Abstract
Biotechnology has revolutionized plant propagation by providing plant tissue culture as a rising alternative platform to conventional methods. In the present scenario, plant tissue culture becomes the key technique for the large-scale production and conservation of germplasm, maintenance of genetic stability, and meets out the economic demands of plant and plant products. It is the science of artificially growing test tube plantlets under optimized microclimatic conditions isolated from the external environment. This micro-scale environment resembles the natural environment consisting of culture vessels, humidity, gases, light irradiation, spectrum, temperature, nutritional media, and its constituents as major growth-affecting factors. The efficient architectural development of any genotype depends on these factors and requires a specifically optimized climate. Here, we are reviewing the interaction of physical factors of in vitro artificial climate including culture vessel, light, and temperature with the developing plantlets. The outcomes can increase the understanding of the developmental response of plants toward their environment in the recently changing climatic conditions. Further, this can also eliminate the challenges of cost, labor, and timings and introduce the advancements of efficient large-scale production of plant and plant products which will be effective in fulfilling the population demands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The stability of life is a remark of a balanced and working ecosystem. This primarily depends upon the interaction between its biological, chemical, and physical components. However global warming and other changes in environmental conditions have greatly influenced these interactions and led to a drastic change in biodiversity. According to the SDG report 2023, if the current scenario continues then 575 million people and 2/3 of countries of the world remain with extreme poverty. Globally 45 million children are wasting, and 148 million people are suffering from stunted growth, and food insecurity. However, plant diversity has a priceless contribution to human welfare as 80,000 plant species, cultivated or noncultivated [1], have been used by society for their food and economic demands [2]. Recent studies specified that biodiversity (Plant diversity) has been decreased due to imbalanced climatic factors like temperature and precipitation [3, 4]. An increase of 1% in precipitation causes a 2.04% loss of plant diversity [4]. There is a need to understand the altered physiological and metabolic characteristics of plants towards changing climate to rescue declining plant diversity. Plant tissue culture techniques provide an artificially controlled working microecological system to recognize the altered mechanisms of plants. This microclimate mainly includes nutritional media (minerals, plant growth regulators, and other organic compounds, supporting agents), light, temperature, gases, culture vessels, and timing (subculture period, dosage) factors for plant propagation [5]. On this platform, the growth and development of plants could be controlled by manipulating surrounding factors instead of dealing with naturally growing plants [6].
G. Haberlandt was the first to come up with the concept of plant tissue culture after a study on the totipotency of plant cells under artificial climatic conditions to understand morphogenesis [7]. During the last 3–4 decades, plant tissue culture techniques became alternative to conventional propagational methods after the discovery and advancement in the studies related to cell division in culture (callus) [8], plant growth regulators (PGRs) like auxin and cytokinin, the contribution of PGR to in vitro morphogenesis [9] and universally accepted nutritional media composition [10]. Further, the successful development of virus-free plants from shoot tip culture [11], somatic embryogenesis in carrot tissues [12], complete plantlets from a single cell [13], plantlet from microspore culture [14], and cryopreservation concept [15], etc. are also key findings for large scale in vitro production of plants [7, 16]. Apart from cryopreservation (− 196 °C), plant tissue culture technique also provides a platform for the storage of plantlets at the temperature of 0 to 15 °C during the in vitro multiplication stage that is used to conserve genetic resources and virus-free plant collections [17]. These advancements in plant tissue culture techniques are applied worldwide in the horticulture, agriculture, and forestry industries for large-scale production of pathogen-free plantlets, genetically and physiologically identical or uniform and cost-effective plantlets without harming the wild population in a minimum time period [18,19,20,21,22]. The commercialization of plant tissue culture began in 1970 with orchid industry which increased production from 130 to 500 million during the 1990s with 5 major industrial units. Presently worldwide production has raised up to 8 million plants from 21 units in Germany, 193 million plants from 78 industrial units in India, 62 million plants from 67 commercial units in the Netherlands, and 212 million plants from 37 units in Western Europe, etc. [23]. Apart from industries plant tissue culture techniques also flourished the other branches of science like molecular biology, developmental biology, agronomy, medicine, and transgenic sciences (Fig. 1).
However successful in vitro propagation desires high survivability of field-transferred plantlets. Still, the rate is low because plantlet growth needs optimization of several factors like nutritional requirement, hormonal dosage, humidity, gases composition, light conditions, temperature, and head space in culture vessels [24] (Figs. 1, 2). The interaction of biological and physical factors with each other is a function of natural ecosystem as well as artificial ecosystem [5]. At artificial platform the isolated container called culture vessels decide the availability of other required physical factors including light, gasses, and humidity [25, 26]. Further, the available irradiance influences the endogenous hormonal concentration that determines in vitro development through axillary shoot production, and apical dominance followed by the establishment of cultures at initial stages [27]. The post-storage vitality of cultures also depends upon plant genotype, explant type, nutrient medium, light, temperature, time of storage, and interactions of these factors [17]. The imbalance of these required factors adversely affects the physiology, morphology, and anatomy of in vitro growing plantlets [24, 28]. These malformed products enhance the production and labor cost that limits the commercialization of this technique and warranted more strategies to make micropropagation economical [26, 29]. Here, we are reviewing the interaction of physical factors including culture vessels, light, and temperature that influence the in vitro growing plantlets. It will improvise the understanding of plant development and developmental influence by factors interaction. It will fruitful to sort out the modulation of plant growth under changing climatic conditions.
