Abstract
Phycobiliproteins, carotenoids and fucoxanthin are photosynthetic pigments extracted from microalgae and cyanobacteria with great potential biotechnological applications, as healthy food colorants and cosmetics. Phycocyanin possesses a brilliant blue color, with fluorescent properties making it useful as a reagent for immunological essays. The most important source of phycocyanin is the cyanobacterium Arthrospira platensis, however, recently, the Rhodophyta Galdieria sulphuraria has also been identified as such. The main obstacle to the commercialization of phycocyanin is represented by its chemical instability, strongly reducing its shelf-life. Moreover, the high level of purity needed for pharmaceutical applications requires several steps which increase both the production time and cost. Microalgae (Chlorella, Dunaliella, Nannochloropsis, Scenedesmus) produce several light harvesting carotenoids, and are able to manage with oxidative stress, due to their free radical scavenging properties, which makes them suitable for use as source of natural antioxidants. Many studies focused on the selection of the most promising strains producing valuable carotenoids and on their extraction and purification. Among carotenoids produced by marine microalgae, fucoxanthin is the most abundant, representing more than 10% of total carotenoids. Despite the abundance and diversity of fucoxanthin producing microalgae only a few species have been studied for commercial production, the most relevant being Phaeodactylum tricornutum. Due to its antioxidant activity, fucoxanthin can bring various potential benefits to the prevention and treatment of lifestyle-related diseases. In this review, we update the main results achieved in the production, extraction, purification, and commercialization of these important pigments, motivating the cultivation of microalgae as a source of natural pigments.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Phycobiliproteins
Phycobiliproteins (PBPs) are brilliantly colored protein-pigments of the photosynthetic light-harvesting antenna complexes produced by some algae, such as Rhodophyta, Cryptomonads, Glaucophytes and, particularly, by Cyanobacteria. The biological importance of these compounds in Cyanobacteria is evidenced by the great amount of PBPs they produce, which can represent up to 50–60% of the total soluble proteins in some organisms [1], such as Arthrospira platensis or A. maxima. In fact, PBPs are the principal accessory pigments of cyanobacteria. In these organisms, PBPs are also nitrogen storage compounds, and are decomposed when nitrogen starvation occurs [2, 3]. PBPs are generally classified based on their absorption maximum (Amax) in the visible region of the spectrum. The most common PBPs are phycocyanin (PC, Amax = 610–625 nm, blue), allophycocyanin (APC, Amax = 650–660 nm, blue green) and phycoerythrin (PE, Amax = 490–570 nm, red). Their structure is shown in Fig. 1 [4,5,6,7,8]. The main components of these pigments are protein subunits α and β, which form the “monomeric” unit (αβ). Various units self-assemble into the PBPs, which, in vivo, are organized on the thylakoid membrane to form larger protein complexes, the phycobilisomes [9] (Fig. 2a [10, 11]). The number and type of PBPs composing the phycobilisome is variable both in individual organisms of a species (due to environmental acclimation of the organism) and among species [12]. The general phycobilisome structure is sketched in Fig. 2b. The protein’s brilliant color is due to the prosthetic groups: covalently bound tetrapyrrole derivatives (such as phycocyanobilin (Fig. 3a) or phycoerythrobilin, (Fig. 3b)), protected by the protein backbone and blocked in a geometry (extended conformation) suitable to efficiently funnel light energy (from PE to PC and, finally, to APC) towards the chlorophyll photosystem center [9]. In the phycobilisome, the pigment with the highest energy state, PE, always occupies the outermost position of the complex. Inwards follows PC and, finally, next to the photosynthetic chlorophyll center, the pigment with the lowest energy state, APC, as sketched in Fig. 2b.
When extracted and not engaged in the energy transfer processes, these molecules exhibit a fluorescence characterized by a very high quantum yield. Thanks to their remarkable characteristics, PBPs have several biotechnological applications in food, cosmetic, pharmaceutical, and biomedical sectors. Among PBPs, PC has by far the largest market. The increasing utilization of these water-soluble pigments is boosted by their safe and healthy nature, as it is evidenced by the numerous papers published in the last years [1, 3, 13,14,15,16,17,18,19,20,21].
PBPs are used as natural cosmetic dyes or as fluorescent probes (APC and PE in particular) in flow cytometry and immunoassays. A. platensis (Spirulina) blue aqueous extracts, containing PC and APC, are approved by EFSA (Regulation (EU) No. 1333/2008 and No. 231/2012) as foodstuff coloring. The US FDA classifies PC (21CFR73.1530) as a natural food color additive. PC and PBPs therapeutic activities as antioxidant, anti-inflammatory, neuroprotective, anti-cancer and immunomodulatory have been reported [1, 3, 13, 14, 18, 19, 21, 23,24,25,26,27].
The commercial value of a PBP is strongly dependent on its purity grade, which is usually evaluated by the ratio between the value of PBP absorption maximum in the visible region of the spectrum and the absorbance value at 280 nm, which is related to the total amount of proteins detectable in the product. PBP purity greater than 0.7 is considered as food grade, greater than 1.5 as cosmetic grade, greater than 3.9 as reactive grade and greater than 4.0 as analytical grade. Usually, a purity greater than 3.0 is required to be used as fluorescent agent, while analytical grade PC is required for biomedical and therapeutic applications [28, 29]. Despite the increasing demand of safe natural products, the widespread use of PBPs is still limited by the costs of large-scale biomass production [30,31,32] as well as of extraction and purification methods.
1.1 Extraction and purification methods
Both extraction and purification are still too expensive, rather complex, and time-consuming, while industry needs simple, cheap and easily scalable, procedure to achieve a sustainable production of PBPs [3, 14, 22, 29, 33,34,35]. In addition, climate change due to the increase in emissions of carbon dioxide and other greenhouse gases, and the worldwide growing environmental deterioration, due to heavy pollution, are requiring industries to urgently align with current guidelines and regulations towards a progressive reduction of carbon dioxide emissions as well as energy consumption, boosting a “green revolution” based on the adoption of sustainable processes. Thus, there is a high industrial and commercial interest for sustainable, environmentally friendly, green chemistry extraction/purification processes to provide PBPs (particularly PC) of various purity grades on large scale while using a minimum number of steps, due to increasing world demand of safe, non-toxic, healthy bio-products.
Tan et al. [17] recently reviewed the research trends of PBPs using a bibliometric approach. They found that the principal issues addressed in the documents were the optimization of microalgae culture parameters and PBPs extraction methods. Moreover, bioactivity properties and extraction of PBPs turned out to be the future research priorities.
Indeed, a quick bibliography survey can easily show the growing interest on the extraction and purification of these compounds, that is proved by the growing number of published documents over the last years. As an example, a survey made on 1st August 2022, based on the Scopus database (combining the terms phycobiliprotein, phycocyanin, phycoerythrin, allophycocyanin, extraction and purification, and eliminating spurious documents) evidenced that in 2021 the number of published documents [83] was more than four time higher that ten years before [19]. In Fig. 4 is reported the number of documents per year from 2000 to 2021 retrieved in the survey, while in Fig. 5 is reported the number of documents retrieved in the same period, using the same database, in relation to the extraction (Fig. 5a) and purification (Fig. 5b) method adopted. Most often various extraction and, particularly, various purification methodologies are combined and used in sequence to achieve a better result.
