Abstract
The intestinal barrier (IB) is a system of diffusion barriers separating the intestinal chyme and blood. The aim of the review is to identify the role of IB dysfunction in the formation of critical states of the body and to substantiate ways to prevent these states. Toxic substances produced by normal intestinal microflora are characterized. The involvement of endotoxin and ammonia in the pathogenesis of sepsis, acute circulatory disorders, secondary acute pulmonary lesions, and acute cerebral insufficiency is shown. Approaches to protect the IB in critical states of the body are proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Legal analysis of the cause of death of a patient gives a choice of four possibilities: untimely, incorrect, insufficient provision of medical care, or a lack of connection with the outcome of the disease (Grishin, 2018). One source of error in this choice is the unpredictability of complications of acute diseases, which became evident during the COVID-19 epidemic (Ponti et al., 2020). The unpredictability is manifested by the sudden development of critical states of the body: sepsis, acute circulatory disorders, secondary acute pulmonary lesions, and acute cerebral insufficiency. They do not occur in all patients and are variable in manifestation. The causes for this variability must be detailed
Previously, we showed (Ivnitsky et al., 2020) that in acute poisoning, the probability of lethal complications increases in accordance with the initial level of vascular endothelial dysfunction. Some of the humoral factors that cause it are waste products of the normal intestinal microflora (Liu et al., 2017). In a healthy person, the ability of these products to overcome the intestinal barrier (IB), the system of structures that separate intestinal chyme and blood, is insignificant. The leakage of waste products of the intestinal microflora into the blood is involved in the pathogenesis of a number of chronic diseases (Tang et al., 2017). The consequences of a massive single intake of such substances into the blood against the action of an exogenous toxicant are little studied.
It can be assumed that in acute diseases, direct or indirect damage to the IB causes secondary IB dysfunction and creates the prerequisites for the entry of metabolites and cellular components of bacteria into the blood at doses that affect the outcome of diseases. A priori, the composition of the mixture of these substances depends on the composition of the intestinal microflora, which is individually variable (Role of Gut Bacteria…, 2005), and, according to some, unique (Choi et al., 2020). This variability may determine the unpredictability of complications of acute diseases. The subject of this review is the data that allow one to test these hypotheses and trace the relationship between some critical states of the body and changes in the IB state that occur during these states.
The aim of the review is to identify the role of IB dysfunction in the formation of critical states of the body and to substantiate approaches for their prevention.
ESSENTIAL INFORMATION ABOUT THE STRUCTURE AND FUNCTION OF THE IB
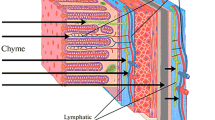
Even 10 years ago, the term “IB” was used to mean intestinal mucosal epithelium (Rao and Wang, 2011, p. 4). Today, the term “IB” also includes the symbiotic bacteria associated with the epithelium, mucin, and endothelium of the blood and lymphatic capillaries of the submucosal layer (Liu et al., 2021). Substances that have escaped absorption by the capillary network of the intestinal wall must overcome additional IB elements on their way into the blood, these are: the smooth muscles of the intestine, two layers of peritoneal mesothelium (visceral, parietal), a layer of peritoneal fluid with an average thickness of 0.5 mm, and the endothelium of the hemo- and lymphocapillaries of the parietal peritoneum.
The layer of mucin with a thickness of 150 μm, consisting of hydrated glycoproteins, separates epithelial cells from the aggressive environment, chyme. The formation of mucin is impaired during mucosal ischemia, which is most dangerous for the large intestine with its high density of microbial colonization. Normally, symbiotic aerobic bacteria displace pathogenic microflora from the apical surface of entero- and colonocytes and provide the latter with the necessary substrates (Role of Gut Bacteria…, 2005). The main function of the monolayer of epitheliocytes with a thickness of 20 μm is selective absorption of substances from the chyme.
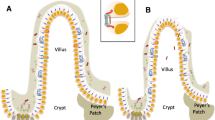
Due to the presence of villi in the small intestine and crypts in the large intestine, the absorption surface area of the mucous membrane of the gastrointestinal tract (GIT) is close to 200 m2 (Lopetuso et al., 2015). Normally, absorption of substances into the GIT occurs mainly through the transcellular route. The percentage of paracellular transport is presumably proportional to the percentage of the surface area of the mucous membrane, which is composed of intercellular contacts, that is: 0.1% (Toxicology…, 2007). Intercellular contacts of two types, tight and adhesive, consist of actin and provide strength to the epithelium by linking the plasma membrane of adjacent cells with their cytoskeleton (Lopetuso et al., 2015). Intercellular contacts are a biotarget for a number of xenobiotics (Lechuga et al., 2020), the importance of paracellular transport increases with the damaging effect of these xenobiotics.
The IB of a healthy person is impenetrable for bacteria living in the intestine. Intestinal microflora (Escherichia coli, Bacteroides) DNA is found in the blood plasma only when the IB is damaged (Shi et al., 2014). Substances to be removed from the body penetrate from the gastrointestinal chyme into the blood by passive diffusion. Their absorption is approximately described by Fick’s first law of diffusion for membranes: J = D(Ci – C0), where J is the density of the substance flux, mol m−2 s−1; D is the membrane permeability coefficient, m s−1; and Ci and C0 are the concentration of the substance from the epithelial and endothelial sides of the membrane, mol m−3. The bioavailability of toxic waste products of the intestinal microflora increases with an increase in the factors (Ci – C0) or D. The first of which is limited by the content of these substances in the chyme, and the second by the state of the epithelium and the vascular endothelium of the GIT. Presumably, absorption increases with an increase in the hydrostatic pressure of the chyme as a result of increased gas formation or smooth muscle spasm (Lin and Pimentel, 2005).
A portion of the substances formed in the chyme that overcome the epithelium enters the hemo- and lymphocapillary networks of the submucosal layer and further into the vessels of the v. portae and ducti thoracici basins. Normally, this is the main path. The other portion, passing through the visceral and parietal layers of the peritoneum, enters the lymphatic vessels of the ducti thoracici basin or the hemocapillary network of the v. cavae inferioris basin. This process of transperitoneal diffusion in the large intestine is facilitated by the absence of a continuous longitudinal muscle layer, which is concentrated in narrow taenie coli and therefore is not an obstacle to diffusion (Magnotti et al., 1998). Transperitoneal diffusion of ammonia (Schäfer et al., 2011) and bacterial lipopolysaccharides (Manani et al., 2020) has been shown. Substances involved in transperitoneal diffusion on their way into the general circulation avoid first pass metabolism in the liver. In portal hypertension, the level of transperitoneal diffusion may increase, since diffusion of substances from the intestinal lumen into the basin of v. portae slows down (Møller et al., 2020). Transperitoneal diffusion is used for detoxification by peritoneal dialysis (Komarov et al., 1981).
Thus, the functions of the IB are to maintain the concentration gradient of substances between the gastrointestinal chyme and blood, as well as the selective absorption of these substances. The movement of substances along this gradient is schematically shown in Fig. 1.
NORMAL MICROFLORA OF THE GASTROINTESTINAL TRACT
Microorganisms of more than 400 species colonize the GIT of a healthy person. Their total number exceeds the number of eukaryotic cells, and their mass is estimated at 0.3% of the host body mass (Sender and Fuchs, 2016). The density of bacterial colonization of the distal GIT is higher than that of the proximal GIT (Table 1).
Fasting samples of gastric contents are normally at pH ≤ 3.0 and are almost sterile. In the presence of food or in hypochlorhydria, bacteria in the gastric lumen are more numerous and are represented mainly by obligate anaerobes, streptococci, lactobacilli, neisseria, and staphylococci. At pH > 5.0 (in old age, pernicious anemia, atrophic gastritis), the microbial composition of gastric contents is indistinguishable from that of the small intestine. On the gastric mucosa of many clinically healthy people (up to 2/3 of the 51–60-year-old subpopulation), the ammonia-producing gram-negative bacterium Helicobacter pylori is detected in the mucin layer. In people infected with Helicobacter pylori for a long time with pangastritis, the pH value of the gastric contents is increased, and the bactericidal function of the stomach is reduced. As a result, in helicobacteriosis, the microflora composition of the GIT structures that are more distal has unfavorable differences from that in healthy individuals (Hunt et al., 2015).
In the duodenum and jejunum, the vegetation of bacteria is balanced by their active removal as a result of digestive secretion and motility, as well as the bactericidal action of bile. Gram-positive cocci (Streptococci, Peptococci) and rods (Lactobacilli, Bifidobacteria) predominate.
The bacterial flora of the terminal ileum is similar to the microflora of the caecum due to reflux from the latter (Role of Gut Bacteria…, 2005).