1.1 Effect of culture vessels, humidity and gaseous composition
in vitro propagation needs uniform, cost-effective, and non-phytotoxic containers with appropriate storage space, called culture vessels [30]. Culture vessels offer primary platform for in vitro growing plantlets by creating an artificial micro environment isolated from the external environment. These vessels also check the entry of invading microorganisms to make contamination-free surroundings for plantlets. Several vessels such as roller tubes, stationary test tubes, tumbler tubes, carrel flasks, Erlenmeyer flasks, small flattened rectangular T -flasks, large roux bottles, petri dishes, magenta vessels, magenta GA-7 vessels, phytacon, and phytatray have been used since 1897 with the advancement of the technique [25]. The physical parameters like material (glass, polypropylene, polyvinyl glycine, and polycarbonate), closure types (porous and nonporous), shape, and size (15–500 ml) of culture vessels are affecting factors [31]. They assures the availability of other required physical factors light, gases, and humidity in this artificial microenvironment [26]. For example, the round shape of culture vessels showed uniformity for light transmittance. On the other hand, gas exchange rate of vessels varies from 0.03 to 0.041 h−1with their size and shape as lower size triangular glass flask had a comparatively lower gas exchange rate of 0.0145 h−1 than the GA-7 magenta box and rectangular box [32, 33]. The size and volume of vessels facilitates the outcomes by providing enough head space for growing plantlets. According to [26] screw cap jars with a 300 ml volume enhanced the production of adventitious shoot than culture tubes with a volume of 40 ml.
The closure type of vessels such as Screw lids, Tight-or loose-fitting Snap-on lids, Plastic film (Parafilm or polypropylene film) fixed to the vessel top, Petri dish with cover, Cotton plug, Lids with small holes or slits to promote aseptic ventilation, and Fluorocarbon polymer film also plays a significant role in maintaining the gaseous composition and light transmittance of vessel’s internal environment [26, 34].Ventilation of vessels is a critical factor that upholds gases exchange and internal relative humidity of culture vessels. in vitro plantlets of Capsicum and Solanum tuberosum showed lower fresh weight, higher dry weight and shorter size of shoots with lower stomatal density in ventilated vessels whereas non-ventilated vessels had vitrified plantlets with malfunctioned leaf anatomy [35, 36]. Non-ventilated culture vessel-grown plantlets suffered from higher relative humidity and accumulated by-products of growing plantlets like carbon dioxide, ethylene, ethane acetaldehyde, and ethanol under in vitro conditions [34]. Due to the higher water content of medium and poor gases exchange with the outer environment, relative humidity dominated the internal microclimate and affected the growth of plants within culture vessels [37]. Meanwhile, plantlets exhibited less mechanical tissues and trichomes, expanded cells with higher intercellular spaces, and lower photosynthetic tissues or starch granules [38]. These poorly developed anatomical characteristics resulted in malfunctioned physiological processes like transpiration, mineral uptake, and photosynthesis, and hindered the acclimatization and hardening of in vitro raised plantlets [39].
High relative humidity enhanced the ethylene production within the culture vessels by slowing down the Ca2+ ion mobility [40]. Ethylene is a very commonly accumulating gas in in vitro environment which accumulation depends on the volume and closure type of culture vessels [41,42,43] described closure material and gelling agents of nutritional media as major abiotic ethylene producers besides biotic (explant) producers. The author also suggested that accumulated ethylene enhanced the shoot proliferation as well as the quality of produced shoots. Apart from internal ethylene concentration, additional exposure of ethylene to in vitro developing rose plantlets at a specific concentration significantly improved the axillary shoot proliferation [41]. However, ethylene could act either as a growth enhancer or silencer according to in vitro developing plant species like in vitro axillary branchlets of Bromeliad were increased while the growth of Mini-roses was negatively affected [34, 42, 44]. The accumulation of ethylene is higher in tightly sealed and non-permeable vessels and acts as in vitro multiplication rate inhibitor by introducing malfunctioned organogenesis such as leaf epinasty, shoot number, and length reduction [41, 45]. The authors also suggested that ethylene was not the sole gas that affects the in vitro differentiation but the interference outcomes were the result of the interaction of ethylene and CO2 with the metabolism of developing plantlets. Although there is no antagonist effect of CO2 on ethylene production and its impairments on development.
in vitro, growing plantlets suffer from insufficiency of CO2 and known as photo mixotrophic depends on a nutritive medium for sugar and other nutrition. [46] found CO2 was the only limiting factors for the in vitro proliferation of shoot of Theobroma cacao. This plant species remained recalcitrant under conventional CO2 supply due to lack of sufficient nutrition. The increased level of CO2 from conventional 100 µmol mol−1 concentration to 400 µmol mol−1 improved in vitro plantlets development [47, 48]. in vitro increased CO2 concentration resulted in high small stomata density that improved water retention capacity during ex vitro desiccation and acclimatization of the plant [49].Ventilated and large vessels, gas-permeable filter discs, and forced aseptic ventilation were used to increase the level of CO2 to create a photoautotrophic micropropagation system that makes plants photoautotrophic [48, 50]. Photoautotrophic micropropagation system improved the multiplication rate, physiological growth of plantlets, humidity level (85–90%), and ethylene permeability [47, 51]. However, this CO2 enrich environment is not effective for the plant species having C4 and Crassulacean Acid Metabolism (CAM) photosynthetic pathways. Findings of various authors on effect of culture vessels on in vitro plant propagation are summarized in Table 1.