The literature survey evidenced that ultrasound-assisted extraction was the most used PBPs extraction method (Fig. 5a). Ultrasound-assisted extraction is considered a green process [36]. It requires short operation time and low solvent consumption, allowing to achieve high yields, but the increase of temperature in the PBPs samples, which are temperature-sensitive compounds, must be controlled and avoided to preserve PBPs’ functionalities [3, 34]. Ultrasound-assisted extraction is, in principle, suitable to large scale PBPs extraction, but a relatively low purity degree is generally achieved [37,38,39]. Other green methodologies applied to PBPs extraction are high pressure processes, pulsed electric field-assisted and microwaves-assisted extraction. Although it is reported that high pressure processes (such as hydrostatic pressure processing, in which pressures around 300–600 MPa are applied, and high-pressure homogenization, in which lower pressures, usually up to 400 MPa, are applied [40]) may exhibit low selectivity [34], good results have been reported for PC extraction [41]. In addition to being considered eco-friendly, this methodology is also suitable to large scale production of PBPs. Other two processes considered green are pulsed electric field-assisted and microwaves-assisted extraction. However, the increase in temperature of the samples, caused by both methods, and the possible electrode corrosion and metal leaking in pulsed electric field-assisted extraction, are drawbacks limiting the application of these methods to the extraction of PBPs [34, 36]. Enzymatic-assisted extraction and repeated freeze–thawing are other two popular methods (Fig. 5a). Enzymatic-assisted extraction can ensure high yield, but it is not particularly suitable to large scale production of PBPs because of the high cost of the commercially available enzymes [36]. On the contrary, repeated freeze-thawing, though time consuming, it is particularly attractive thanks to its simplicity, reproducibility, and moderate cost. Therefore, it is commonly used to treat small quantity of biomass. In fact, repeated freeze–thawing efficiently lyses cells and ensures a higher purity respect to other approaches [3, 34]. However, it cannot be considered a general method since the cells of some cyanobacterial or algal species are too stiff to be broken by freeze–thawing and require a different approach. Indeed, a single preferrable PBPs extraction method does not exist, instead it must be chosen and optimised for each species. In addition, the type of biomass (fresh, spray-dried, freeze-dried, oven-dried, etc.) used as pigment source must be considered when choosing the optimal extraction method.
As concerns purification methods, Fig. 5b shows how ammonium sulfate precipitation (or salting out) was the most exploited one. Indeed, ammonium sulfate salting out is widely used in protein purification procedures [42], including PBPs [43], and is most often applied as pre-purification step, to obtain highly purified PBPs [44] (see Table 1). The highest PBPs purity is usually achieved by column chromatography, particularly ion exchange chromatography, or exploiting liquid (especially aqueous) two-phase systems [1, 35]. However, column chromatography is difficult to scale up, while aqueous two-phase systems are more suitable for large scale PBPs production [18]. Very recently membrane chromatography has been advantageously exploited to obtain analytical grade PBPs [29, 45, 46]. This methodology is interesting because fundamentally “green”, cost-effective, environmentally friendly, and suitable to be used on an industrial scale [47]. Table 1 shows a summary of PBP extraction and purification procedures available in the literature.
1.2 Main environmental factors affecting the productivity of phycocyanin
As previously mentioned, currently, among the PBPs, PC occupies the largest market area as it is widely used in the food sector. Nowadays, commercial production of PC relies almost exclusively on Arthrospira (Spirulina) biomass. Arthrospira extracts were approved for use in candy, chewing gum and other types of confectionery in the US in 2013 and 2014 [104] while EU approved Arthrospira extracts in 2013 as food coloring [105]. The decision was based upon the fact that Arthrospira has been consumed for centuries and its consumption is considered safe worldwide [106]. Therefore, information on production, extraction and purification of PC come mostly from Arthrospira cultures.
1.2.1 Light intensity and spectra
Both light intensity and wavelength can strongly modify the productivity and concentration of PC in the biomass. In general, acclimation to low light, achieved either by exposing cells to low intensity incident light or by increasing the density of cultures, entails a raise in both chlorophyll and PC contents [31]. In A. platensis cultures, during the logarithmic growth phase, the percentage of chlorophyll a (Chl a) doubled from 0.8% to 1.6% of dry weight, while the percentage of PC increased from an initial value of 3% up to 12–14% depending on the strain and light spectra at which cultures were grown [32].
Considering the high market value of PC, artificial light is often used for the production. Fluorescent lamps and light emitting diodes (LEDs) are used for the microalgal cultivation, with different spectrum and energy conversion efficiency. Fluorescence tubes have the advantage to deliver light irradiance at a 360 degree angle and are usually characterized by much larger emission bands compared to LEDs. However, due to small size, light weight, durability efficiency of energy conversion and longer operating life, LEDs have attracted the interest of algal biomass producers. LEDs have several advantages that have promoted their diffusion as the light source for the purpose of microalgae growth, and recent studies on the growth of A. platensis were conducted using fluorescent lamps [32, 107].
The cyanobacterium A. platensis grown under illumination with lights of different colors (white, orange and blue) shows absorption peaks in the blue and red part of the spectrum (440 and 680 nm), due to Chl a, and in the orange part (620 nm) due to PC (Fig. 6). The ratio between the PC peak at 620 nm and that of Chl a at 680 nm is higher for cells grown under blue light than under the orange and white light, indicating an increase in PC content under blue light.
In-vivo light absorption spectra of A. platensis cells grown under white, orange and blue lights, adapted from [32]
It has been proposed that blue light creates an unbalance between the two photosystems, with an excess of energy at the PSI side and a deficiency at the PSII side of the photosynthetic electron transport chain of A. platensis [32, 108]. It is well known that cyanobacteria invest much more of their chlorophyll a in PSI than in PSII [109, 110]. This unbalance between the two photosystems is compensated for by the light-harvesting phycobilisomes (PBSs) which are mainly associated to PSII [111]. In this way cyanobacteria maintain a balanced excitation distribution between the two photosystems enabling the production of both ATP and NADPH necessary for growth. Under blue light, the PBSs do not absorb blue photons efficiently, as their short wavelength of 450 nm does not coincide with the absorption spectrum of PC (peaking at 620 nm). Therefore, under blue light, PBSs hardly transfer any energy to PSII. On the other hand, Chl a, which is more abundant in PSI, can transfer energy to PSI efficiently. Moreover, in cyanobacteria β-carotene, which absorbs blue light efficiently, is more abundant in PSI than in PSII, thus further contributing to photosynthesis light harvesting by PSI. Therefore, under blue light, PSII experiences a severe shortage of photons in comparison to PSI, with a strong limitation in the linear electron transport. Consequently, cell acclimate to blue light by enhancing the production of PBSs normally serving the PSII, so to restore the balance between the two photosystems. However, this strategy is ineffective towards growth since PBSs do not absorb blue light. These facts strongly support the findings that cultures grown under blue light have a much lower growth rate compared to orange and white lights, but a significantly higher PC content [32]
There is a consensus that blue light increases PC concentration in Arthrospira cells, while it is less effective in promoting growth which was much higher when culture was grown under white or orange lights [32, 112]. Therefore, a two-step strategy to produce Arthrospira biomass enriched in PC could be employed, starting by growing the culture under orange or white light, and once the culture has reached the stationary phase, shifting the light to blue to further enhance PC content. As synthesis of PC requires high amount of nitrogen, and the exposure to blue light can stimulate protein synthesis [113, 114], it is important to avoid nitrogen deprivation during the accumulation step, which may occur at the end of the growth phase. The lack of nitrogen in this step may even cause a reduction of the PC concentration, since PC would be used by cells as source of nitrogen [2]. Therefore, it is advisable to use a two-step process to improve the economic feasibility of the process [115].
1.2.2 Influence of nitrogen source on productivity of phycocyanin
PC is an intracellular nitrogen source, and it can be used in prolonged nitrogen-limited conditions [2, 32]. Thus, the content of PC can be strongly affected by the nature of the nitrogen source and its concentration in the culture medium. Depletion of nitrate in the culture medium leads to the reduction of PC while an excess of nitrate could lead to its inhibition [116]. According to [116], the optimal nitrate concentration for high PC concentration is in the range between 1200 and 1600 mg/l. Addition of glutamate and succinic acid to Zarrouk medium enhanced the PC concentration by about 20% [116]. The reason for this increase of PC content is that glutamate and succinyl-coenzyme A are among the intermediate metabolites in the biosynthesis of tetrapyrroles including phycobilin in cyanobacteria cells [116]. The type of nitrogen source plays an important role in the production and concentration of PC in the biomass. Studies carried out in A. platensis using different sources of nitrogen (NaNO3, KNO3, NH4NO3, (NH4)2SO4, NH4Cl, and urea), have shown that the highest PC production was attained by using (NH4)2SO4. Higher biomass productivity was also accompanied by high PC content (11.3%) [117].