In the chyme of the large intestine, bacteria account for an average of 27% of its dry mass (Sender and Fuchs, 2016). Anaerobic bacteria (with predominance of Escherichia coli, Bacteroides fragilis, Lactobacilli, and Bifidobacteria) in the colon are 1000 times more common than aerobic bacteria (Role of Gut Bacteria…, 2005). Anaerobes are represented by gram-positive (Bifidobacteria, Eubacteria, Propionibacteria) and gram-negative (Bacteroides, Fusobacteria, Enterobacteria) bacteria. The large intestine mucosa is colonized not only by symbiotic, but also opportunistic bacteria (mainly from the Enterobacteriaceae family) with urease activity (Walker, 2012).
The composition of normal intestinal microflora depends on diet, age, intake of antibacterial drugs, and a number of uncontrolled conditions (Role of Gut Bacteria…, 2005; Choi et al., 2020). The formation of toxic substances in the intestine is facilitated by the predominance of representatives of Proteobacteria and Fusobacteria over Bacteroidetes, while Lactobacilli and Bifidobacteria counteract the formation of toxic substances. The number of microorganisms in the GIT is limited by its propulsive activity. Normally, the transit time for the stomach is 10–48 min, for the small intestine 2.5–4 h (O’Grady et al., 2020), and for the large intestine 25–40 h (Sender and Fuchs, 2016). The duration of chyme passage through the large intestine is why it has the greatest contribution of microbiota to the formation of toxic substances, especially in constipation (Metchnikoff, 1907; Role of Gut Bacteria…, 2005).
TOXIC WASTE PRODUCTS OF NORMAL INTESTINAL MICROFLORA
The normal intestinal microflora produce both essential substances and those toxic to the host (Rodríguez-Hernández et al., 2020). The validity of the hypothesis formulated more than a century ago (Metchnikoff, 1907) about the ability of compounds formed from nutrients by normal intestinal microflora to cause systemic pathological processes under certain conditions is fully proven now. Over the past 10 years, the number of annual publications available with the keywords gut-liver axis, gut-brain axis, gut microbiota, gut microbiota and metabolome, gut microflora, and human gut microbiome has grown exponentially. On the PubMed.gov website, it increased by 12, 14, 17, 30, 37, and 61 times, respectively, from 2010 to 2019, while it remained virtually unchanged during the previous decades. Even the dissonant term fecal volatilome, denoting a mixture of volatile waste products of the intestinal microflora, the content of which in exhaled air is given diagnostic significance in systemic diseases, has appeared (Rodríguez-Hernández et al., 2020). Substances of bacterial origin that possess systemic toxicity, and the degradation products of nitrogen-containing nutrients (proteins, amino acids, amino alcohols, phospholipids) and lipopolysaccharides are considered below.
Ammonia. The GIT accounts for at least 2/3 of the ammonia produced in the body. In the enterocytes, the main mechanism of ammonia formation is the glutaminase reaction, and in the large intestine, it is the metabolic activity of bacteria: deamination of amino acids and nitrogenous bases, and hydrolysis of urea diffusing to the luminal surface of the mucous membrane from the blood (Ivnitskii et al., 2012). The ureolytic activity of microorganisms associated with the colonic mucosa is the source of half of the ammonia produced by the intestinal microflora (Walker, 2012). The content of ammonia in the chyme of the rat large intestine is three times higher than that of the small intestine: 45 and 15 mmol/kg, respectively (Agostini et al., 1972).
From the intestinal chyme, ammonia enters the general circulation both through the portal vein and via transperitoneal diffusion (Schäfer et al., 2011). During a day, in a healthy person, about 4 g of ammonia enters the blood (mainly via the portal vein) from the intestines (Summerskill and Wolpert, 1970). The ammonia level in the blood of the hepatic vein depends on the level in the portal blood (Dobson et al., 1990), and the latter depends on the content of ammonia in the chyme (Gips et al., 1973). Due to neutralization in the liver, the concentration of ammonia in the blood plasma of the hepatic vein is 2–3 times lower than in the blood plasma of the portal vein (Dobson et al., 1990), and an order of magnitude lower than in the blood of the mesenteric veins draining the colon (Role of Gut Bacteria…, 2005). In the plasma of arterial blood, the normal concentration of ammonia ([NH3] + [NH4+]) is 30 µM (Ali and Nagalli, 2021). An increase in ammonia concentration is accompanied by neurotoxicity, as first described in the laboratory of I.P. Pavlov (Hahn et al., 1893). At ([NH3] + [NH4+]) values of 50–100 μM in the blood plasma, hyperammonemia is asymptomatic; vomiting, ataxia, irritability, and hyperactivity are noted at 100–200 μM; and coma is observed at 200 μM (Pagana, K.D. and Pagana, T.J., 2014). With an average volume of the large intestine chyme of 0.4 L (Metchnikoff, 1907) and ammonia content of 5.7–39.0 mM (Agostini et al., 1972), the total intestinal pool of ammonia is 2.3–15.6 mmol. Simultaneous uniform distribution of this amount of ammonia in the blood (5 L), lymph (2 L), and chyme of the large intestine would increase the ammonia content in the blood to 311–2108 μM, that is, to values far exceeding the coma threshold.
Ammonia enters the cells in the non-ionized form, NH3. At pH = 7.36, which is typical for normal blood plasma (taking into account the basicity constant of ammonia pKa = 9.15 at t = 37°C), the proportion of the NH3 form is 1.6%. In the cytoplasm, pH is lower, which causes diffusion of ammonia into the cells even at its normal content in the blood (Ivnitsky et al., 2012). In metabolic acidosis or gaseous alkalosis, the pH difference between the blood plasma and cytoplasm increases, which intensifies the supply of ammonia to cells along the NH3 concentration gradient (Dobson et al., 1990; Scott et al., 2013). In these cases, toxic effects are possible even in the absence of hyperammonemia.
In addition to neurotoxicity, ammonia exhibits endothelial toxicity, shown both in vitro and in vivo. In cultured endotheliocytes of cerebral capillaries, it caused oxidative stress, accumulation of NO and arachidonic acid peroxidation products (Skowrońska and Albrecht, 2013), increased the permeability of the plasma membrane for the fluorescein-isothiocyanate dextran derivative, and increased expression of the arginine transporter, a substrate for NO synthesis (Skowrońska et al., 2012 ). The cultivation medium of endotheliocytes treated with ammonia, when added to a culture of astrocytes, caused swelling of the latter, which indicates the possible involvement of the vascular endothelium in the formation of cerebral edema in hyperammonemia (Jayakumar and Norenberg, 2018). Increasing the ammonia concentration in the cultivation medium destroyed the extracellular matrix and increased the paracellular permeability of the endotheliocyte monolayer (Skowrońska et al., 2012).
In the endotheliocytes, ammonia activates Toll-like receptor 4 responsible for the inflammatory response to endotoxin, which leads to overproduction of oxygen and nitrogen free radicals, swelling of the endotheliocytes, and an increase in their permeability to substances involved in the pathogenesis of hepatic encephalopathy (Jayakumar and Norenberg, 2018). Hyperammonemia caused endothelium-dependent NO-mediated dilatation of cerebral arterioles, which could contribute to increased intracranial pressure and cerebral edema in acute liver failure (Scott et al., 2013). Intravenous administration of ammonium acetate to rabbits increased the permeability of the blood-brain barrier (BBB) to polyethylene glycol PEG 400 (McClung et al., 1990). According to positron emission tomography, BBB permeability is also increased in patients with chronic hepatic encephalopathy (Ochoa-Sanchez and Rose, 2018). Thus, the intensification of ammonia entry into the endothelial cells of cerebral vessels causes oxidative stress, inflammatory damage, increases permeability, and disrupts local regulation of blood flow.
Trimethylamine-N-oxide (TMAO) is an ammonia derivative rudimentary for humans, the useful function of which (as an osmolyte) was preserved in marine animals. It is formed in the liver by oxidation of trimethylamine, a decomposition product of L-carnitine, choline, lecithin, or betaine by Clostridium, Escherichia, Enterobacter, Acinetobacter and Proteus in the colon lumen (Zhang and Davis, 2016). The concentration of trimethylamine in the blood plasma is 38 times lower than that of TMAO. Data on the concentration of TMAO in the blood plasma of healthy people vary from 0.17 to 34.62 μM with six-month daily intake of 1.5 g L-carnitine (Olek et al., 2019). It also increases with an increase in the proportion of Gammaproteobacteria in the intestinal microbiota (Bonitenko et al., 2010). The content of TMAO in the blood of mice increases when they are transferred to a diet enriched with choline. The blood of gnotobiotic (bacteria free) C57BL/6J mice lacks TMAO even when they receive a choline-rich diet. The transfer of such mice to non-sterile conditions leads to the appearance of TMAO in their blood (Zhu et al., 2016).