1.2 Effect of light
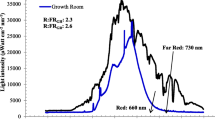
The overall growth of either ex-vitro or in vitro regenerated plants is regulated by light spectra and irradiance. Function of light is not limited only to the photosynthesis process but also augmented the morphogenesis (photomorphogenesis) or architectural development of plants. Plants have several photoreceptors for different wavelength (UV to far red) of light that determines the growth through photomorphogenesis [63]. in vitro growing plantlets also require optimized quality of light and photoperiod that are identical to natural light irradiance or spectrum. From several decades fluorescent light, halide metal, high-pressure solid, and incandescent, have been used as conventional light source for plant tissue culture research. In recent years, LED lights have turned out to be a favorable light source due to various level of advancement including durability, relatively cool emitting surface, wavelength specificity, small size, long operating lifetime, a photon output that is linear with the electrical input current, and the ability to control spectral composition over conventional lights [63, 64]. However, according to [65] light sources have their own impact on developing plantlets. The authors suggested that fluorescent light induced somatic embryogenesis while shoot multiplication and rooting stages favored by LED (Red and Blue) lights without any adverse acclimatization effect on plantlets. Further according to [66] photosynthetically active region (PAR) of light promoted multiplication and acclimatization over the white cool fluorescent light in both Rosa damascena and Rhynchostylis retusa. Blue and red LEDs were found to be more effective for in vitro regeneration [67, 68] as well as for biochemical production than conventional white cool fluorescent lights [69]. The in vitro microenvironment needs optimization of available light to achieve well-developed and healthier plantlets as light is an important physical factor that governs various metabolic pathways and growth [70]. The period of light perception and intensity decides the development of stomata, photosynthetic pigment and growth of plantlets under in vitro conditions. Higher intensity of light favored the stomata development while decreased the level of pigments in Alocasia amazonica [71]. The lowest intensity could retard the growth whereas the highest intensity results in photodamage to the plantlets. Reports mentioned that 1000 lux was optimal intensity for date palm multiplication as the higher intensity lowered the shoot bud proliferation rate with increasing shoot length [72]. According to [73] standard (40 µmol m−2 s−1) light intensity of micropropagation system is inappropriate for in vitro propagation of shaded growing Acer saccharum and Capsicum chinense while higher intensity favored the net photosynthesis rate of in vitro plantlets [29, 74]. in vitro plantlets growing under everlasting low light conditions are not able to withstand to high irradiance during acclimatization due to malformed photoprotective and photosynthetic apparatus [75]. Similarly leaf internal structures well developed under higher irradiance and promote ex vitro survival of sugarcane plantlets [61]. In case of Araujia sericifera, light worked as a dominant factor to promote somatic embryogenesis and mask the effect of polyamine inhibitors [76].
The wavelength or quality of light (Green/Red/Blue) controls the stages of embryogenesis by altering the level of abscisic acid formation [77]. Red light stimulated embryogenesis while blue showed inhibitory effect on embryogenesis even in the presence of red light [78]. Red and blue spectra showed an antagonist effect of each other on in vitro plantlets of Cymbidium and Betula pendula [79, 80]. Red light stimulated the leaves growth with decreasing chlorophyll content whereas blue light reversibly turned these effects of red light. [81] also found antagonist effect of red and white spectrum where red LED stimulated the root formation by lowering some key phytocompounds concentration and white LED raised the concentration of these same compounds. Besides this, the combination of photosynthetically active spectra (Red and Blue) in a particular ratio improvised the production of high-quality plantlets of Camellia oleifera [82], Pyrus communis [83], and Fragaria × ananassa [84]. In the spectral combination of blue and red, blue light does not involve in shoot growth rather it works as a function of plantlet quality, where its small amount is enough for healthier shoot formation [84].
Light spectrum altered the rate of plant regeneration as well as their phytocompound composition [85]. The green light was found appropriate for shoot numbers, and yellow light for shoot length and antioxidant compound synthesis. White light increased the p-coumaric acid, rosmarinic acid, and cinnamic acid production. Blue and red wavelength favored gentisic acid, syringic acid and vanillic acid from in vitro cultured plantlets [86]. The various range of light check the in vitro production of phytocompounds. Green, red and blue lights in a ratio of (10:7:3) maximize the biomass of Nasturtium officinale micro shoots with significant concentration of medicinally active compound glucosinolates and phenolics [87]. Similarly, the light spectrum found responsible for in vitro production of antioxidative enzyme, photosynthetic pigments and antioxidant compounds in Artemisia absinthium callus culture [88]. The quality of light raised the quantity of medicinally active phtyocompounds like ginsenosides, alkaloids and polysaccharides in Cistanche deserticola, Dendrobium candidum, and Perilla plants [89,90,91]. The light intensity also influences the phytochemical composition of in vitro Plectranthus amboinicus plantlets and cardenolides production in Digitalis mariana [60, 92]. The in vitro development of various plantlets under different light ranges is summarized in Table 2.
1.3 Effect of temperature
Temperature affects the growth, development and physiological process of naturally growing plant as well as the micropropagating plantlets [101]. In various studies, temperature range from 21 °C to 27 °C found appropriate for effective in vitro morphogenesis of plantlets while the lower or higher temperature from this range showed deleterious effects on every studied stage of morphogenesis such as shoot bud, shoot, callus and root formation [102].The range of temperature varies widely with growing stages of in vitro plantlets. The increasing temperature favored shoot elongation and callus proliferation while reduced the rate of multiplication [103]. The often-optimum temperature for tissue culture conditions is 25 °C for the C3 metabolism and sometimes the higher range is for the Crassulacean acid metabolism. This optimum temperature of the artificial environment correlates and facilitates the CAM which decides the multiplication rate of in vitro plantlets [26]. The 25 °C temperature was found suitable for in vitro multiplication and highest plantlet production of Acanthostachys strobilacea, Mentha spp, Nopalxochiaa ckermannii, Pyrus communis, and Saccharum officinal varieties [27, 104,105,106] In several studies authors have also reported the connection of temperature in epigenetic modification of in vitro cultures which leads phenological changes in tree species [107].