Ammonium is a more reduced form of nitrogen than nitrate and this may account for the better culture performance, particularly in the cyanobacterium Arthrospira whose protein content is very high (60–70%). However, at a pH of above 9, typical of Arthrospira cultures, ammonia (NH3) is the dominant chemical species, and its diffusion through the cell membrane results in ammonia accumulation inside the cell and consequent toxic effect [118]. Ammonia causes damage to the manganese (Mn) cluster of the oxygen-evolving complex of the PSII complex, causing a considerable increase of sensitivity of PSII to photodamage [119]. Chlorophyll fluorescence measurements showed that the PSII damage is related to the light intensity, and that the inhibition of the PSII performance by ammonia is not relieved neither by re-incubating the cells in the dark, nor by reducing the ammonia concentration [120]. A. platensis cultures with high biomass density and without pH regulation resulted less susceptible to ammonia inhibition, most likely through the faster assimilation of ammonia present in the medium [121]. Nonetheless, care is needed when ammonium salts are used as a nitrogen source in Arthrospira cultures, particularly when pH is uncontrolled as it may occur in large scale ponds. Moreover, at high pH a consistent amount of ammonia is lost by outgassing, contributing to the increase of greenhouse gas released in the atmosphere, and to an increase in costs. For these reasons, ammonium should be supplied in a fed-batch way to prevent accumulation. The most used nitrogen source remains NaNO3 as it can be supplied in a large amount to the culture (2.5 g/l in Zarrouk medium) without incurring in toxicity problems. Similar problems can be foreseen for the use of digestate for biomass production of cyanobacteria. This waste represents an excellent source of nitrogen and phosphorus as well as other minor nutrients. The amount of ammoniacal nitrogen in digestates ranges between 500 and 2000 mg/l, therefore it is inevitable to operate a dilution with fresh water. At the present the use of digestate in EU countries seems to be restricted to the production of biomass for use as feed. The legislation however doesn’t specifically allow or prohibit the use of slurry to grow algae as it is permitted for land plants used in the feed chain [122].
1.2.3 Effect of high dissolved oxygen concentrations and light–dark cycle
Large raceway ponds equipped with paddle wheels are usually characterized by low turbulence, and in Arthrospira cultures, faster mixing achieved by increasing to rotation of the paddle wheels or by other means can cause breakage of filaments, and foam formation, and a consistent increase in production costs. Oxygen removal is therefore usually limited and O2 concentration of 300–400% of air saturation are very frequent particularly in the afternoon [123]. Mass transfer coefficient can vary greatly in each section of the reactor: Kla is higher in proximity of the paddle wheel (about 164 h−1) while in the channel and bends it drops dramatically (below 1 h−1). This is a clear indication that cultures are poorly mixed, not allowing for an adequate stripping of oxygen [124]. Oxygen stripping can be improved by placing a sump where air is injected. However, care is needed with filamentous cyanobacteria such as Arthrospira, as the injection of air under pressure may result a fragmentation and flotation of the trichomes.
The negative effect of oxygen on culture productivity of mass cultures has been overlooked for many years. Torzillo et al. [125] reported evidence that, in Arthrospira cultures, a concentration of oxygen above 30 mg L−1 had a negative effect on both growth and biomass protein content. However, the problem of oxygen toxicity becomes evident when cultivating microalgae in closed systems [126]. Only a few studies have addressed the potential role of singlet oxygen as a marker for resilience to excess light environments, and to find out whether singlet oxygen resilience correlates with a strain's robustness in excess light environments.
Hiday and Belay [127] followed the daily time course in the photosynthetic pigments in cultures of Arthrospira grown in large scale ponds (0.5 ha). Larger variations in the PC content correlated with higher dissolved oxygen concentrations in the pond. PC content fluctuated according to an opposite trend to that of the oxygen concentration. It was evident that the lowest level of PC coincided with that of the peak of oxygen [127].
The concentration of PC can also change during the day as the illumination conditions vary, decreasing as light increases. Changes in PC are particularly evident in low density cultures, particularly in summer. The PC content is usually higher in the morning particularly in low density cultures. Usually, in Arthrospira cultures exposed to high light, there is an accumulation of carbohydrates during the day. Because of nocturnal respiration of carbohydrates, the concentration of PC can result significantly higher in the morning [127, 128]. Therefore, the time of biomass harvesting can affect the PC content in the biomass.
1.2.4 Effect of temperature
Because of seasonal and diurnal fluctuations, light and temperature represent the major biological limitations for biomass production of microalgae. However, as pointed out by Borowitzka [129], temperature plays the most important role on productivity, and therefore it should probably be the first parameter for strain selection. In the case of A. platensis, laboratory experiments have shown that the maximum biomass yield is obtained when Arthrospira is grown at the optimal temperature of 35 °C [130,131,132,133]. However, outdoors, in open ponds, culture temperature can fluctuate from 15 °C in the early morning hours to about 35 °C in the middle of day, causing a significant reduction in productivity and changes of the biomass composition [127]. Using different strains acclimated to the different temperatures at different times of the year can be useful to extend the cultivation period and attain higher productivities year-round. This strategy has been successfully tested with Arthrospira at Earthrise Farm in California, USA [134].
Studies carried out on Synechococcus sp. PCC 7002, whose optimal temperature for growth is 38 °C, reported that when the strain was grown at 15 °C there was a loss of blue color by the cell in a similar manner to nutrient starvation [135]. The amount of PBP and chlorophyll decreased while there was a strong accumulation of glycogen. By contrast, when the cells were grown with urea as a nitrogen source, cells grew normally without any sign of chlorosis. The authors hypothesized that the defect in nitrogen assimilation was most likely due to an inefficiency of transport of nitrate across the cytoplasmic membrane.
Summing up, the PC production by cyanobacteria (and by Arthrospira in particular) strongly depends on maintaining the optimal conditions of temperature and light intensity at which cells are exposed, two environmental factors that can strongly change along the year and during the day. The culture system can affect the temperature profile and light uptake. The control of temperature is more easily achieved in a closed system, and more difficult to implement in open ponds. In this system, it is frequently observed that temperature does not rise fast enough in the morning in comparison to light which goes up very fast, and this desynchronization between the raise in light and temperature may causes cell photoinhibition [136, 137].
1.3 Strain selection
Surprisingly, while the cultivation of Arthrospira for industrial purposes, dates several decades back, the progress made in the selection of newer more productive strains is minimal. Indeed, any enhancement in strain performance would reflect immediately in industrial applications. Factors discouraging from planning a systematic strain selection activity are the necessity to have a large collection of strains which requires considerable manpower for its maintenance, and the too large variation in PC content determined by environmental factors which makes more complicate the evaluation of differences between strains. Moreover, it is worth to point out that because of the high morphological plasticity of Arthrospira strains, the selected laboratory strains once transferred in mass cultured may perform significantly different compared to the original laboratory strain. Indeed, outdoors, strains are subject to strong variability in the environmental factors, particularly light, temperature and oxygen exposure, and to hydrodynamic stress caused by mixing, which can strongly modify the morphology of trichomes and their biochemical composition. Prolonged cultivation of the same strain can generate phenotypes with improved performance, with higher resilience to temperature and light stress. This may justify the reluctance of the grower to change their strain, together with the necessity to guarantee a constant production and consistent biochemical characteristic of the product. In addition, employing higher PC content strains for industrial production must prove to be economically advantageous, compared to conventional strains. In other words, the increase in PC content should not be achieved at the expense of a reduced biomass production. Therefore, biomass productivity of the strain, is thus an important aspect that should not be overlooked during strain selection. Moreover, it often happens that the producer of the pigment is usually not the grower, and producers are interested in cheaper biomass rather than high concentration of the pigment in the biomass (Vonshak, personal communication).