The systemic toxicity of TMAO is manifested by systemic inflammation (MacPherson et al., 2020). When evaluating the ability of an increased level of TMAO in the blood to cause oxidative stress in the endothelium, conflicting data were obtained, with both the presence (Brunt et al., 2020) and the absence (Olek et al., 2019) of signs of such found. In vitro, the ability of TMAO to induce vasospasm was shown (Restini et al., 2020). Elevated plasma TMAO levels are associated with cardiovascular diseases (Marchenko and Laryushina, 2017), hepatic steatosis (Tan et al., 2019), kidney fibrosis (Zhang and Davis, 2016; Cosola et al., 2018), and thrombosis (Zhu et al., 2016), and an increased risk of thromboembolism in atrial fibrillation (Gong et al., 2019). Some publications showed an association of circulatory disorders with the level of trimethylamine, and not with TMAO (Jaworska et al., 2019).
The role of intestinal microflora in the formation of TMAO-mediated endothelial dysfunction is described by the third postulate of R. Koch for pathogens: the pathogen causes the disease when introduced into a healthy animal. Transplantation of cecal chyme from nonthrombotic NZW/LacJ mice into gnotobiotic mice fed a choline-rich diet did not alter the blood levels of TMAO, or blood clotting in the recipients. Transplantation of the chyme from mice of prothrombotic C57BL/6J line into gnotobiotic mice caused a significant increase in both TMAO content and blood clotting (Zhu et al., 2016). Oral antibiotics blocked the rise in blood TMAO levels after loading with lecithin (Tang et al., 2013) or carnitine (Koeth et al., 2013).
Thus, high levels of TMAO and/or trimethylamine in the blood are associated with an increased risk of systemic inflammation, vasospasm, thrombus formation, liver damage, and kidney damage.
Indoxyl sulfate, indoxyl acetate. Indole is a metabolite of tryptophan, for which Clostridium sporogenes that metabolizes it to 3-indole propionic acid, Lactobacillus that metabolizes tryptophan to indole-3-aldehyde, and a number of bacteria that express tryptophanase but do not metabolize indole, compete in the large intestine. In the latter case, free indole enters the liver, where it is acylated to form indoxyl sulfate and indoxyl acetate. Unlike 3-indole propionate and indole-3-aldehyde, which exhibit neuroprotective and immunomodulating properties, respectively (Zhang and Davis, 2016), indoxyl sulfate and indoxyl acetate are endotheliotoxic. Their accumulation in the blood is associated with chronic renal failure and the risk of cardiovascular disease (Zhang and Davis, 2016; Cosola et al., 2018). The content of indoxyl sulfate in the blood plasma ranges from 10−7 to 2.4 × 10−6 M under normal conditions and from 2.5 × 10−4 to 5.0 × 10−4 M in chronic renal failure (Matsumoto et al., 2019).
p-Cresyl sulfate is formed in epithelial cells of the large intestine and in hepatocytes by sulfation of p‑cresol, a product of deamination and decarboxylation of the aromatic amino acids tyrosine and phenylalanine with the participation of bacteria of the families Bacteroidaceae, Bifidobacteriaceae, Clostridiaceae, Enterobacteriaceae, Enterococcaceae, Eubacteriaceae, Fusobacteriaceae, Lachnospiraceae, Lactobacillaaceae, Porphyromonaceae Staphylococcaceae, Ruminococcaceae, and Veillonellaceae. Every day, 50–100 mg of p-cresol is formed in the large intestine (Role of Gut Bacteria…, 2005). p-Cresyl sulfate possesses endotheliotoxicity at the plasma levels observed in chronic renal failure, 116–568 μM, while the normal level is 15–35 μM (Gryp et al., 2017). The content of p-cresyl sulfate in the blood of patients with pulmonary edema is increased. Administration of p-cresyl sulfate to mice increased pulmonary capillary permeability, activated leukocytes, increased the production of free radical oxygen species, and caused cell death and interstitial pulmonary edema (Chang et al., 2018).
Indoxyl sulfate, indoxyl acetate and p-cresyl sulfate have low renal clearance, so they are accumulated in the blood in renal failure (Matsumoto et al., 2019). Normally, the plasma pool of these substances is 95–97% represented by complexes with albumin. In renal insufficiency, their content in the free form increases, which is accompanied by an increase in endotheliotoxicity. This is manifested by inflammation, vasospasm, ischemia, thrombus formation, the development of tissue insulin resistance and multiorgan failure, and an increased risk of cardiovascular complications of existing diseases (Cosola et al., 2018).
Monoamines are formed in the intestinal chyme from amino acids under the action of inducible decarboxylases, some of which are pyridoxal phosphate-dependent. The highest decarboxylase activity is shown by Enterobacteria (Role of Gut Bacteria…, 2005). In the intestinal chyme of newborns, the content of monoamines is insignificant, but increases with bacterial colonization of the GIT (Suárez et al., 2019). From L-tyrosine, the microflora forms p-tyramine and p-octopamine, from L-tryptophan it forms tryptamine and serotonin, from histidine it forms histamine. The amount of monoamines entering the portal system normally does not exceed the ability of the liver to neutralize them, mainly by conjugation (Role of Gut Bacteria…, 2005). In IB dysfunction, intensification of synthesis, and (or) impaired conjugation of monoamines in the liver, monoamine content in the blood is increased (Zhang and Davis, 2016).
Tyramine, tryptamine, octopamine, and serotonin, when released into the blood, have a vasoconstrictive effect. Octopamine infusion induced hypoxemia in pigs with hyperventilation and increased pulmonary veno-arterial shunting (Nespoli et al., 1983). The accumulation of serotonin in the blood causes serotonin syndrome, the symptoms of which are tachycardia, arterial hypertension, hyperthermia, increased blood clotting, and in severe cases, rhabdomyolysis and multiorgan failure (Volkov, 2014). Histamine causes bronchospasm, is involved in the pathogenesis of inflammation, increases blood viscosity, and impairs microcirculation (Zhang and Davis, 2016).
Thus, the systemic biological activity of monoamines produced by the intestinal microflora is characterized by their effect on blood circulation, gas exchange processes in the lungs, blood coagulation, and (or) inflammation. This determines the possibility of monoamine involvement in the pathogenesis of acute poisoning complications, that is: cardiovascular disorders, sepsis, coagulopathy, and secondary acute lung lesions.
Bacterial endotoxin. The content of a mixture of gram-negative bacterial lipopolysaccharides (called endotoxins) in adult colon chyme is 2.5 g/L (Bested et al., 2013). In the blood plasma, it is 9 orders of magnitude less: 10 ng/L, which is the threshold for inflammatory activation of macrophages and endotheliocytes. This value is moderately increased in periodontitis, diabetes mellitus, liver cirrhosis, and Alzheimer’s disease, and reaches 500 ng/L in sepsis (Brown, 2019). Elevated levels of endotoxin in the blood plasma can be defined as endotoxemia. Plasma levels of endotoxin and TMAO are positively correlated (MacPherson et al., 2020). In the blood plasma, endotoxin is contained both in the biologically active free form and in the form of complexes with blood proteins, which are not always detected in laboratory studies (Komarov et al., 1981).
Endotoxins have pronounced endotheliotoxicity, which is manifested by oxidative stress, destruction of the glycocalyx, adhesion of leukocytes, vasospasm and thrombus formation (Iba et al., 2018), and increased BBB permeability (Minami et al., 2007). The content of endotoxin in the blood that is typical for sepsis, leads to cytokine storm and septic shock (Pfalzgraff and Weindl, 2019). Chronic exposure at lower concentrations is accompanied by chronic systemic inflammation (Morris et al., 2015). Escherichia coli lipopolysaccharides have much greater proinflammatory properties than Bacteroides dorei or Bacteroides vulgatus lipopolysaccharides (Yoshida et al., 2020).
Endotoxins cause thromboxane-dependent pulmonary vasoconstriction, and at higher concentrations, typical for septic shock, cause systemic vasodilation and pulmonary hypertension (Corrêa et al., 2020). Endotoxinemia increases BBB permeability for both endotoxins and other substances, increases the permeability of pulmonary capillaries, causes noncardiogenic pulmonary edema (Wang et al., 2019), and impairs the functions of the liver (Solé et al., 2021) and kidneys (Nežić et al., 2020).
MECHANISMS OF IB DYSFUNCTION IN ACUTE DISEASES
Direct damage to IB. Xenobiotics, infectious agents, and ionizing radiation directly damage IB, disturbing its barrier function. Enterotoxicity is inherent not only to chemically aggressive substances that enter the body orally, but also to many systemically acting toxicants.