Many reports concluded the importance of high temperature in optimum in vitro multiplication of woody tree plant species. The increased temperature from ideal range improvised the in vitro bud sprouting, shoot proliferation, and rooting [108, 109]. The higher temperature of in vitro conditions also enhanced the number of internodes and represent normal leafy shoots rather than a stoloniferous habit with scale leaves in potato plantlets [110] [111, 112]. The stimulation of warmer temperature during in vitro embryogenesis delayed bud set in autumn and advanced bud burst in spring in Norway spruce and Abies nordmanniana respectively [107]. Similarly, warmer temperature stimulation during dark period enhanced the in vitro rooting even in the absence of root stimulating compound phloroglucinol [58]. However, an increased root system with a higher temperature reduced the transport from per unit area rather than at a lower temperature, the increased transport from per unit area indicated inhibitory effects of decreasing temperature [113]. Although, in case of rose, the lower temperature enhanced the root initiation and ceased the multiplication of plantlets [114].
Lowering the temperature (28 °C/15 °C light/dark) during dark period, determined the changes in plantlets like fewer root, high dry weight, and small thicker leaves with high chlorenchyma and hypodermal tissue according to CAM pathway. The enzymatic activities also decided by temperature such as the abscisic acid (ABA) level raised in light period while indole-3-acetic (IAA) found in higher amount in dark period with PEPCase activity [115]. The lower temperature enhanced the timing of seeds germination and further decreased the leaves and root number from these in vitro raised seedlings. in vitro plantlets of Alcantarea imperialis accumulated sugars like raffinose, trehalose, and stachyose instead of starch to defense temperature generated abiotic stress [116]. The exposure to lower temperature either before or after inoculation on explant enhances the survival rate of in vitro as well as ex vitro plantlets. The pre chilling treatment of antioxidant solution on explant prior to inoculation enhanced shoot regeneration by removing inhibitory effect of phenolic exudation [109]. Further stimulation of pre chilling treatment before the acclimation phase to plantlets also enhanced the survival rate. The authors reported that chilling treatment influenced the major physiological processes such as translocation, stomatal conduction, photosynthesis, and transpiration of these plantlets through improved vascular bundles, stomatal density, and leaf anatomy especially mechanical tissue deposition on the leaf surface. All of these anatomical and physiological changes improves the ex vitro survival rate of plantlets [117]. Temperature also plays a critical role in germplasm conservation. in vitro storage of micro shoots in a confined area at lower temperature slows down the growth of plantlets including leaf and root length, shoot fresh and dry mass [118] and frost tolerance [119] during multiplication phase. This leads to an increase in the duration of subculturing and make the conservation cost effective without any harmful impact on the acclimatization and survival of these plantlets [118]. Further the influence of the temperature range on plantlets is briefed in Table 3.
2 Conclusion
There is a need of industrialization, cost effective, and nutritious food production in a short time period to fulfil population demands. Plant tissue culture technique can combat the barrier and fulfil the requirements by developing green industries and reducing the cost and timings of production. On this platform the growth of plants could be controlled and increased by manipulating surrounding physical factors. The selection of culture vessels with enough volume and ventilation improvised the quality of plantlets by maintaining light transmittance and maintain gaseous composition, humidity and aseptic microclimatic conditions. The exposure of LED lights with various wavelength and higher intensity enhances the rate of multiplication of plantlets and their products while lower temperature (10–15 °C) facilitates long term storage of these in vitro plantlets. However, an ideal range of light and temperature (25–30 °C) is required, beside this range plantlets suffered from malfunctioning and lower survivability in external environment. Nevertheless, the understanding of interrelationship of these factors can provide more strategies to improve the micropropagation mechanism that minimize the barrier of large-scale production. In the future, this could be used to optimize the in vitro microclimatic conditions and these would be also effective to understand the developmental and conservational biology of plants under changing climatic conditions.
References
Rahmatov M, Lazarte CE. Role of neglected plant foods in achieving dietary diversity, zero hunger and good health Exploring and valorizing nature to feed hunger. Berlin: Springer; 2023. https://doi.org/10.1007/978-3-031-37077-9_2.
Thattantavide A, Sreedharan S, Sharma N, Uthirchakkavu I, Surendran A, Kumar A. An introduction to wild food plants for zero hunger and resilient agriculture in wild food plants for zero hunger and resilient agriculture. Berlin: Springer; 2023.
Gren IM, Campos M, Gustafsson L. Economic development, institutions, and biodiversity loss at the global scale. Reg Environ Chang. 2016;16:445–57.
Habibullah MS, Din BH, Tan SH, Zahid H. Impact of climate change on biodiversity loss: global evidence. Environ Sci Pollut Res. 2022;29(1):1073–86.
McCown BH, Sellmer JC. General media and vessels suitable for woody plant culture in cell and tissue culture in forestry: general principles and biotechnology. Berlin: Springer; 1987.
Carvalho SD, Ortega M, Orellana M, Rodríguez M, Folta KM, de Torres M. In vitro propagation of the Amazonian medicinal plant guayusa (Ilex guayusa) and effects of light in the growth and development of this shade tolerant plant. Plant Cell Tissue Organ Cult. 2021;147:503–17.
Read PE. Micropropagation past present and future. Acta Hortic. 2007. https://doi.org/10.17760/ActaHortic.2007.748.1.
Gautheret RJ. Culture du tissu cambial. 1934.
Skoog F. Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Symp Soc Exp Biol. 1957;11:118–31.
Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–97.
Morel GT. Guérison de dahlias atteints d’une maladie à virus. CR Acad Sci Paris. 1952;235:1324–5.
Steward FC, Mapes MO, Mears K. Growth and organized development of cultured cells II organization in cultures grown from freely suspended cells. Am J Bot. 1958;45:705–8.
Vasil V, Hildebrandt AC. Differentiation of tobacco plants from single, isolated cells in microcultures. Science. 1965;150(3698):889–92.
Guha S, Maheshwari SC. Cell division and differentiation of embryos in the pollen grains of Datura in vitro. Nature. 1966;212(5057):97–8.