Strain selection of a NaCl tolerant mutant of A. platensis, showed that the PC production was 12.2% as compared to its wildtype counterpart when cultivated in a nitrate and bicarbonate sufficient medium (40 and 60 mM, respectively) at pH 9.0 under phototrophic conditions [138]. Among 13 cyanobacteria species investigated, Arthrospira sp., Pseudanabaena sp. and Synechococcus elongatus might be the promising candidates for PC, PE and APC sources, respectively, for commercial production purposes [139]. Interestingly, the specific growth rate and biomass productivity of Synechocystis sp. were significantly higher than the other cyanobacteria. This was attributed to a higher surface to volume ratio (S/V) that allows this cyanobacterium to uptake more nutrients [140].
A comparison of the biochemical composition of different A. platensis strains from different geographical areas (Algeria, Chad, USA) was carried out. The Arthrospira strain from USA showed the highest PC content (% of dry weight) compared to the other strains [141].
In Table 2 shows the PC content found in different Arthrospira strains. To make the comparison among the different strains easier, the culture conditions are specified. The geographic origin of the strain and depositary institution is also indicated.
1.4 Production of C-PC with Galdieria sulphuraria: an alternative to Arthrospira
Galdieria sulphuraria is a red (Rhodophyta) polyextremophilic microalga which can tolerate very low pH (as low as 0.2), temperature up to 57 °C [77, 143, 144], and high osmotic pressure, up to 400 g L−1 of sugar, and 2–3 M of salt. Similarly, to Arthrospira, it contains only chlorophyll a, and accessory photosynthetic pigments are represented by blue PBPs, PC and APC. This organism can growth heterotrophically (in the dark), in which organic substrates represent the source of both carbon and electrons, as well as autotrophically (in the light) in which reducing power is generated via water splitting by PSII, while the source of carbon is CO2. It can also grow mixotrophically, that is, a combination of heterotrophy and autotrophy [145,146,147,148]. Their rather unique ability to cope with very harsh growth conditions strongly reduce the risk of contamination even in heterotrophy, making this organism very attractive to produce PC and proteins (up to 72%), which are also richer in essential amino acids compared to those from Arthrospira and Chlorella [146]. Since this organism, contrary to Arthrospira, does not have a record history of its use as food and feed, it is considered a potential novel food by the EU regulation, therefore it needs to go through the procedure of admission as novel food before the approval [148]. Autotrophic cultivation of Galdieria requires a careful choice of the cell density and of the light supply to avoid risk of photoinhibition [146]. This phenomenon can occur after a strong dilution of a dense culture where most cells are acclimated to low light and thus may suffer of the suddenly raise in the specific light supply. Although this problem can be easily managed in laboratory by temporarily reducing the light supply, it may represent a problem when working with large scale bioreactors under solar light. The risk of photoinhibition is less relevant with mixotrophic cultures, particularly when they are grown according to an “oxygen balanced” regime, in which the supply of substrate, and thus the oxygen uptake is balanced with photosynthesis [145]. This culture mode allows to maintain the reactor closed and thus reduces the risk of contamination which may occur through the injection of air in the reactor.
Under heterotrophic conditions, PC content is very low, ranging from 0.3 to 0.6% DW, in cultures grown on glucose and glycerol, respectively. The PC content resulted 40-fold higher and 20-fold higher in cultures grown under autotrophically [147]. These results indicated that heterotrophy inhibits pigment synthesis particularly in cells grown on glucose. [148]. Heterotrophic cells grown on glucose became yellowish while those grown on glycerol still maintained the green color. The pigmentation was restored in glucose grown cells when it is totally consumed. PC content ranging between 0.8 and 1.2% of dry weight was reported by [149] in G. sulphuraria strain 074G under heterotrophy conditions in carbon-limited cultures and nitrogen sufficient cultures, while the PC content dropped to 0.1% in nitrogen deficient cells. Therefore, despite the much higher volumetric productivity attained with heterotrophic cultures, the PC content in this biomass is too low for economic extraction, with limitation of the commercial applications. Indeed, according to EU regulations, the concentration of pigment in the source determines whether the extract can be considered as a food extract with coloring property (> 3% of dry weight) or just a natural food additive (< 3% dry weight) [150]. Moreover, while food extracts are considered food ingredients and are used in clean label food products, the natural additives require an “E” number. Therefore, due to PC content, PC extracts obtained from heterotrophic Galdieria biomass fall into the additives categories, while PC extracts from autotrophic and mixotrophic cultures would enter in the first category, not requiring any labelling.
A strategy to conjugate the high biomass accumulation with high level of PC in G. sulphuraria was proposed [151]. It consists of sequential production of biomass in heterotrophy followed by dilution and exposure of cells to light to induce synthesis of PC. By this way it was possible to raise the PC content up to 13.88%, that is, higher than that attained by growing cells autotrophically or mixotrophically. Although this strategy may be appealing, the economic comparison between autotrophic, mixotrophic, and sequential heterotrophic/autotrophic needs to be assessed. The sequential heterotrophic/autotrophic strategy entails the need to strongly dilute the culture before exposure to light which increases the cost of harvesting, particularly with unicellular microalgae (Table 3).
Another thermoacidophile red microalga, Cyanidioschyzon merolae, belonging to the order Cyanidiophyceae of the phylum Rhodophyta, has been proposed for PC production [152]. Similarly to G. sulphuraria, it thrives at pH between 0.5 and 3, and tolerates temperatures as high as 56 °C. Indeed, expressed sequence tags and high-throughput genomic sequence reads covering > 70% of the G. sulphuraria genome were compared to the genome of Cyanidioschyzon merolae, and more than 30% of the Galdieria sequences did not relate to any of the Cyanidioschyzon genes [153]. Contrary to Galdieria which has a large metabolic flexibility, C. merolae is an obligate photoautotrophic organism, which restricts its cultivation to autotrophic conditions (light and CO2). Interestingly, it lacks a cell wall, which makes it possible to extract PC with high degree of purity (up to 9.9) just using osmotic shock and centrifugation. The PC of C. merolae resulted stable to pH 4–5 up to 80 °C. Because of the high degree of purity of PC produced using this species, without applying time consuming and expensive purification procedures, its employment may be evaluated for production of analytical/reagent grade PC. However, the effective performance of this species needs to be assessed in mass cultures, where hydrodynamic stress could strongly hamper its cultivation.
1.5 Attempts to enhance phycocyanin stability
PC molecule proved to be very sensitive to environmental factors such as temperature, pH, and light, which can cause blue color to fade up to a total loss of color. This fact strongly hampers its utilization in food and cosmetics. Therefore, several efforts have been devoted to finding out the optimal conditions preventing PC degradation and thus increase its shelf-life. Different stabilization techniques have been proposed, by using preservatives as well as formulation processes. Food grade PC is usually used to assess its degradation, which is commonly determined by UV–vis spectrophotometry at 620 nm. The stability of the PC molecule is also influenced by its purity. A comparison between reactive grade PC and food grade, determined a higher thermostability in food grade PC [154]. However, it is worth noting that a hard purification procedure can affect the pigment stability. In general, gentle extraction and purification procedures are expected to better preserve PC stability. Most of the studies on PC stability have been carried out on PC extracted from Arthrospira (Spirulina) platensis, which is nowadays the major source of PC utilized worldwide.