The epithelium of the small intestine and, to a lesser extent, the stomach and large intestine is a typical system of cell renewal with high proliferative activity. This makes it highly sensitive to cytostatic drugs, which cause interphase death of enterocytes and, as a result, denudation of the epithelium. Differences in the enterotoxic action of such substances are determined only by the differentiation level of the predominantly affected cells. Thus, adriamycin causes apoptosis of enterocytes mainly in positions 4–5 from the stem cell located at the lower point of the intervillous crypt. Under the action of isopropyl methane sulfonate, nitrogen mustard, or actinomycin D, enterocytes die in an interphase manner at positions 6–7; fluorouracil, myleran, cyclophosphamide, or cycloheximide, at positions 7–9; and vincristine or hydroxyurea, at positions 10–11 (Ijiri and Potten, 1983). In rats with acute intoxication with cyclophosphan, the IB permeability for methylene blue, mannitol, and lactulose is increased (Schäfer et al., 2011). The enterotoxicity of cytostatics used in hematopoietic stem cell transplantation is one of the factors limiting patient survival (McMillen et al., 2021).
Direct enterotoxicity is inherent to ethanol (Bishehsari et al., 2017), a number of mycotoxins, arsenic, and salts of heavy metals (Kutsenko, 2004). The specific toxicity of nonsteroidal anti-inflammatory drugs made them a means of experimental modeling of acute gastroenteritis (Karádi et al., 2001), and dextran sulfate and 2,4,6-trinitrobenzenesulfonate are useful as a means of experimental modeling of acute colitis (Ishisono et al., 2019). Some mycotoxins cause inflammatory damage to the IB by potentiating the effect of endotoxins on it (Ge et al., 2020). Mycotoxins T-2 and deoxynivalenol increase IB permeability to polyethylene glycol PEG 4000 (Semenov et al., 2019).
An increase in IB permeability is not only the result of direct damage to the epithelium and vascular endothelium of the intestine by a xenobiotic or its metabolic products. The same may be due to the effects of acute hypoxia, smooth muscle spasm of GIT organs, and gastrointestinal stasis, often observed in acute poisoning.
Acute intestinal hypoxia in critical states of the body is caused by disturbances in external respiration and blood circulation. In acute severe poisoning, such states include exotoxic shock, and in the absence of respiratory support, depression of the respiratory center, neuromuscular block, and broncho-obstructive syndrome (Bonitenko et al., 2010). As a result of the blood circulation centralization, the GIT experiences a deeper hypoxia than the most important organs for maintaining the vital activity of the body that is, the brain, lungs, and heart.
The aerobic energy metabolism of enterocytes predisposes them to disruption of oxidative resynthesis of ATP. This is evidenced by intestinal damage during high-altitude hypoxia, manifested by inflammation, ulceration, and bleeding, which are life-threatening states complicating altitude sickness (Khanna et al., 2019). Gas hypoxia provoked an increase in the level of cytokines in the blood and increased IB permeability in rats when running on the treadmill (Hill et al., 2020). IB permeability is increased in hemolytic anemia (Abuga et al., 2020) and acute blood loss (Khazoom et al., 2020).
Some cytotoxicants inhibit enzymes of energy metabolism or uncouple oxidative phosphorylation (Kutsenko, 2004), which exacerbates the energy-deficient state of biological tissues in acute poisoning with these substances. Uncoupling of oxidative phosphorylation in colonic epithelial cells disrupts their barrier function against Escherichia coli (Saxena et al., 2018). Mice with more active oxidative phosphorylation in the mucosa of the large intestine are more resistant to damage by dextran sulfate or trinitrobenzene sulfonate (Bär et al., 2013).
The submucosal hemocapillary plexus is better developed in the small intestine than in the large intestine (Magnotti et al., 1998), which explains the higher sensitivity of the latter to ischemic injury (Zvenigorodskaya et al., 2010). During hypoxia, the formation of mucin in the intestine is reduced, which leads to inflammatory changes in the mucous membrane (Zvenigorodskaya et al., 2010).
Smooth muscle spasm of GIT organs is one of the characteristic symptoms of poisoning with cholinesterase inhibitors and serotonergic drugs (Kutsenko, 2004), but it can also be a reaction to acute hypoxia caused by blood circulation centralization, or the result of a spasmogenic effect of serotonin produced by Enterobacteria on the smooth muscles of the intestine. Serotonergic stimulation of the large intestine is also accompanied by arterial spasm of the microvasculature in addition to spasm of its own smooth muscles. A hypothesis about the muscle spasm–ischemia–pain triad as a factor in increased IB permeability in irritable bowel syndrome has been put forward (Uno, 2019). In acute poisoning, the IB reaction to such changes may be similar.
Gastrointestinal stasis is a potentially lethal complication in patients of resuscitation and intensive care departments. This is a toxicity manifestation of opioids, cholinergic antagonists, and serotonin agonists (Toxicology…, 2007). It is typical for severe poisoning accompanied by coma (Ivnitsky et al., 2006), and for acute poisoning with alkylating substances (Schäfer, 2015).
In stasis, two prerequisites for the intensification of diffusion of substances produced by the intestinal microflora through IB arise: damage and increased gas formation in the GIT. One of the factors of IB damage during stasis is a change in the composition of the intestinal microflora. With a decrease in the propulsive activity of the GIT, the vegetation of bacteria is not compensated by their removal, resulting in an increase in the luminal concentration of toxic substances, in particular, ammonia. Damage to the colonocytes is determined by an increase not only in the total concentration of ammonia ([NH3] + [NH4+]), but also in the pH of the chyme, and hence, the proportion of ammonia present in the highly penetrating form NH3 (Agostini et al., 1972); its cytotoxic effect on colonocytes reduces their life cycle and reduces mucin production (Zhou et al., 2020).
Thus, in acute severe diseases, secondary IB dysfunction develops: an increase in IB permeability to metabolites and cellular components of the intestinal microflora. The pathogenetic mechanisms of secondary IB dysfunction in acute diseases are direct damage, gastrointestinal stasis, hypoxia, and smooth muscle spasm of GIT tissues. Secondary IB dysfunction, increased production of toxic substances by the intestinal microflora, and gas formation are prerequisites for intensifying the flow of toxic substances from the intestinal chyme into general circulation with the formation of endotoxemia.
ENDOTOXEMIA AND ENDOTOXICOSIS IN CRITICAL STATES OF THE BODY
Endotoxemia is the accumulation in the blood of biologically active substances formed in the body. Endotoxicosis is a set of clinical manifestations of endotoxemia. Based on the idea of the body as an ecosystem, of which the intestinal microbiota is an integral part (Role of Gut Bacteria…, 2005; Tang et al., 2017), compounds produced by the normal intestinal microflora are classified as endogenous substances. Such substances contribute to the development of critical states of the body, complicating acute diseases. These conditions can be considered as endotoxicosis, a set of clinical manifestations of endotoxemia.
Acute circulatory disorders. Severe endotoxinemia and hyperammonemia are typical for shock. An increased level of endotoxin in the blood was observed in septic (Luna et al., 2021), traumatic, and hemorrhagic (Hu et al., 2019) shock. Experimentally, septic shock is reproduced by the administration of endotoxins to animals (Fujivara et al., 2020).
The concentration of ammonia in the blood plasma of patients admitted to the intensive care department due to cardiac arrest is 4.8 times higher than in patients with spontaneous circulation (Lin et al., 2013). The content of ammonia in the blood plasma and brain tissue of dogs and rabbits is increased many times during insulin and heat shock. The main symptoms of both insulin and heat shock are reproduced by the administration of ammonium salts to animals (Kozlov, 1971).
In hemorrhagic shock, the source of endotoxemia is the intestine. Endotoxemia proceeds in three stages: (1) an increase in the content of substances of low and medium molecular weight in tissues of the intestine and the blood of the portal vein; (2) the beginning of the increase in their content in the systemic circulation; (3) achieving equally high blood toxicity of the portal vein and carotid artery (Khramikh and Dolgikh, 2007).
Sepsis is a systemic inflammatory response to endotoxinemia and bacteremia most frequently complicating acute poisoning in alcohol dependent individuals (Chaung et al., 2019). In massive acute methanol poisoning, sepsis developed in 6.4% of victims (Kumar et al., 2019). In 33% of patients with sepsis, infectious agents were not detected in the blood (Novosad et al., 2016). However, sepsis is characterized by endotoxinemia. The leading mechanism of development of which is IB dysfunction caused by the activation of one of the DNA repair enzymes, poly (ADP-ribose) polymerase-1, in enterocytes. This depletes their NAD+ pool, which leads to the disruption of oxidative phosphorylation, death of the enterocytes, and increased permeability of the intestinal epithelium (Fink, 2002).
Experimentally, sepsis is well reproduced by the administration of endotoxins to animals (Chen et al., 2020). Endotoxins at blood levels typical for sepsis damages the kidneys, heart, and liver and is the main etiological factor in multiorgan failure (Luna et al., 2021). Under the influence of endotoxins in sepsis, the blood coagulation cascade is activated and microvascular thrombosis occurs. Perivascular edema and coagulopathy in sepsis impede tissue perfusion, leading to tissue hypoxia, multiorgan failure, pulmonary edema, and shock (Pool et al., 2018).