Nag KK, Street HE. Carrot embryogenesis from frozen cultured cells. Nature. 1973;245(5423):270–2.
Thorpe TA. History of plant tissue culture. Mol Biotechnol. 2007;37:169–80.
Orlikowska T. Effect of in vitro storage at 4 C on survival and proliferation of two apple rootstocks. Plant Cell, Tissue Organ Cult. 1992;31:1–7.
Gamborg OL. Plant tissue culture biotechnology milestones. Vitr Cell Dev Biol Plant. 2002;38(2):84–92.
Rathore JS, Rathore V, Shekhawat NS, Singh RP, Liler G, Phulwaria M, et al. Micropropagation of woody plants. Plant Biotechnol Mol Markers. 2005. https://doi.org/10.1007/1-4020-3213-7_13.
Onay A, Yildirim H, Tokatli YO, Akdemir H, Suzerer V. Plant tissue culture techniques—Tools in plant micropropagation. Curr Opin Biotechnol. 2011;22:S130.
Aitken-Christie J, Kozai T, Smith MAL. Automation and environmental control in plant tissue culture. Berlin: Springer Science Business Media; 2013.
Dias MI, Sousa MJ, Alves RC, Ferreira ICFR. Exploring plant tissue culture to improve the production of phenolic compounds: a review. Ind Crops Prod. 2016;82:9–22.
Patil SM, Kumari VBC, Sumana K, Sujay S, Tejaswini M, Shirahatti PS, et al. Sustainable development of plant tissue culture industry: the Indian scenario. J Appl Biol Biotechnol. 2021;9(2):18–27.
Hazarika BN. Morpho-physiological disorders in in vitro culture of plants. Sci Hortic. 2006;108(2):105–20.
Zobayed SMA, Afreen F, Kubota C, Kozai T. Evolution of culture vessel for micropropagation: from test tube to culture room. In: Transplant Production in the 21st Century: Proceedings of the International Symposium on transplant production in closed system for solving the global issues on environmental conservation, food, resources and Energy. Springer. Berlin. 2000. p. 231–7.
Amoo SO, Finnie JF, Van Staden J. Effects of temperature, photoperiod and culture vessel size on adventitious shoot production of in vitro propagated Huernia hystrix. Plant Cell, Tissue Organ Cult. 2009;99:233–8.
Wang Q. The effect of light, darkness and temperature on micropropagation of the pear rootstock BP10030. J Hortic Sci. 1992;67(6):869–76.
Chandra S, Bandopadhyay R, Kumar V, Chandra R. Acclimatization of tissue cultured plantlets: from laboratory to land. Biotechnol Lett. 2010;32:1199–205.
Barrales-López A, Robledo-Paz A, Trejo C, Espitia-Rangel E, La Rodríguez-De OJL. Improved in vitro rooting and acclimatization of Capsicum chinense Jacq. plantlets. Vitr Cell Dev Biol. 2015;51:274–83.
Loberant B, Altman A. Micropropagation of plants Biosep cell technol. New York: Wiley; 2010.
Zobayed SMA. In vitro propagation of Lagerstroemia spp from nodal explants and gaseous composition in the culture headspace. Environ Control Biol. 2000;38(1):1–11.
Chen C, Chen JJ. Measurement of gas exchange rates in plant tissue culture vessels. Plant Cell Tissue Organ Cult. 2002;71:103–9.
Huang C, Chen C. Physical properties of culture vessels for plant tissue culture. Biosyst Eng. 2005;91(4):501–11.
Kavanagh K, Drew AP, Maynard C. The effect of the culture vessel on micropropagation. High-Tech Micropropag. 1991;I:202–11.
Mohamed MAH, Alsadon AA. Influence of ventilation and sucrose on growth and leaf anatomy of micropropagated potato plantlets. Sci Hortic. 2010;123(3):295–300.
Mohamed MAH, Alsadon AA. Effect of vessel type and growth regulators on micropropagation of Capsicum annuum. Biol Plant. 2011;55:370–4.
Chen C. Humidity in plant tissue culture vessels. Biosyst Eng. 2004;88(2):231–41.
Majada JP, Tadeo FR, Fal MA, Sánchez-Tamés R. Impact of culture vessel ventilation on the anatomy and morphology of micropropagated carnation. Plant Cell Tissue Organ Cult. 2000;63:207–14.
Pasqualetto PL. Vitrification in plant tissue culture In plant aging: basic and applied approaches. Berlin: Springer; 1990.
Teixeira da Silva JA, Nezami-Alanagh E, Barreal ME, Kher MM, Wicaksono A, Gulyás A, et al. Shoot tip necrosis of in vitro plant cultures: a reappraisal of possible causes and solutions. Planta. 2020;252:1–35.
Kevers C, Boyer N, Courduroux JC, Gaspar T. The influence of ethylene on proliferation and growth of rose shoot cultures. Plant Cell Tissue Organ Cult. 1992;28:175–81.
Biddington NL. The influence of ethylene in plant tissue culture. Plant Growth Regul. 1992;11:173–87.
Mensuali-Sodi A, Panizza M, Tognoni F. Quantification of ethylene losses in different container-seal systems and comparison of biotic and abiotic contributions to ethylene accumulation in cultured tissues. Physiol Plant. 1992;84(3):472–6.
Proft MP de, Broek G van den, Dijck R van. Implications of the container-atmosphere during micropropagation of plants. 1985;
Jackson MB, Abbott AJ, Belcher AR, Hall KC, Butler R, Cameron J. Ventilation in plant tissue cultures and effects of poor aeration on ethylene and carbon dioxide accumulation, oxygen depletion and explant development. Ann Bot. 1991;67(3):229–37.
Figueira A, Whipkey A, Janick J. Increased CO2 and light promote in vitro shoot growth and development of Theobroma cacao. J Am Soc Hortic Sci. 1991;116(3):585–9.