1.5.1 Effect of temperature and use of preservatives to enhance the thermal stability
Temperature influences the stability of PC [[155] and references therein]. The stability of total PBPs from 13 isolated cyanobacteria after 24 h of incubation at − 20 °C resulted variable. The loss of PBP content ranged between 4.88% and 15.94%. The highest stability of total PBPs was observed in Pseudanabaena sp. (reduced by 4.88%), whereas PBP content from Desertifilum sp. extracts decreased the most by 15.94% over 24 h [156]. These findings may indicate that the stability of PBPs may be related to cyanobacteria species and/or strains. Recently, heterologous expression of phycocyanin derived from the thermophilic cyanobacterium Thermosynechococcus elongatus, was applied in the mesophilic cyanobacterium Synechocystis sp. 6803 to produce thermostable PC [103]. Importantly, the yields of PC in the Synechocystis mutant strains resulted comparable to that of native PC in wild-type Synechocystis, while the thermostability properties of PC matched those from T. elongatus [103].
Very often, experiments on the effect of temperature are associated to the use of preservatives to improve the pigment stability. Incubation of PC from Arthrospira biomass at temperatures between 47 and 64 °C, in a pH range of 5.5–6.0, caused a reduction in the concentration by 50% [157]. Adding 20–40% glucose or sucrose and heating to 60 °C for 15 min, the CR (relative concentration) value of PC at pH 7.0 was maintained at around 62–70%, and the half-life increased from 19 min to 30–44 min. The addition of 2.5% sodium chloride had an even better preservative effect on PC at pH 7.0 since 76% of the PC concentration was retained [157].
PC was found to be stable at temperature as high as 45 °C, but the stability decreased proportionally between 45 and 70 °C. Sodium chloride (20% w/w) was an effective stabilizing agent for PC, and its efficacy was higher at higher concentrations used [158].
Consistently to other studies, at temperature of 74 °C the degradation of PC was increased (t½ 9.7 min, pH 6.0) [159]. However, stability of PC can vary significantly within the species studied. A different thermostability was found between the extracts of the cyanobacterium A. platensis and the polyextremophile G. sulphuraria (Rhodophyta) incubated at temperatures between 25 and 55 °C. In this Rhodophyta the PC absorbance remained stable (95% of initial value) until 55 °C and decreased steadily within 55 and 75 °C, down to 39% of the initial value. In the case of A. platensis, PC extracts started to degrade already at 45 °C losing 18% of absorbance at 620 nm, while at 75 °C the absorbance was reduced to only 20% of initial value [146]. Similar findings were also reported by [77], and by [160] who found that PC from G. sulphuraria was more stable than that from A. platensis under all conditions, especially in the range of 50–65 °C, and in neutral environment of pH 7. After 30 min incubation at 60 °C and pH 7, the color preservation rate of PC from G. sulphuraria and A. platensis were 86.66% and 60.83%, respectively.
The higher thermostability showed by the PC extracted from G. sulphuraria is likely the result of a long adaptation process of the organism to the extreme environment (temperature up to 57 °C) where it still thrives. In conclusion, temperatures lower than 45 °C are generally considered optimal to preserve PC stability.
1.5.2 Effect of pH
The pH of the PC extract is another environmental factor that can destabilize the pigment. The pH of the solution can also modify the color of the PC solution. At neutral pH, the PC color is perceived as blue, while at acidic pH it is green. The optimal pH range for PC was found to be between 5.0 and 6.0 [158]. Usually, acid compounds are added to beverages to provide tartness and tangy taste to balance the sweetness of sugar added to beverages. It was reported that Arthrospira extracts lost 60% of the absorbance at 620 nm moving from pH 5.5 to 3.5, while within the same range of pH, Galdieria extract maintained 100% of the absorbance. Thereafter, the stability declined in a linear manner reaching about 30% of the initial value at pH 1.5. [146] As said above, this significantly greater tolerance to high acidity showed by G. sulphuraria PC extract is probably the result of a long-term adaptation of this organism to extremely acidic environments, with pH as low as 0.2.
1.5.3 Effect of light
Several studies have assessed the degradation kinetics of aqueous solutions of PC evaluating temperature or light as accelerating factors using a first order kinetic model, and both environmental factors have been studied separately to evaluate the effect [158, 161]. Pérez-Rico et al. [162], have developed an empirical model able to predict the effect of temperature and light combined in the degradation ratio of this pigment at selected storage condition. They reported that exposure of PC to a photon flux density of 50 and 100 µmol m−2 s−1 resulted in a decrease in the concentration according to a dose-dependent pattern. The light-induced degradation resulted dependent on pH; at pH 6, the pigment degradation was lower compared to pH 5 and pH 7.0. The final protein concentration declined by 20% after continuous exposure to a photon flux density of 100 µmol m−2 s−1 for 36 h, independently of the pH of the solution. Therefore, to preserve PC, the best storage condition is in the dark.
1.5.4 Uses of preservatives
Since degradation of the protein structure of PC strongly affects its color and bioactivity, preservatives are added to ensure a longer shelf life of the product [[155] and references therein]. Both the chemical composition and the preservative concentration are relevant aspects since the resulting mixture must not affect the human health. The most used preservatives are mono or di-saccharide (glucose, fructose, saccharose, lactose, maltose) and the polyol sorbitol [71, 154], organic acids (citric, ascorbic, benzoic), and sodium chloride and calcium chloride among inorganic salts. Among the acidic preservatives, citric acid performs better. Indeed, with citric acid, 67% of the PC remained stable after 45 days compared to less 3% for the control [163]. This result is explained by both the decrease of pH of the solution caused by the addition of citric acid, and by its chelating property.
1.6 Market
The commercial value of PC is strongly dependent on its purity grade. The commercial price of PC as a colorant in food and cosmetics industries (the largest market) is about 0.35 US$ and 135 US$ per gram respectively, and can reach 4600 US$ per gram for therapeutic and diagnostic applications [169].
Figure 7 reports the production volume (tons) forecasted within the period 2020–2027 [22]. By 2027 the total volume of PC production is expected to raise 7.2-fold in Middle East & African countries, to a maximum near ninefold for European countries. During the same period, the calculated CAGR (compound annual growth rate) is expected to range within about 33% for Middle East & Africa countries to near 41% for European Union (Fig. 7 insert).
Production volume of phycocyanin by countries. AP, Asia–Pacific; NA, North America; EU, European Union; MEA, Middle East & Africa. Insert shows the CAGR (%) calculated within the period 2000–2027. Data source [21] adapted
The increasing demand of powder is due to its use as alternative for synthetic color in food and beverages, being PC preferred by consumers attracted by organic and natural ingredient-based food products, and by regulations which are posing increasing constraints to the use of synthetic colors. Western Europe is the biggest consumer of this product (around 33%) and that 80% of PC produced is used in the food industry. Pharmaceutical, is another promising sector which will likely see a sizeable increase by 2030. In 2020, PC turnover accounted for more than $12 million, and it’s expected to rise to 36 million $ with a CAGR of 10%. Other than the generally recognized antioxidant properties attributed to PC, its use for cancer diagnosis generates further demand in pharmaceutical industry. By 2030 the turnover of the global PC market is expected to exceed $400 Million with an estimate CAGR of 9.6% within 2021–2030 [164].
Figure 8 shows the share of PC among food, cosmetic and analytical/reactive recorded in 2020. It can be seen, the largest part of PC is destinated to food, while the part used for pharmaceutical purposes, is incomparably lower (only 4.4 kg). Yet, when the comparison is made based on market value, the analytical-reactive grade surpasses that of cosmetic, owing to its much higher price.
Comparison of the different PC sectors and their respective market value. Due to its very low production size (4.4 kg), the analytical/reactive grade market volume cannot be appreciated (A/R = analytical/reactive grade). Data source [21], adapted
The key players of the PC market include Earthrise Nutritionals LLC, Bluetec Naturals Co., DDW Inc., DIC Corporation, Japan Algae Co. Ltd., Phyco-Biotech Laboratories, Cyanotech corp, Parry Nutraceuticals Qingdao ZolanBio Co. Ltd., Sigma-Aldrich Corporation, and Yunnan Green A Biological Project Co. Ltd, BueBiotech int. GmbH, Algosource.