Ammonia is also involved in the pathogenesis of multiorgan failure in sepsis. The concentration of ammonia in the blood plasma of patients diagnosed with sepsis upon admission to the intensive care department is increased and positively correlates with the likelihood of developing multiorgan failure over the next 28 days (Zhao et al., 2020). In patients with positive test for bacteremia, the plasma levels of ammonia are twice as high as those with negative test (Numan et al., 2018).
Secondary acute lung lesions are noncardiogenic pulmonary edema, shock and wet lung syndromes (Bonitenko et al., 2010). The involvement of endotoxins in the pathogenesis of increased permeability of the alveolar-capillary barrier is evidenced by endotoxemia in patients with this condition (Maniatis et al., 2008) and the ability to simulate it by exposure to endotoxins both in vitro and in vivo (Wang et al., 2018). Endotoxins also cause hemodynamic disturbances in the lungs, leading to the development of edema: injection of Escherichia coli endotoxins into the cavity of the right atrium of pigs increased blood pressure in the pulmonary artery (Corrêa et al., 2020). Hyperammonemia precedes the development of pulmonary hypertension and is the best laboratory indicator for its prediction in patients with a portocaval shunt (Bloom et al., 2020).
Acute cerebral insufficiency (ACI) is a set of syndromes and conditions resulting from acute CNS dysfunction caused by diffuse brain damage. It occurs in severe poisoning with substances with different mechanisms of action. It is clinically manifested by syndromes of impaired and clouded consciousness, movement disorders, accelerated catabolism, and acute respiratory and circulatory disorders of central origin. It is characterized by a lack of specificity of both clinical and morphological features. It is a critical state of the body, since it is characterized by a gross disturbance of vital functions. ACI pathogenesis is poorly understood. It is thought to involve acute hypoxia and, later, mediator chaos (Shilov et al., 2010).
The biological activity of representatives of the bacterial metabolome suggests their involvement in ACI pathogenesis as factors aggravating the effect of exogenous toxicant and hypoxia. Hyperammonemia is typical for acute liver failure complicated by cerebral edema and increased intracranial pressure (Sheikh et al., 2018). Intracranial pressure, neurological disorders, and mortality are positively associated with serum ammonia levels in patients with nonhepatic hyperammonemia (Bested et al., 2013). The content of ammonia in the blood plasma and brain tissue was repeatedly increased when diabetic coma was simulated in dogs and rats (Kozlov, 1971). In rats with acute cyclophosphamide intoxication (600 mg/kg), the aggravation of hyperammonemia by intragastric administration of ammonium acetate intensified the accumulation of ammonia and glutamine in the blood and brain, depleted the tissue pool of pyruvate (Ivnitsky et al., 2019), accelerated the development of neurological disorders that are clearly similar to symptoms of acute poisoning with ammonium salts, and reduced the life span of animals (Ivnitsky et al., 2011). Neurological manifestations of acute poisoning with ammonium salts correspond to the definition of ACI (Kozlov, 1971).
Endotoxins sensitized animals to acute cerebral hypoxia. Administration of Escherichia coli endotoxins to piglets potentiated neuronal loss, metabolic disorders, and increased the likelihood of brain death under subsequent carotid artery occlusion (Pang et al., 2020). Administration of endotoxins to ferrets aggravated their brain damage under carotid artery occlusion (Wood et al., 2019). Endotoxin potentiated cerebral edema during high-altitude hypoxia (Zhou et al., 2017).
Thus, the data available in the literature indicate the involvement of endotoxins and ammonia produced by normal intestinal microflora in the pathogenesis of a number of pathological conditions that can complicate acute poisoning, such as sepsis, acute circulatory disorders, secondary acute lung lesions, and acute cerebral insufficiency.
PROSPECTS FOR IB PROTECTION IN CRITICAL STATES OF THE BODY
Means for systematic IB protection. Normal intestinal microflora is the main factor of protection against colonization by pathogenic microorganisms. In the small intestine, it activates glycosyltransferases, which are responsible for the formation of brush border glycoconjugates. Bacteria adhering to the mucosa form a mechanical barrier separating it from the chyme. Symbiotic anaerobic gram-positive bacteria: Lactobacilli, Bifidobacteria, and Propionobacteria, suppress the development of opportunistic intestinal microflora: Escherichia coli, Clostridium botulinum, and Clostridium dificile (Role of Gut Bacteria…, 2005). The mechanisms of the protective effect of probiotics on IB include (Bested et al., 2013) normalization of the composition of the intestinal microflora, suppression of the formation of ammonia, amines, and uremic toxins, inhibition of the production of inflammatory cytokines, synthesis of neurotrophic factors, improved absorption of carbohydrates and essential nutrients, and an increase in the pain threshold. For systematic IB protection, probiotics, prebiotics and other low toxicity drugs designed for long-term use are applicable. Thus, the possibility of using of the plantain juice to correct the hyperammoniemic and neurotoxic effects of cyclophosphamide was experimentally shown (Schäfer et al., 2015).
Means of emergency IB protection. The beneficial effect of prebiotics on IB has been reviewed in a number of detailed reviews (Role of Gut Bacteria…, 2005; Bested et al., 2013; Liu et al., 2017; Tang et al., 2017). As a means of emergency prevention of damage, those that are able to form a shielding film on the mucous membrane are promising. When pectin is administered orally, a protective film of calcium pectinates is formed on the mucous membranes (Ishisono et al., 2019). This prevents the entry of substances formed by the microflora from the chyme into the blood. Gelatin tannate also has a similar property (Çağan et al., 2017). The protective effect due to the shielding action is expected during the period of enterosorbent transit in the small intestine, that is, from 10–48 to 160–288 minutes after administration.
In critical states of the body, information about their etiological factor is usually not enough, so treatment is aimed at correcting the general mechanisms of the pathogenesis. These include acute hypoxia of the GIT, so early oxygen therapy may be useful for the prevention of endotoxicosis. This is consistent with the data on the potentiation of the toxic effect of ammonia by acute hypoxia (Kozlov, 1971) and the anti-inflammatory effect of hyperbaric oxygen therapy in sepsis (Rinaldi et al., 2011).
A valuable addition to oxygen therapy of hypoxic states of the body is antihypoxants (Ivanov et al., 2020). These include gas mixtures containing inert gases at low concentrations, nitric oxide (II), hydrogen, and hydrogen sulfide. Xenon, nitric oxide (II), and hydrogen have neuroprotective and cardiotonic properties, while argon and hydrogen sulfide have only neuroprotective properties (Alshami et al., 2020). An important element of the treatment is the elimination of inflammation. Hydrocortisone at a dose of 2.8 mg/kg prevented IB dysfunction and endotoxemia in rats in a model of sepsis by ligation and cecal puncture (Assimakoppoulos et al., 2021).
CONCLUSIONS
The normal intestinal microflora produces substances whose systemic toxicity profile, when administered at high doses, corresponds to a number of critical states of the body: acute circulatory disorders, sepsis, secondary acute lung lesions, and acute cerebral insufficiency. The amount of these substances contained in the intestinal chyme of a healthy person provides the occurrence of the listed pathological states when there is complete or significant loss of their concentration gradients between the chyme and blood. The IB is a system of diffusion barriers that separate intestinal chyme and blood, maintaining the concentration gradients of toxic substances reaching up to 109. In severe acute diseases, there are prerequisites for damage to the IB and its secondary dysfunction, leading to the development of endotoxemia and endotoxicosis.
Both IB dysfunction and endotoxemia in a number of critical states of the body are documented by the data of experimental and clinical studies. The involvement of bacterial endotoxins and ammonia of intestinal origin in the pathogenesis of sepsis, acute circulatory disorders, secondary acute lung lesions, and acute cerebral insufficiency, which complicate such poisoning, has been shown.
Measures of systematic IB protection from damage include correction of the composition of the intestinal microflora, aimed at suppressing its formation of cytotoxic substances and suppressing the production of inflammatory cytokines. Emergency prevention of secondary IB dysfunction involves elimination of hypoxia and inflammation of the GIT organs, and protection of the mucous membrane using drugs that shield it from the chyme.
REFERENCES
Abuga, K., Muriuki, J., Williams, T., and Atkinson, S., How severe anaemia might influence the risk of invasive bacterial infections in African children, Int. J. Mol. Sci., 2020, vol. 21, no. 18, art. ID 6976.
Agostini, L., Down, P., Murison, J., and Wrong, O., Faecal ammonia and pH during lactulose administration in man: comparison with other cathartics, Gut, 1972, vol. 13, no. 11, pp. 859–866.
Ali, R. and Nagalli, S., Hyperammonemia, Treasure Island: StatPearls, 2021.
Alshami, A., Einav, S., Skrifvars, M., and Varon, J., Administration of inhaled noble and other gases after cardiopulmonary resuscitation: a systematic review, Am. J. Emerg. Med., 2020, vol. 38, no. 10, pp. 2179–2184.
