mapping population construction (CC, TT, AV). Pathotype screening (CC, MJ, BQ, AL, SK, SK). Gene cloning and sequence analysis (CC, BZ). Manuscript preparation (CC, CJ, BZ). All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
The resistance reactions of JHN and different IRBLs to 132 Philippines isolates. The 42 representative isolates used for the cluster analysis of QTL1 and QTL11 with different target R genes in IRBLs were indicated in superscript letter a. IRBL: International Rice Research Institute bred lines; R: resistance; S: susceptible. (DOC 385 kb)
Additional file 2: Figure S1.
The diagrammatic flowchart for the generation of mapping population and monogenic lines of QTL1-C and QTL11-C. The heterozygous F2 plants which harbored either QTL1 or QTL11 were selected for self-pollination to produce F3 generation for mapping analysis. (DOC 69 kb)
Additional file 3: Table S2.
The genetics of QTL1 and QTL11 in JHN by analyzing F3 population derived from selfing of a single F2 progeny containing either QTL1 or QTL11. (DOC 29 kb)
Additional file 4: Figure S2.
Polymorphism analysis of 5 simple sequence repeat (SSR) markers between JHN (J) and CO39 (C). RM224 and RM144 were used as flanking markers for QTL11whereas RM212 and RM11744 was used for QTL1. (DOC 55 kb)
Additional file 5: Figure S3.
The gene structure of Pi7-J-1 (a) and Pi7-J-2 (b). The size of each intron and exon was indicated. Black boxes represented exons and lines represented introns. The figure was not drawn in scale. (DOC 43 kb)
Additional file 6: Table S3.
Protein sequence similarity of Pi7-J-1 and Pi7-J-2 with other Pi7-1 and Pi7-2 alleles. (DOC 34 kb)
Additional file 7: Table S4.
Primers used in this study. (DOC 41 kb)
Additional file 8: Table S5.
132 Philippine rice blast isolates used in the experiment. (DOC 123 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chaipanya, C., Telebanco-Yanoria, M.J., Quime, B. et al. Dissection of broad-spectrum resistance of the Thai rice variety Jao Hom Nin conferred by two resistance genes against rice blast. Rice 10, 18 (2017). https://doi.org/10.1186/s12284-017-0159-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-017-0159-0