1.6.1 Concluding remarks
PBPs are valuable, safe and healthy bio-compounds with many bio-technological applications and an increasing global market. However, their large-scale production is still restrained by the complexity and high cost of the available extraction and purification methods. In fact, PBPs extraction and purification procedures usually involve numerous steps, which reduce product yield and increase the costs, impairing the exploitation at a large scale. Most of the proposed procedures are often time-consuming, and can affect the quality of the final product, since PBPs are photo- and temperature-sensitive.
A few published works have addressed these critical issues. For example, proposed procedures are characterized by a minimum number of steps so as to reduce the production time and cost. Generally, in those studies, the ordinary approach characterized by two distinct phases (extraction followed by purification) was avoided. For example, stirred fluidized bed chromatography [63, 165], aqueous two-phase systems [166], glycerol-based natural deep eutectic solvents [167], three-dimensional printed anion exchange monoliths [168] and three phase partitioning processes [88, 97] have been successful applied directly on cyanobacteria or algae biomass, obtaining crude extracts of commercial interest, having good purity grade. Recently, an innovative extraction/purification method, based on ultrasound cell lysis in ammonium sulphate solution, was exploited to obtain PC from fresh A. platensis biomass [169]. In this process PBPs extraction was decoupled from biomass cell lysis, and the pigment recovered in a subsequent extraction step, obtaining a crude PC extract of elevated purity grade and concentration. However, the economic feasibility of this procedure should be proved on an industrial scale. New PC microalgal strains need to be isolated and selected from nature. An example is represented by the Rhodophyta G. sulphuraria which has recently attracted the attention of researchers owing to the large metabolic flexibility, and its capacity to grow under prohibitive conditions for the majority of other PC producers. However, the production cost with other PC producers, such as Arthrospira need to be addressed.
2 Primary carotenoids in microalgae
2.1 Metabolic pathways of carotenoids synthesis in microalgae
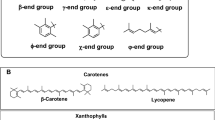
Carotenoids exist in a large variety of colors ranging from yellow to orange, red and purple. They are composed of eight isoprene units with a 40-carbon skeleton, generally consisting of a polyene chain with nine conjugated double bonds and an end group at both ends of the polyene chain. The five-carbon (C5) ubiquitous precursor metabolites isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) are necessary to produce carotenoids. Two distinct routes, the MEP (methylerythritol phosphate) pathway and the MVA (mevalonate) pathway, contribute in the synthesis of these compounds in biological systems [170].
The biosynthetic pathway of carotenoids is reported in Fig. 9. Isopentenyl pyrophosphate (IPP, C5) and its isomer dimethylallyl diphosphate (DMAPP, C5) are converted at a ratio of 3:1 to geranylgeranyl pyrophosphate (GGPP, C20) via the methylerythritol phosphate pathway (MEP) in the chloroplast. In a further reaction, phytoene synthase condenses two molecules of GGPPS into (15Z)-phytoene (C40), which is then desaturated and isomerized into lycopene via phytoene desaturases, ζ-carotene isomerase, ζ-carotene desaturase, and carotene isomerase [171].
Scheme of carotenoid synthesis pathways in microalgae. The microalgae Dunaliella sp. (red border, diameter: 9–11 μm), Nannochloropsis sp. (green border, diameter: 2–4 μm), Scenedesmus (yellow border, 11–18 μm long, 3.5–7 μm wide) and Chlorella sp. (orange border, diameter 2–10 μm) are presented as principal producers of β-carotene, violaxanthin, lutein, and lycopene, respectively. Dashed lines indicate still unclear pathways
The initial step of carotenogenesis, which enables the creation of both α-carotene and β-carotene in algae, involves lycopene. A crucial branching point is the determination of the proportion of lutein coming from α-carotenoids, and β-carotene, coming from β-carotenoids. Zeaxanthin, which can derive from β-carotene, is epoxidized to violaxanthin, via antheraxanthin (xanthophyll cycle of violaxanthin). The other xanthophyll derived from violaxanthin are: (i) neoxanthin, by a violaxanthin de-epoxidase-like enzyme recently reported [172], which can be converted to dinoxanthin, which, in turns, generates vaucheriaxanthin and peridin, (ii) diadinoxanthin, that can be de-epoxidated to diatoxanthin (diadinoxanthin cycle). Both diadinoxanthin and dinoxanthin can be converted into fucoxanthin, by an enzymatic pathway still unclear.
Carotenoids may be divided into two classes based on their chemical composition: carotenes and xanthophylls. While xanthophylls, which include β-cryptoxanthin, lutein, zeaxanthin, astaxanthin, fucoxanthin, neoxanthin, and peridinin, are carotenoids with oxygen atoms, carotenes, such as lycopene, α-carotene, β-carotene and γ-carotene, lack oxygen.
Carotenoids are divided into primary and secondary. The major carotenoids, such as certain xanthophylls and ß-carotene, are often found in the chloroplast and are directly involved in photosynthesis due to their function in the collection of light energy.
Under stress conditions (nutrient deficiency, high light, salt stress, high/low temperature), the photosynthetic system would not be able to effectively use the absorbed light energy, and the dissipation process might be activated. Additionally, a consistent supply of secondary carotenoids can be developed to serve as a free radical and reactive oxygen species quencher.
2.2 Main microalgal strains high producer of carotenoids
When microalgal cells receive a signal of high reductive state level inside the cells, specific actions are started to dissipate the accumulated electrons. These methods entail the synthesis of antioxidant carotenoids such as lutein, the xanthophyll cycle pigments violaxanthin, antheraxanthin, and zeaxanthin, as well as loroxanthin and fucoxanthin, which are largely found in the marine microalgae Phaeodactylum and Isochrysis [173,174,175].
The xanthophyll cycle is activated by the thylakoid lumen acidification, with the activation of the enzyme violaxanthin-de-epoxidase, leading to the synthesis of zeaxanthin, via antheraxanthin [176, 177]. In diatoms and dinoflagellate, such as Phaeodactylum and Isochrysis, respectively [173] an additional xanthophyll cycle can be found, consisting in diadinoxanthin, which can be de-epoxidized to diatoxanthin [178]. Thanks to the induction of these cycles, the reduction of the singlet oxygen inside the cell is activated, and the cellular damage can be avoided or reduced. The carotenoids production of some of the most studied and productive microalgal strains is reported in Table 4.
Haematococcus pluvialis, Chlorella species, Dunaliella salina, Scenedesmus species, Botryococcus braunii, Nannochloropsis species, Coelastrella striolata, Chlorococcum species, and some diatoms are known for producing β-carotene, lutein, canthaxanthin, astaxanthin, and fucoxanthin among the microalgae [171, 185, 195, 196].
The green microalga Chlorella vulgaris is well-known for its use as supplemental nutrition and for having a high protein and chlorophyll content in its biomass. A significant quantity of lutein, β -carotene, and zeaxanthin (1.18 mg g−1, 0.31 mg g−1, and 0.24 mg g−1, respectively) with good bioavailability have also been recorded for this microalga [197]. Interesting results have been reported in Chlorella strains for the production of lutein, as primary carotenoids, under heterotrophic conditions [198]. In particular, up to 5.3 mg g−1 of lutein has been extracted from heterotrophic culture, and around 7.5 mg g−1 in mixotrophic cultivation [199, 200]. Lutein production and choice of culture medium may depend on the strain and the cultivation conditions: for C. protothecoides and C. pyrenoidosa, under heterotrophic conditions, glucose is preferred, while under phototrophic conditions the highest lutein increase is obtained with acetate.