Assimakoppoulos, S., Papadopoulou, L., Bantouna, D., et al., Fecal microbiota transplantation and hydrocortisone ameliorate intestinal barrier dysfunction and improve survival in a rat model of cecal ligation and puncture-induced sepsis, Shock, 2021, vol. 55, no. 5, pp. 666–675.
Bär, F., Bochmann, W., Widok, A., et al., Mitochondrial gene polymorphisms that protect mice from colitis, Gastroentherology, 2013, vol. 145, no. 5, pp. 1055–1063.
Bested, A.C., Logan, A.C., and Selhub, E.M., Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II—contemporary contextual research, Gut Pathog., 2013, no. 5, art. ID 3.
Bishehsari, F., Magno, E., Swanson, G., et al., Alcohol and gut-derived inflammation, Alcohol Res., 2017, vol. 38, no. 2, pp. 163–171.
Bloom, P., Rodriguez-Lopez, J., Witkin, A., et al., Ammonia predicts hepatic involvement and pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia, Clin. Transl. Gastroenterol., 2020, vol. 11, no. 1, art. ID e00118.
Bonitenko, E.Yu., Bonitenko, Yu.Yu., Bushuev, E.S., et al., Ostrye otravleniya lekarstvennymi sredstvami i narkoticheskimi veshchestvami (Acute Poisoning with Drugs and Narcotic Substances), St. Petersburg: ELBI-SPb, 2010.
Brown, G.C., The endotoxin hypothesis of neurodegeneration, J. Neuroinflammation, 2019, vol. 16, art. ID 180.
Brunt, V., Gioscia-Ryan, R., Casso, A., et al., Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans, Hypertension, 2020, vol. 76, no. 1, pp. 101–112.
Çağan, E., Ceilan, S., Mengi, S., and Çağan, H., Evaluation of gelatin tannate aganist symptoms of acute diarrhea pediatric patients, Med. Sci. Monit., 2017, vol. 23, pp. 2029–2034.
Chang, J., Liang, S., Thanasekaran, P., et al., Translational medicine in pulmonary-renal crosstalk: therapeutic targeting of p-cresyl sulfate triggered nonspecific ROS and chemoattractans in dyspneic patients with uremic lung injury, J. Clin. Med., 2018, vol. 7, no. 9, art. ID 266.
Chaung, W., Brenner, M., Yen, H., et al., Recombinant human milk fat globule-EGF factor VIII (rhMFG-E8) as a therapy for sepsis after acute exposure to alcohol, Mol. Med., 2019, vol. 25, art. ID 52.
Chen, S., Xiu, G., Zhou, J., et al., Role of high mobility group box1 in intestinal mucosal barrier injury in rat with sepsis induced by endotoxin, Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 2020, vol. 32, no. 7, pp. 803–807.
Choi, T., Choi, Y., and Koo, J., Mental disorders linked to crosstalk between the gut microbiome and the brain, Exp. Neurobiol., 2020, vol. 29, pp. 403–416.
Corrêa, T., Pereira, A., Takala, J., and Jakob, S., Regional venous-arterial CO2 to arterial-venous O2 content difference ratio in experimental circulatory shock and hypoxia, Intensive Care Med. Exp., 2020, vol. 8, no. 1, art. ID 64.
Cosola, C., Rocchetti, M.T., Cupisti, A., and Gesualdo, L., Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease, Pharmacol. Res., 2018, vol. 130, pp. 132–142.
Dobson, G.P., Veech, R.L., Passoneau, J.V., and Huang, M.T., In vivo portal-hepatic venous gradients of glycogenic precursors and incorporation of D-[3-3H]glucose into liver glycogen in the awake rat, J. Biol. Chem., 1990, vol. 265, pp. 16350–16357.
Fink, M., Bench-to-bedside review: cytopathic hypoxia, Crit. Care, 2002, vol. 6, no. 6, pp. 491–499.
Fujivara, Y., Ohnishi, K., Horlad, H., et al., CD163 deficiency facilitates lipopolysaccharide-induced inflammatory responses and endotoxin shock in mice, Clin. Transl. Immunol., 2020, vol. 9, no. 9, art. ID e1162.
Ge, L., Lin, Z., Le, G., et al., Nontoxic-dose deoxynivalenol aggravates lipopolysaccharides-induced inflammation and tight junction disorder in IPEC-J2 cells through activation of NF-κB and LC3B, Food Chem. Toxicol., 2020, vol. 145, art. ID 111712.
Gips, C.H., Qué, G.S., and Wibbens-Alberts, M., The arterial ammonia curve after oral and intraduodenal loading with ammonium acetate. Absorption in the stomach, Neth. J. Med., 1973, vol. 16, pp. 14–17.
Gong, D., Zhang, L., Zhang, Y., et al., Gut microbial metabolite trimethylamine N-oxide is related to thrombus formation in atrial fibrillation patients, Am. J. Med. Sci., 2019, vol. 358, no. 6, pp. 422–428.
Grishin, S.M., Crimes committed by medical workers as a result of improper performance of their professional duties (based on the case-law of the European part of Russia 2015–2017), Meditsina, 2018, no. 1, pp. 1–14.
Gryp, T., Vanholder, R., Vaneechoutte, M., and Glorieux, G., p-Cresyl sulfate, Toxins, 2017, vol. 9, no. 2, art. ID 52.
Hahn, M., Massen O., Nencki M., and Pawlow J., Die Eck’sche Fistel zwischen der unteren Hohlvene und der Pfortader und ihre Folgen für den Organismus, Arch. Für Exp. Pathol. Pharmakol., 1893, Bd. 32, S. 161—210.
Hill, G., Gillum, T., Lee, B., et al., Prolonged treadmill running in normobaric hypoxia causes gastrointestinal barrier permeability and elevates circulating levels of pro- and anti-inflammatory cytokines, Appl. Physiol., Nutr. Metab., 2020, vol. 45, no. 4, pp. 376–386.
Hu, C., Sun, J., Du, J., et al., The Hippo-YAP pathway regulates the proliferation of alveolar epithelial progenitors after acute lung injury, Cell Biol. Int., 2019, vol. 43, no. 10, pp. 1174–1183.
Hunt, R.H., Camilleri, M., Crowe, S.E., et al., The stomach in health and disease, Gut, 2015, vol. 64, no. 10, pp. 1650–1668.
Iba, T., Levy, J.H., Hirota, T., et al., Protection of the endothelial glycocalyx by antithrombin in endotoxin-induced rat model of sepsis, Thromb. Res., 2018, vol. 171, pp. 1–6.
Ijiri, K. and Potten, C., Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation, Brit. J. Cancer, 1983, vol. 47, no. 2. pp. 175–185.
Ishisono, K., Mano, T., Yabe, T., and Kitaguchi, K., Dietary fiber pectin ameliorates experimental colitis in a neutral sugar side chain-dependent manner, Front. Immunol., 2019, vol. 10, art. ID 2979.
Ivanov, M.B., Shustov, E.B., Litvintsev, B.S., et al., Endothelial dysfunction as a link in COVID-19 pathogenesis, Medline.Ru., 2020, vol. 21, pp. 884–903.
Ivnitsky, Ju.Ju., Rejniuk, V.L., Ivanov, M.B., et al., Vascular endothelium in acute poisoning, Medline.Ru., 2020, vol. 21, pp. 976–1004.
Ivnitsky, Ju.Ju., Rejniuk, V.L., Schäfer, T.V., and Malakhovsky, V.N., Fulminant hyperammonaemia induced by thiopental coma in rats, Toxicology, 2006, vol. 224, no. 3, pp. 184–190.
Ivnitsky, Ju.Ju., Schäfer T.V., Tyaptin, A.A., and Rejniuk, V.L., Changes in the chemical composition of blood and brain of rats under the conditions of modeling of the myeloablation regimen of cyclophosphamide administration, Toksikol. Vestn., 2019, vol. 156, no. 3, pp. 13–18
Ivnitsky, Ju.Ju., Schäfer, T.V., and Rejniuk, V.L., Endogennyi ammiak v toksicheskom protsesse. Razvitie kontseptsii endotoksikoza (Endogenous Ammonia in the Toxic Process. Development of the Concept of Endotoxicosis), Palmarium Acad. Pub., 2012.
Ivnitsky, Ju.Ju., Schäfer, T.V., and Rejniuk, V.L., Promotion of the toxic action of cyclophosphamide by digestive tract luminal ammonia in rats, ISRN Toxicol., 2011, vol. 2011, art. ID 450875.
Jaworska, K., Bielinska, K., Gawrys-Kopczynska, M., and Ufnal, M., TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts hemodynamic effects-implications for interpretation of cardiovascular actions of gut microbiome, Cardiovasc. Res., 2019, vol. 115, no. 11, pp. 1948–1949.