Kozai T, Xiao Y. A commercialized photoautotrophic micropropagation system. Plant tissue Cult Eng. 2008. https://doi.org/10.1007/1-4020-3694-9_19.
Nguyen QT, Xiao Y, Kozai T. Photoautotrophic micropropagation. Plant Fact. 2020. https://doi.org/10.1016/B978-0-12-816691-8.00023-6.
Vahdati K, Asayesh ZM, Aliniaeifard S, Leslie C. Improvement of ex vitro desiccation through elevation of CO2 concentration in the atmosphere of culture vessels during in vitro growth. HortScience. 2017;52(7):1006–12.
Kozai T. Micropropagation under photoautotrophic conditions micropropagation: technology and application. Berlin: Springer; 1991.
Xiao Y, Niu G, Kozai T. Development and application of photoautotrophic micropropagation plant system. Plant Cell, Tissue Organ Cult. 2011;105:149–58.
McClelland MT, Smith MAL. Vessel type, closure, and explant orientation influence in vitro performance of five woody species. HortScience. 1990;25(7):797–800.
Patel AK, Lodha D, Ram K, Shekhawat S, Shekhawat NS. Evaluation of physiochemical factors affecting high-frequency plant regeneration of Blyttia spiralis (synonym: Pentatropis spiralis), a threatened climber of medicinal value. Vitr Cell Dev Biol. 2016;52:10–9.
Nguyen QT, Kozai T, Van Nguyen U. Effects of sucrose concentration, supporting material and number of air exchanges of the vessel on the growth of in vitro coffee plantlets. Plant Cell Tissue Organ Cult. 1999;58:51–7.
Murphy KP, Santamaria JM, Davies WJ, Lumsden PJ. Ventilation of culture vessels Increased growth in vitro and survival ex vitro of Delphinium. J Hortic Sci Biotechnol. 1998;73(6):725–9.
Fal MA, Majada JP, Sánchez TR. Physical environment in non-ventilated culture vessels affects in vitro growth and morphogenesis of several cultivars of Dianthus Caryophyllus L. Vitr Cell Dev Biol. 2002;38:589–94.
Manokari M, Badhepuri MK, Cokulraj M, Dey A, Rajput VD, Minkina T, et al. Differential morphometric and micro-morpho-anatomical responses toward types of culture vessels used in micropropagation of Hemidesmus indicus (L). Plant Cell Tissue Organ Cult. 2022;150:1–8.
Zimmerman RH. Rooting apple cultivars in vitro: Interactions among light, temperature, phloroglucinol and auxin. Plant Cell Tissue Organ Cult. 1984;3:301–11.
Kacar YA, Biçen B, Varol I, Mendi YY, Serçe S, Çetiner S. Gelling agents and culture vessels affect in vitro multiplication of banana plantlets. Genet Mol Res. 2010;9(1):416–24.
Silva ST, Bertolucci SKV, da Cunha SHB, Lazzarini LES, Tavares MC, Pinto JEBP. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour) Spreng. Plant Cell Tissue Organ Cult. 2017;129:501–10.
Neto AR, Chagas EA, Costa BNS, Chagas PC, Vendrame WA. Photomixotrophic growth response of sugarcane in vitro plantlets using different light intensities and culture vessel types. Vitr Cell Dev Biol. 2020;56:504–14.
Lai CC, Lin HM, Nalawade SM, Fang W, Tsay HS. Hyperhydricity in shoot cultures of Scrophularia yoshimurae can be effectively reduced by ventilation of culture vessels. J Plant Physiol. 2005;162(3):355–61.
Batista DS, Felipe SHS, Silva TD, de Castro KM, Mamedes-Rodrigues TC, Miranda NA, et al. Light quality in plant tissue culture: does it matter? Vitr Cell Dev Biol. 2018;54:195–215.
Yeh N, Chung JP. High-brightness LEDs—energy efficient lighting sources and their potential in indoor plant cultivation. Renew Sustain Energy Rev. 2009;13(8):2175–80.
Ferreira LT, de Araújo Silva MM, Ulisses C, Camara TR, Willadino L. Using LED lighting in somatic embryogenesis and micropropagation of an elite sugarcane variety and its effect on redox metabolism during acclimatization. Plant Cell, Tissue Organ Cult. 2017;128:211–21.
Kumar A, Palni LMS. The effect of light source and gelling agent on micropropagation of Rosa damascena Mill and Rhynchostylis retusa (L) Bl. J Hortic Sci Biotechnol. 2003;78(6):786–92.
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Luna-Sánchez IJ. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. South African J Bot. 2017;109:288–93.
Gnasekaran P, Rahman ZA, Chew BL, Appalasamy S, Mariappan V, Subramaniam S. Development of micropropagation system of Zingiber officinale var rubrum Theilade using different spectrum light-emitting diode (LED) irradiation. Ind Crops Prod. 2021;170:113748.
Manivannan A, Soundararajan P, Halimah N, Ko CH, Jeong BR. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic Environ Biotechnol. 2015;56:105–13.
Kozai T, Kubota C, Ryoung JB. Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult. 1997;51:49–56.
Jo EA, Tewari RK, Hahn EJ, Paek KY. Effect of photoperiod and light intensity on in vitro propagation of Alocasia amazonica. Plant Biotechnol Rep. 2008;2:207–12.
Meziani R, Jaiti F, Mazri MA, Anjarne M, Chitt MA, El Fadile J, et al. Effects of plant growth regulators and light intensity on the micropropagation of date palm (Phoenix dactylifera L) cv Mejhoul. J Crop Sci Biotechnol. 2015;18:325–31.
Singh AS, Jones AMP, Shukla MR, Saxena PK. High light intensity stress as the limiting factor in micropropagation of sugar maple (Acer saccharum Marsh). Plant Cell Tissue Organ Cult. 2017;129:209–21.