Dunaliella salina, a green unicellular microalga that primarily produces β-carotene, may also be regarded as a strong carotenoid producer. In this microalga, the pigment begins to build up in lipid structures on one side of the cell, and eventually it fills the entire cell.
It is possible to improve the synthesis of carotenoids in the green microalga Asterarcys quadricellulare, a potential good producer of carotenoids, by regulating the medium composition, pH, salinity, light quality, intensity, and duration [201]. The amount of β-carotene, lutein, astaxanthin, and canthaxanthin it can generate per dry gram of biomass is 47.0, 28.7, 15.5 and 14.0, respectively.
Scenedesmus can produce significant levels of lutein, which turns cells from green to yellow, and is a significant source of antioxidant carotenoids. A strain of S. obliquus isolated from Kapulukaya Reservoir (Kırıkkale, Turkey) has been found to produce a considerable amount of carotenoids, mainly found as -trans forms of lutein, β-carotene, α carotene, zeaxanthin, cis-neoxanthin, 9-or 9’-cis-α-carotene, 13-or 13’-cis-lutein, 13-or 13’-cis-β-Carotene, 9-or 9’-cis-lutein, cis-lutein, 9-or 9’-cis-β-Carotene, high amount of carotenoids, among them, the most abundant were all-trans isomers of lutein and β-carotene, being 83.74% (2.52 mg g−1) in total carotenoids [202]. These results are interesting since they demonstrate that there is a large variety of microalgae that may be considered a valuable source of natural chemicals.
2.3 Main environmental factors affecting the production of primary carotenoids
The over-reduction of the photosynthetic chain and the production of free radicals are caused by variables that have an impact on growth and photosynthetic efficiency. Optimal nutrient composition, and optimal values of light intensity, pH, salinity and temperature, are some of the parameters that vary according to the strain, specie, and origin. Among these parameters, a crucial one is light intensity. The photosynthetic system has various physiological restrictions that prevent it from utilizing light irradiation above a certain level of intensity. Despite being a useful method for accumulating antioxidant compounds, inducing the synthesis of carotenoids through exposure to stress conditions like high light, nutrient limitation-starvation, excessive low or high temperature, or salinity stress is not suitable in terms of biomass productivity, as in these circumstances growth is severely impacted.
2.3.1 Light intensity
The microalga Chlamydomonas reinhardtii, which is regarded as a model organism for physiological and biochemical investigations on photosynthesis, has been used extensively in research in this respect. In this microalga, zeaxanthin production was triggered within 10 min of exposure to 800 mol photons m−2 s−1, while a partial induction of the cycle was seen at 300–350 mol photons m−2 s−1 [177, 203, 204].
With the activation of the other xanthophyll cycle, the synthesis of diatoxanthin by the de-epoxidation of diadinoxanthin, microalgal cells promptly react to excessive light intensities. In P. tricornutum, the conversion of diadinoxanthin to diatoxanthin is very quick, as it can happen in outdoor cultures exposed to direct sunlight in as little as 15 min.
Lutein is among the powerful antioxidant carotenoid reported to be enhanced in high light conditions. As a main carotenoid, this pigment is naturally present in most photosynthetic cells, and under conditions of photo-oxidative stress, its production may rise. Under conditions of 1900 mol photons m−2 s−1 and 35 °C, the microalga Scenedesmus almeriensis in a tubular photobioreactor outdoors produced extremely high levels of lutein (around 5 mg m−2 d−1) [205]. The simultaneous presence of high light and heat was the cause of this increase. In fact, this microalgal strain typically thrives best at temperatures between 25 and 28 °C.
Another key carotenoid that often rises in content with increased light exposure is β-carotene, which is naturally present in microalgal cells but may be over-synthesized in certain conditions.
Among the microalgae with a reputation for being a good producer of this molecule, surely Dunaliella salina is one of the most promising. In a lab setting, with a light intensity of 200–1200 mol photons m−2 s−1, at 30 °C, a production rate of 13.5 mg −1 d−1 has been reported [206].
Studies on a strain of Tetraselmis sp. showed that under a light intensity of 170 µmol photons m−2 s−1, the lutein and β-carotene content increased to 3.17 and 3.21 mg g−1 of dry weight, respectively [207]. In addition to the light stress, this strain was subjected to the quite high temperature of 30 °C, revealing a thermotolerance that is a suitable characteristic for cultivation under extreme conditions.
2.3.2 Nutrient limitation or starvation
Sulfur is essential for maintaining protein synthesis, hence its lack or restriction results in a sharp decrease in protein content, which in turn affects photosynthetic activity and slows development. In closed photobioreactors, the drop in photosynthetic oxygen evolution and the presence of oxygen respiration may result in the formation of extremely low oxygen contents or even anaerobic conditions, a very strong reductive environment [208]. The microalga C. reinhardtii has been the subject of many investigations for its capacity to produce hydrogen in the absence of sulfur. Interestingly, and the xanthophyll cycle was strongly induced with a significant increment in antheraxanthin, zeaxanthin, and violaxanthin levels. The amounts of the other xanthophylls, lutein and β-carotene, also rose [177]. Additionally, anaerobiosis also induced the same reaction over a longer period but in a complete medium (5 h, instead of some minutes). Antheraxanthin and zeaxanthin dropped after aerobic conditions had been achieved, along with lutein and β-carotene, suggesting the occurrence of a recovery.
The xanthophyll cycle is induced in Nannochloropsis gaditana by a sulfur restriction or deprivation, with an increase in violaxanthin and zeaxanthin as was seen in the case of nitrogen [209].
Since nitrogen is an essential component of proteins and enzymes, the lack of this nutrient in the culture medium strongly affects both the growth and biomass composition. Dunaliella's ability to produce β-carotene under conditions of intense light exposure is increased by nitrogen deprivation. The combination of nitrogen starvation and exposure to the light intensity of 200 mol photons m−2 s−1 induced an increment in β-carotene to reach levels of 2.7% of the biomass [210].
Under both severe light stress and nitrogen deficiency, the increase of this pigment has been linked to the production of total fatty acids [211]. The production of this carotenoid and the fatty acid biosynthetic pathway are strongly related, which may be explained by the fact that β-carotene is stored in lipid globules. This element is quite intriguing and demonstrates how the metabolic pathways in microalgae are closely linked to provide an effective and efficient response to stressors.
The xanthophyll cycle is induced in Nannochloropsis gaditana by nitrogen restriction or deprivation, which results in an increase in violaxanthin and zeaxanthin. In [209] the cells underwent sulfur limitation or starvation after cultivation on complete medium to produce a sizeable quantity of biomass. In this instance, xanthophyll pigments were enhanced in the biomass. Given that this pigment participates in the formation of zeaxanthin via antheraxanthin, the violaxanthin rise was less pronounced. The high induction of carotenoids synthesis is signaled by its increase, which points to violaxanthin's de-novo synthesis.
Phosphorus has a crucial role in the cellular composition of DNA, RNA, and phospholipids as well as the control of enzyme activity, metabolic pathways, and ATP synthesis. It can be provided as polyphosphate or orthophosphate, and its inclusion in the medium composition ensures that photosynthetic activity will be effective [212].
The xanthophyll cycle is induced in Nannochloropsis gaditana by phosphorus restriction or deprivation, with an increase in violaxanthin and zeaxanthin, as shown in the case of nitrogen [209].
2.3.3 Salt stress
Salt stress is one of the most significant abiotic variables influencing the development and productivity of microalgae. Some microalgae have developed saline environment adaptation methods, and in these unfavorable environments, they may control the creation of osmoregulatory chemicals that can assist the cells in dealing with stress. Particularly, osmotic stress can cause a rise in the concentration of various polar lipids, such as glycolipids and phospholipids, in chloroplasts and cellular membranes. As previously mentioned, several carotenoids depend on lipid production for their placement, and frequently, lipid production is closely related to the synthesis of carotenoids.