Jayakumar, A. and Norenberg, M., Hyperammonemia in hepatic encephalopathy, J. Clin. Exp. Hepatol., 2018, vol. 8, no. 3, pp. 272–280.
Karádi, O., Nagi, Z., Bódis, B., and Mózsik, G., Atropine-induced gastrointestinal cytoprotection dependences to the intact of vagal nerve against indomethacin-induced gastrointestinal mucosal and microvascular damage in rats, J. Physiol. Paris, 2001, vol. 95, nos. 1–6, pp. 29–33.
Khanna, K., Mishra, K., Chanda, S., et al., Effects of acute exposure to hypobaric hypoxia on mucosal barrier injury and the gastrointestinal immune axis in rats, High Alt. Med. Biol., 2019, vol. 20, no. 1, pp. 35–44.
Khazoom, F., L’Ecuyer, S., Gilbert, K., et al., Impact of uric acid on liver injury and intestinal permeability following resuscitated hemorrhagic shock in rats, J. Trauma Acute Care Surg., 2020, vol. 89, no. 6, pp. 1076–1084.
Khramykh, T.P. and Dolgikh, V.T., Endotoxemia in hemorrhagic hypotension, Politravma, 2007, no. 3, pp. 51–59.
Koeth, R.A., Wang, Z., Levison, B.S., et al., Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis, Nat. Med., 2013, vol. 19, no. 5, pp. 576–585.
Komarov, B.D., Luzhnikov, E.A., and Shimanko, I.I., Khirurgicheskie metody lecheniya ostrykh otravlenii (Surgical Methods of Treatment of Acute Poisoning), Moscow: Meditsina, 1981.
Kozlov, N.B., Ammiak, ego obmen i rol’ v patologii (Ammonia, Its Metabolism and Role in Pathology), Moscow: Meditsina, 1971.
Kumar, M., Kaeley, N., Nagasubramanyam, V., et al., Single center experience of managing methanol poisoning in the hilly state of Uttarakhand: a cross sectional study, Int. J. Crit. Illness Injury Sci., 2019, vol. 9, no. 4, pp. 172–176.
Kutsenko, S.A., Osnovy toksikologii (Fundamentals of Toxicology), St. Petersburg: Foliant, 2004.
Lechuga, S., Naydenov, N., Feygin, A., et al., Loss of β-cytoplasmic actin in the intestinal epithelium increases gut barrier permeability in vivo and exaggerates the severity of experimental colitis, Front. Cell Dev. Biol., 2020, vol. 8, art. ID 588836.
Lin, C., Chi, C., Wu, S., et al., Prognostic values of blood ammonia and partial pressure of ammonia on hospital arrival in out-of-hospital cardiac arrests, Am. J. Emerg. Med., 2013, vol. 31, no. 1, pp. 8–15.
Lin, H. and Pimentel, M., Bacterial concepts in irritable bowel syndrome, Rev. Gastroenterol. Dis., 2005, vol. 5, pp. S3–S9.
Liu, S., Song, P., Sun, F., et al., The concept revolution of gut barrier: from epithelium to endothelium, Int. Rev. Immunol., 2021, vol. 40, no. 6, pp. 401–408.
Liu, R., Hong, J., Xu, X., et al., Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention, Nat. Med., 2017, vol. 23, no. 7, pp. 859–868.
Lopetuso, L., Scaldaferri, F., Bruno, G., et al., The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors, Eur. Rev. Med. Pharmacol. Sci., 2015, vol. 19, no. 6, pp. 1068–1076.
Luna, M., Kamariski, M., Principi, L., et al., Severely ill pediatric patients with Shiga toxin-associated hemolytic uremic syndrome (STEC-HUS) who suffered from multiple organ involvement in the early stage, Pediatr. Nephrol., 2021, vol. 36, no. 6, pp. 1499–1509.
MacPherson, M., Hov, J., Ueland, T., et al., Gut microbiota-dependent trimethylamine N-oxide associates with inflammation in common variable immunodeficiency, Front. Immunol., 2020, vol. 11, art. ID 574500.
Magnotti, L.J., Upperman, J.S., Xu, D.Z., et al., Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock, Ann. Surg., 1998, vol. 228, no. 4, pp. 518–527.
Manani, S., Virzì, G., Guiliani, A., et al., Lipopolysaccharide evaluation in peritoneal dialysis patients with peritonitis, Blood Purif., 2020, vol. 49, no. 4, pp. 434–439.
Maniatis, N.A., Kotanidou, A., Catravas, J.D., and Orfanos, S.E., Endothelial pathomechanisms in acute lung injury, Vasc. Pharmacol., 2008, vol. 49, nos. 4–6, pp. 119–133.
Marchenko, A.B. and Laryushina, E.M., Role of trimethylamine-N-oxide in pathogenesis, diagnosis and forecast of cardiovascular diseases, Med. Ekol., 2017, no. 1, pp. 41–46.
Matsumoto, T., Takayanagi, K., Kojima, M., et al., Acute exposure to indoxyl sulfate impairs endothelium-dependent vasorelaxation in rat aorta, Int. J. Mol. Sci., 2019, vol. 20, no. 2, art. ID 338.
McClung, H., Sloan, H., Powers, P., et al., Early changes in the permeability of the blood-brain barrier produced by toxins associated with liver failure, Pediatr. Res., 1990, vol. 28, no. 3, pp. 227–231.
McMillen, K., Coghlin-Dickson, T., and Adintori, P., Optimization of nutrition support practices early after hematopoietic cell transplantations, Bone Marrow Transplant., 2021, vol. 56, pp. 314–326.
Metchnikoff, E., Essais optimists, Paris: A. Maloine, 1907.
Minami, T., Oda, K., Gima, N., and Yamazaki, H., Effects of lipopolysaccharide and chelator on mercury content in the cerebrum of thimerosal-administered mice, Environ. Toxicol. Pharmacol., 2007, vol. 24, no. 3, pp. 316–320.
Møller, S., Kimer, N., Barløse, M., and Bendtsen, F., Pathophysiological-based treatment of complications of cirrhosis, Scand. J. Gastroentherol., 2020, vol. 55, no. 4, pp. 383–394.
Morris, M.C., Gilliam, E.A., and Li, L., Innate immune programing by endotoxin and its pathological consequences, Front. Immunol., 2015, vol. 5, art. ID 680.
Nespoli, A., Chiara, O., Clement, M., et al., The cardiorespiratory impairment in cirrhosis and sepsis. An experimental interpretation using octopamine infusion, Circ. Shock, 1983, vol. 10, no. 1, pp. 15–30.
Nežić, L., Škrbić, R., Amidžić, L., et al., Protective effects of simvastatin on endotoxin-induced acute kidney injury through activation of tubular epithelial cells’ survival and hindering cytochrome C-mediated apoptosis, Int. J. Mol. Sci., 2020, vol. 21, no. 19, art. ID 7236.
Novosad, S., Sapiano, M., Grigg, C., et al., Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention, Morb. Mortal. Wkly. Rep., 2016, vol. 65, no. 33, pp. 864–869.
Numan, Y., Jawaid, Y., Hirzallah, H., et al., Ammonia vs. lactic acid in predicting positivity of microbial culture in sepsis: the ALPS pilot study, J. Clin. Med., 2018, vol. 7, no. 8, art. ID 182.
O’Grady, J., Murphy, C.L., Burry, L., et al., Defining gastrointestinal transit time using video capsule endoscopy: a study of healthy subjects, Endoscopy Int. Open, 2020, vol. 8, no. 3, pp. E396–E400.
Ochoa-Sanchez, R. and Rose, C., Pathogenesis of hepatic encephalopathy in chronic liver disease, J. Clin. Exp. Hepatol., 2018, vol. 8, no. 3, pp. 262–271.
Olek, R., Samulak, J., Sawicka, A., et al., Increased trimethylamine N-oxide is not associated with oxidative stress markers in healthy aged women, Oxid. Med. Cell. Longevity, 2019, vol. 2019, art. ID 6247169.
Pagana, K.D. and Pagana, T.J., Mosby’s Manual of Diagnostic and Laboratory Tests, St. Louis: Mosby Elsevier, 2014.
Pang, R., Martinello, K., Meehan, C., et al., Proton magnetic resonance spectroscopy lactate/N-acetylaspartate within 48 h predicts cell death following varied neuroprotective interventions in a piglet model of hypoxia-ischemia with and without inflammation-sensitization, Front. Neurol., 2020, vol. 11, art. ID 883.
Pfalzgraff, A. and Weindl, G., Intracellular lipopolysaccharide sensing as a potential therapeutic target for sepsis, Trends Pharmacol. Sci., 2019, vol. 40, no. 3, pp. 187–197.