Talavera C, Contreras F, Espadas F, Fuentes G, Santamaría JM. Cultivating in vitro coconut palms (Cocos nucifera) under glasshouse conditions with natural light, improves in vitro photosynthesis nursery survival and growth. Plant Cell Tissue Organ Cult. 2005;83:287–92.
Sáez PL, Bravo LA, Latsague MI, Toneatti MJ, Sánchez-Olate M, Ríos DG. Light energy management in micropropagated plants of Castanea sativa, effects of photoinhibition. Plant Sci. 2013;201:12–24.
Torné JM, Moysset L, Santos M, Simón E. Effects of light quality on somatic embryogenesis in Araujia sericifera. Physiol Plant. 2001;111(3):405–11.
Michler CH, Lineberger RD. Effects of light on somatic embryo development and abscisic levels in carrot suspension cultures. Plant Cell Tissue Organ Cult. 1987;11:189–207.
D’Onofrio C, Morini S, Bellocchi G. Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult. 1998;53:91–8.
Sæbø A, Krekling T, Appelgren M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995;41:177–85.
Tanaka M, Takamura T, Watanabe H, Endo M, Yanagi T, Okamoto K. In vitro growth of Cymbidium plantlets cultured under superbright red and blue light-emitting diodes (LEDs). J Hortic Sci Biotechnol. 1998;73(1):39–44.
Wu HC, Lin CC. Red light-emitting diode light irradiation improves root and leaf formation in difficult-to-propagate Protea cynaroides L plantlets in vitro. HortScience. 2012;47(10):1490–4.
Nhut DT, Takamura T, Watanabe H, Okamoto K, Tanaka M. Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003;73:43–52.
Lotfi M, Mars M, Werbrouck S. Optimizing pear micropropagation and rooting with light emitting diodes and trans-cinnamic acid. Plant Growth Regul. 2019;88:173–80.
He C, Zeng Y, Fu Y, Wu J, Liang Q. Light quality affects the proliferation of in vitro cultured plantlets of Camellia oleifera Huajin. PeerJ. 2020;8: e10016.
Bach A, Kapczyńska A, Dziurka K, Dziurka M. The importance of applied light quality on the process of shoot organogenesis and production of phenolics and carbohydrates in Lachenalia sp. cultures in vitro. South African J Bot. 2018;114:14–9.
Sayed SS, Gabr AMM, Amin MA, Taha LS. Biochemical characterization of micropropagated Ceratonia siliqua L under effect of growth regulators and light quality. Bull Natl Res Cent. 2020;44:1–7.
Klimek-Szczykutowicz M, Prokopiuk B, Dziurka K, Pawłowska B, Ekiert H, Szopa A. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (watercress). Plant Cell Tissue Organ Cult. 2022;149(1–2):113–22.
Tariq U, Ali M, Abbasi BH. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J Photochem Photobiol B Biol. 2014;130:264–71.
Ouyang J, Wang X, Zhao B, Wang Y. Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci. 2003;165(3):657–61.
Nishimura T, Ohyama K, Goto E, Inagaki N. Concentrations of perillaldehyde, limonene, and anthocyanin of Perilla plants as affected by light quality under controlled environments. Sci Hortic. 2009;122(1):134–7.
Zheng YP, Jiang W, Liao FL, Lu HF. Optimization of light quality for production of alkaloid and polysaccharide in Dendrobium candidum Wall ex Lindl. J Med Plants Res. 2012;6:560–5.
da Silva GM, Mohamed A, de Carvalho AA, Pinto JEBP, Braga FC, de Pádua RM, et al. Influence of the wavelength and intensity of LED lights and cytokinins on the growth rate and the concentration of total cardenolides in Digitalis mariana Boiss ssp heywoodii (P. Silva and M. Silva) Hinz cultivated in vitro. Plant Cell Tissue Organ Cult. 2022;151(1):93–105.
Alvarenga ICA, Pacheco FV, Silva ST, Bertolucci SKV, Pinto JEBP. In vitro culture of Achillea millefolium L quality and intensity of light on growth and production of volatiles. Plant Cell Tissue Organ Cult. 2015;122:299–308.
Zarei A, Behdarvandi B, Tavakouli Dinani E, Maccarone J. Cannabis sativa L photoautotrophic micropropagation: a powerful tool for industrial scale in vitro propagation. Vitr Cell Dev Biol. 2021;57(6):932–41.
Zheng YP. Optimization of light quality for production of alkaloid and polysaccharide in Dendrobium candidum wall ex Lindl. J Med Plants Res. 2012;6(4):560–5.
Wilken D, Jiménez Gonzalez E, Gerth A, Gómez-Kosky R, Schumann A, Claus D. Effect of immersion systems, lighting, and TIS designs on biomass increase in micropropagating banana (Musa spp cv’.Grande naine’AAA). Vitr Cell Dev Biol. 2014;50:582–9.
Klimek M, Barbara S, Kinga P, Bożena D, Halina P. The influence of different wavelengths of LED light on the production of glucosinolates and phenolic compounds and the antioxidant potential in in vitro cultures of Nasturtium officinale (watercress) ferric reducing ability of Plasma. Plant Cell Tissue Organ Cult. 2022;149(1):113–22.
Yu KW, Murthy HN, Hahn EJ, Paek KY. Ginsenoside production by hairy root cultures of Panax ginseng: influence of temperature and light quality. Biochem Eng J. 2005;23(1):53–6.
Ali MB, Hahn EJ, Paek KY. Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ Exp Bot. 2005;54(2):109–20.
In LP. Wu H chiun, Lin C chih. Irradiation Improves Root and Leaf. 2012;47(10):1490–4.
Read PE, Preece JE. Environmental management for optimizing micropropagation. Acta Hortic. 2003;616:49–58.