Ponti, G., Maccaferri, M., Ruini, C., et al., Biomarkers associated with COVID-19 disease progression, Crit. Rev. Clin. Lab. Sci., 2020, vol. 57, no. 6, pp. 389–399.
Pool, R., Gomez, H., and Kellum, J.A., Mechanisms of organ dysfunction in sepsis, Crit. Care Clin., 2018, vol. 34, pp. 63–80.
Rao, J.N. and Wang, J.Y., Regulation of Gastrointestinal Mucosal Growth, San Rafael: Morgan and Claypool Life Sci., 2011.
Restini, C., Fink, G., and Watts, S., Vascular reactivity stimulated by TMA and TMAO: are perivascular adipose tissue and endothelium involved?, Pharmacol. Res., 2020, vol. 163, art. ID 105273.
Rinaldi, B., Cuzzocrea, S., Donniacuo, M., et al., Hyperbaric oxygen therapy reduces toll-like receptor signaling pathway in multiple organ failure, Intensive Care Med., 2011, vol. 37, no. 7, pp. 1110–1119.
Rodríguez-Hernández, P., Cardador, M.J., Arce, L., and Rodríguez-Estévez, V., Analytical tools for disease diagnosis in animals via fecal volatilome, Crit. Rev. Anal. Chem., 2020, vol. 12, pp. 1–16.
Role of Gut Bacteria in Human Toxicology and Pharmacology, Hill, M.J., Ed., London: Taylor & Francis, 2005.
Saxena, A., Lopes, F., and McKay, D., Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli, Inflamm. Res., 2018, vol. 67, no. 10, pp. 829–837.
Schäfer, T.V., Ivnitsky, Ju.Ju., and Rejniuk, V.L., The influence of the plantain juice on the manifestations and outcome of acute cyclophosphamide intoxication in rats, Biopreparaty, 2015, vol. 55, no. 3, pp. 61–63.
Schäfer, T.V., Pathogenetic bases of drug correction of early manifestations of acute resorptive action of mustard gas. Development of the concept of endotoxicosis, Doctoral (Med.) Dissertation, St. Petersburg: VMedA, 2015.
Schäfer, T.V., Rejniuk, V.L., Krasnov, K.A., and Ivnitsky, Ju.Ju., Increased permeability of a small intestine to luminal hydrophilic medium-sized molecules in cyclophosphamide-treated rats, Medline.Ru., 2011, vol. 12, pp. 1437–1449.
Schäfer, T.V., Ivnitsky, J.J., and Rejniuk, V.L., Cyclophosphamide-induced leakage of gastrointestinal ammonia into the common bloodstream in rats, Drug Chem. Toxicol., 2011, vol. 34, no. 1, pp. 25–31.
Scott, T., Kronsten, T., Hughes, R., and Shawcross, D., Pathophysiology of cerebral oedema in acute liver failure, World J. Gastroenterol., 2013, vol. 19, no. 48, pp. 9240–9255.
Semenov, E.I., Mishina, N.N., and Papunidi, K.Kh., Unaccounted anaphylactic reaction to effect of mycotoxins, Medline.Ru., 2019, vol. 20, pp. 36–43.
Sender, R. and Fuchs, S., Revised estimates for the number of human and bacteria cells in the body, PLoS Biol., 2016, vol. 14, no. 8, art. ID e1002533.
Sheikh, M., Unni, N., and Agarwal, B., Neurological monitoring in acute liver failure, J. Clin. Exp. Hepatol., 2018, vol. 8, no. 4, pp. 441–447.
Shi, K., Wang, F., Jiang, H., et al., Gut bacterial translocation may aggravate microinflammation in hemodialysis patients, Dig. Dis. Sci., 2014, vol. 59, pp. 2109–2117.
Shilov, V.V., Aleksandrov, M.V., Vasil’ev, S.A., et al., Acute cerebral failure at the serious poisoning, Medline.Ru., 2010, vol. 11, art. 25, pp. 315–321.
Skowrońska, M. and Albrecht, J., Oxidative and nitrosative stress in ammonia neurotoxicity, Neurochem. Int., 2013, vol. 62, no. 5, pp. 731–737.
Skowrońska, M., Zielińska, M., Wójcik-Stanaszek, W., et al., Ammonia increases paracellular permeability of rat brain endothelial cells by a mechanism encompassing oxidative/nitrosative stress and activation of matrix metalloproteinases, J. Neurochem., 2012, vol. 121, no. 1, pp. 125–134.
Solé, C., Guilly, S., Da Silva, K., et al., Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis, Gastroenterology, 2021, vol. 160, pp. 206–218.
Suárez, L., Moreno-Luque, M., Martínez-Ardines, I., et al., Amine variations in faecal content in the first weeks of life of newborns in relation to breast-feeding or infant formulas, Br. J. Nutr., 2019, vol. 122, no. 10, pp. 1130–1141.
Summerskill, W.H.J. and Wolpert, E., Ammonia metabolism in the gut, Am. J. Clin. Nutr., 1970, vol. 23, no. 5, pp. 633–639.
Tan, X., Liu, Y., Long, J., et al., Trimethylamine N-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease, Mol. Nutr. Food Res., 2019, vol. 63, no. 17, art. ID e1900257.
Tang, W.H., Wang, Z., Levison, B.S., et al., Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk, N. Engl. J. Med., 2013, vol. 368, no. 17, pp. 1575–1584.
Tang, W., Kitai, T., and Hazen, L., Gut microbiota in cardiovascular health and disease, Circ. Res., 2017, vol. 120, no. 7, pp. 1183–1196.
Toxicology of the Gastrointestinal Tract, Gad, S.C., Guy, R.C., and Jacoby, H.I., Eds., New-York: Taylor & Francis Group, 2007.
Uno, Y., Hypothesis: mechanism of irritable bowel syndrome in inflammatory bowel disease, Med. Hypoth., 2019, vol. 132, art. ID 109324.
Volkov, V.P., Yatrogennye psikhoneirosomaticheskie sindromy (Iatrogenic Psychoneurosomatic Syndromes), Tver: Triada, 2014.
Walker, V., Severe hyperammonaemia in adults not explained by liver disease, Ann. Clin. Biochem., 2012, vol. 49, no. 3, pp. 214–228.
Wang, W., Weng, J., Yu, L., et al., Role of TLR4-p38 MAPK-Hsp27 signal pathway in LPS-induced pulmonary epithelial hyperpermeability, BMC Pulm. Med., 2018, vol. 18, no. 1, art. ID 178.
Wang, L., Cao, Y., Gorshcov, B., et al., Ablation of endothelial Pfkfb3 protects mice from acute lung injury in LPS-induced endotoxemia, Pharmacol. Res., 2019, vol. 146, art. ID 104292.
Wood, T., Moralejo, D., Corry, K., et al., A ferret model of inflammation-sensitized late preterm hypoxic-ischemic brain injury, J. Vis. Exp., 2019, vol. 153, art. ID e60131.
Yoshida, N., Yamashita, T., Kishino, S., et al., A possible beneficial effect of Bacteroides on faecal lipopolysaccharide activity and cardiovascular diseases, Sci. Rep., 2020, vol. 10, no. 1, art. ID 13009.
Zhang, L.S. and Davis, S.S., Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions, Genome Med., 2016, vol. 8, no. 1, art. ID 46.
Zhao, J., He, Y., Xu, P., et al., Serum ammonia levels on admission for predicting sepsis patient mortality at D28 in the emergency department: a 2-center retrospective study, Medicine (Baltimore), 2020, vol. 99, art. ID e19477.
Zhou, Y., Huang, X., Zhao, T., et al., Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice, Brain Behav., Immun., 2017, vol. 64, pp. 266–275.
Zhou, W., Cheng, Y., Zhu, P., et al., Implication of gut microbiota in cardiovascular diseases, Oxid. Med. Cell. Longevity, 2020, vol. 2020, art. ID 5394096.
Zhu, W., Gregory, J.C., Org, E., et al., Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk, Cell, 2016, vol. 165, no. 1, pp. 111–124.
Zvenigorodskaya, L.A., Samsonova, N.G., and Toporkov, A.S., Chronic ischemic disease of the digestive system: an algorithm for diagnosis and treatment, Rus. Med. Zh., 2010, no. 9, pp. 544–548.
Funding
The study was conducted without financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that there is no conflict of interest.
Statement on the welfare of animals. In all cited articles, all applicable international, national and/or institutional guidelines for the care and use of animals were followed when conducting experiments.
Statement of compliance with standards of research involving humans as subjects. This article does not contain any studies involving humans as subjects of research.
Additional information
Translated by D. Novikova
Rights and permissions
About this article
Cite this article
Schäfer, T.V., Vakunenkova, O.A., Ivnitsky, J.J. et al. Gut Barrier in Critical States of the Body. Biol Bull Rev 12, 392–405 (2022). https://doi.org/10.1134/S2079086422040077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079086422040077