Durán-Vila N, Gogorcena Y, Ortega V, Ortiz J, Navarro L. Morphogenesis and tissue culture of sweet orange (Citrus sinensis (L) Osb): effect of temperature and photosynthetic radiation. Plant Cell Tissue Organ Cult. 1992;29:11–8.
Chauvin JE, Salesses G. Advances in chestnut micropropagation (Castanea Sp). Acta Horticulturae. 1988. https://doi.org/10.1660/ActaHortic.1988.227.6.
Islam MT, Dembele DP, Keller ERJ. Influence of explant, temperature and different culture vessels on in vitro culture for germplasm maintenance of four mint accessions. Plant Cell Tissue Organ Cult. 2005;81:123–30.
Sengar K, Sengar RS, Garg SK. The effect of in-vitro environmental conditions on some sugarcane varieties for micropropagation. Afr J Biotechnol. 2011;10(75):17122–6.
Deng Q, Deng Q, Wang Y, Liu S, Liu Y, Yang Q, et al. In vitro micropropagation of Nopalxochia ackermannii Kunth IOP Confernces Series: Earth and Environmental Science. Bristol: IOP Publishing; 2018.
Lobo A, Find JI, Hansen JK, Ræbild A, Kjaer ED. Effect of temperature and osmotic stress during somatic embryogenesis on phenology and physiology of abies nordmanniana emblings. For Ecol Manage. 2022;514: 120212.
Shekhawat NS, Rathore TS, Singh RP, Deora NS, Rao SR. Factors affecting in vitro clonal propagation of Prosopis cineraria. Plant Growth Regul. 1993;12:273–80.
Gupta AK, Harish RM, Phulwaria M, Agarwal T, Shekhawat NS. In vitro propagation, encapsulation, and genetic fidelity analysis of Terminalia arjuna: A cardioprotective medicinal tree. Appl Biochem Biotechnol. 2014;173(6):1481–94.
Hussey G, Stacey NJ. In vitro propagation of potato (Solanum tuberosum L). Ann Bot. 1981;48(6):787–96.
Caligari PDS, Powell W. Variability in response of potato cultivars to micropropagation In vitro performance. Ann Appl Biol. 1989;115(1):115–21.
Tadesse M, Lommen WJM, Struik PC. Effects of in vitro treatments on leaf area growth of potato transplants during acclimatisation. Plant Cell Tissue Organ Cult. 2000;61:59–67.
Clarkson DT, Hopper MJ, Jones LHP. The effect of root temperature on the uptake of nitrogen and the relative size of the root system in Lolium perenne I solutions containing both NH+ 4 and NO3−. Plant Cell Environ. 1986;9(7):535–45.
Bressan PH, Kim YJ, Hyndman SE, Hasegawa PM, Bressan RA. Factors affecting in vitro propagation of rose1. J Am Soc Hortic Sci. 1982;107(6):979–90.
Nievola CC, Kraus JE, Freschi L, Souza BM, Mercier H. Temperature determines the occurrence of CAM or C3 photosynthesis in pineapple plantlets grown in vitro. Vitr Cell Dev Biol - Plant. 2005;41(6):832–7.
Mollo L, Martins MCM, Oliveira VF, Nievola CC, Figueiredo L. Effects of low temperature on growth and non-structural carbohydrates of the imperial bromeliad Alcantarea imperialis cultured in vitro. Plant Cell Tissue Organ Cult. 2011;107:141–9.
Malik S, Kumar R, Vats SK, Bhushan S, Sharma M, Ahuja PS. Regeneration in Rheum emodi Wall a step towards conservation of an endangered medicinal plant species. Eng Life Sci. 2009;9(2):130–4.
de Carvalho V, dos Santos DS, Nievola CC. In vitro storage under slow growth and ex vitro acclimatization of the ornamental bromeliad Acanthostachys strobilacea. South African J Bot. 2014;92:39–43.
Jouve L, Franck T, Gaspars T, Cattivelli L, Hausman JF. Poplar acclimation to cold during in vitro conservation at low non-freezing temperature: metabolic and proteic changes. J Plant Physiol. 2000;157(1):117–23.
dos Santos DS, Cardoso-Gustavson P, Nievola CC. Stem elongation of ornamental bromeliad in tissue culture depends on the temperature even in the presence of gibberellic acid. Acta Physiol Plant. 2017;39:1–8.
Carvalho CP, Hayashi AH, Braga MR, Nievola CC. Biochemical and anatomical responses related to the in vitro survival of the tropical bromeliad Nidularium minutum to low temperatures. Plant Physiol Biochem. 2013;71:144–54.
Tadesse M, Lommen WJM, Struik PC. Development of micropropagated potato plants over three phases of growth as affected by temperature in different phases. NJAS Wageningen J Life Sci. 2001;49(1):53–66.
Pedroso ANV, Lazarini RA, Tamaki V, Nievola CC. In vitro culture at low temperature and ex vitro acclimatization of Vriesea inflata an ornamental bromeliad Brazilian. J Bot. 2010;33(407):14.
Acknowledgements
We acknowledge the financial support of UGC, New Delhi in the form of BSR Start up Grant to the author AKG and as JRF to the authors Nikita Gautam and Priyanka Faroda. We are also thankful to Prof. NS Shekhawat for his continuous motivation and support.
Author information
Authors and Affiliations
Contributions
AKG, H and TA conceptualize this review article. NG and PF wrote the original draft and prepared the figures and tables. AKG, H and TA edited the manuscript and prepared the final draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gautam, N., Faroda, P., Agarwal, T. et al. Effect of microclimatic physical factors on in vitro morphogenesis of plants: a systematic review. Discov Agric 2, 13 (2024). https://doi.org/10.1007/s44279-024-00022-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00022-5