In fact, an excess of the ions Na+ and Cl− reduces the osmotic potential of the environment, which in turn reduces water intake, resulting in a wide range of bioenergetic and biochemical alterations in photosynthetic species under salt stress. The effects of salt stress on microalgal cells result in modifications and effects at several levels, including changes in membrane permeability, ion homeostasis blockade, increased catabolism of lipids and biopolymers, and altered yield of energy-producing activities.
Salt stress can be used as a strategy to encourage the synthesis of carotenoids, since like other stressors it may have a significant influence on the growth rate of microalgal cultures and also lower photosynthetic activity. An increase in NaCl concentration inhibited the growth of Chlorella zofingiensis while the quantity of carotenoids was enhanced [213].
The effect on lutein production in C. vulgaris has been reported by [214]. The results showed that using different levels of salinity stress, the highest increase of lutein content (11.36 mg g−1) was induced by a salt concentration of 35 ppt, the highest tested. The influence of high light intensity and salt stress was investigated also in Scenedesmus sp. by [215]. The authors reported the production of 6.45 mg g−1 of lutein, with NaCl 156 mmol L−1, under exposure to 160 µmol photons m−2 s −1. In a strain of Scenedesmus grown on 10 g L−1 of NaCl the increase of carotenoids content was observed, particularly the xanthophyll component [216].
According to [217] salt stress in the range of 0.34 M to 0.51 M caused a decline in the growth rate in C. vulgaris. This decline may be related to an imbalance in ion homeostasis, which reduces photosynthetic activity, affects light utilization and the metabolism of carbohydrates, which is involved in osmotic regulation, and leads to the accumulation of reactive oxygen species. As with nitrogen, sulfur, or phosphorus restriction, this is thought to be a catalyst for the induction of carotenoids synthesis, therefore in this instance, the poor biomass productivity can also be linked to a high carotenoids production.
2.4 Extraction of carotenoids from microalgal cells
Conventional solvent extraction techniques using organic solvents are used to obtain carotenoids from microalgae. The polarity, solubility, and chemical stability of the carotenoids to be extracted determine whether conventional extraction should be carried out using organic or aqueous solvents.
Non-polar solvents (n-hexane, dichloromethane, dimethyl ether, diethyl ether) or polar solvents (acetone, methanol, ethanol, biphasic combinations of various organic solvents) can be utilized according to the polarity of the target carotenoid,
For the recovery of carotenoids from microalgae, the use of green solvents (environmentally safe and non-toxic solvents) like ethanol, limonene, and biphasic mixes of water and organic solvents has been examined. Due to the high cost of manufacturing and extensive solvent use, the economic feasibility of carotenoid extraction from microalgal species is currently low.
Thus, interest in the use of unconventional extraction techniques has grown recently. These unconventional extraction techniques have quick extraction times, little solvent usage, improved recovery, and more selectivity, among other benefits [218,219,220,221,222,223,224,225,226]. The most used extraction methods are briefly described.
2.4.1 Microwave-assisted extraction (MAE)
Due to the presence of algaenan and sporopollenin in their cell walls, breaking down microalgal cells can be a challenging task [224]. Additionally, the efficiency of traditional cell disruption and extraction methods is poor. When microwave radiation is used at a frequency close to 2.45 GHz, polar molecules vibrate, resulting in inter- and intramolecular friction.
Two marine microalgae, Dunaliella tertiolecta (Chlorophyta) and Cylindrotheca closterium (Bacillariophyta), were subjected to microwave irradiation for the extraction of pigments. [219] examined the effectiveness of this method in comparison to traditional methods. All techniques used on D. tertiolecta produced quick pigment extraction. The optimum extraction method for C. closterium pigments was determined to be MAE, bringing benefits in speed, repeatability, homogenous heating, and high extraction yields.
2.4.2 Ultrasound-assisted extraction (UAE)
UAE is based on ultrasound cavitation. Ultrasound waves of high strength and low frequency are used to perform ultrasonic extraction. Low intensity-high frequency (100 kHz–1 MHz) and high intensity-low frequency (> 1 MHz) ultrasound can be distinguished from one another (20–100 kHz). UAE is affordable, it dramatically shortens the extraction time, and increases extraction yields.
4.66 mg of β-carotene per g of dry weight was extracted using ultrasonic assistance from the microalga Arthrospira platensis [38]. For enhanced extraction, several parameters (amplitude, duty cycle, sonication period, and depth of horn immersed in solution) were tuned.
The ideal parameters for the greatest amount of β-carotene extraction from this alga were 80% amplitude, 33% duty cycle, 0.5 cm of horn depth in the solution, and 10 min of ultrasonication. The extraction of lutein, β -carotene, and α-carotene from Chlorella vulgaris has also been carried out using UAE [227]. The highest extraction levels were, respectively, 4.844 ± 0.780, 0.258 ± 0.020, and 0.275 ± 0.040 mg g−1 of dry weight biomass.
2.4.3 Subcritical fluid extraction
Subcritical fluid extraction uses liquefied subcritical fluids as the extraction solvent. Compared to supercritical fluid extraction, subcritical fluid extraction operates at comparatively modest temperatures and pressures. Moreover, using ethanol modified subcritical 1,1,1,2-tetrafluoroethane, the carotenoids and chlorophyll-a can be efficiently extracted [170].
2.4.4 Electrotechnologies-assisted extraction
Electrotechnologies including pulsed electric field (PEF), moderate electric field (MEF), and high-voltage electric discharges (HVED), are other non-thermal and environmentally friendly methods of extraction which target intracellular chemicals from bio-suspensions.
Carotenoids extracted from Chlorella vulgaris with pulses of milliseconds (5 kV/cm–40 ms) or microseconds (20 kV/cm–75 s) were extracted with an 80% greater efficiency. Up to 73% of carotenoid extraction was achieved in the microalgae Heterochlorella luteoviridis when MEF and ethanol were used as the solvent (180 V, 75 mL/100 mL of ethanol solution) [228].
2.4.5 Pressurized liquid extraction (PLE)
The primary benefit of employing PLE is that it enables quick extraction while consuming less solvent. In PLE, solvents are extracted under pressure and temperature conditions that are always below their critical points.
Carotenoids have been extracted using PLE from freeze-dried macro- and microalgal biomass. Pressurized liquid extractions produced extraordinary levels of extraction of fucoxanthin in Phaeodactylum tricornutum, up to 26.1 mg g−1 DW [229]. In the instance of Neochloris oleoabundans, pressurized liquid extraction was used to recover the bioactive carotenoids lutein, carotenoid monoesters, and violaxanthin [230].
In comparison to maceration, soxhlet extraction, and ultrasound-assisted extraction, pressurized liquid extraction (PLE) demonstrated greater extraction efficiency for the extraction of carotenoids and chlorophylls from the green microalga Chlorella vulgaris (UAE) [231].
2.4.6 High pressure homogenization (HPH) treatment and enzyme-assisted extraction
Since microalgal cells are not easy to disrupt, recovering carotenoids can be aided by a physical or enzymatic pre-treatment before extraction. One such approach is HPH, which uses high intensity fluid stress to cause cell breakdown (50–400 MPa). It has important advantages over conventional physical milling methods, including simplicity of use, commercial viability, repeatability, and high throughput [170].
2.4.7 Supercritical fluid extraction (SFE)
SFE includes extraction utilizing supercritical fluids, or fluids at a temperature and pressure above their critical limit. Due to their low viscosity and high diffusivity, supercritical fluids have better solvating and transport qualities than liquids.
SFE was used to produce the maximum carotenoid output in Scenedesmus obliquus at 250 bar and 60 °C [232].
2.5 Purification of carotenoids
To ensure that the carotenoids maintain their qualities, when they reach the consumer, further methods are required after the process of extracting the carotenoids. These strategies include purifying the compounds of interest, removing any leftover cell debris, and preserving the molecules [233]. Carotenoids obtained from microalgae are as pure as those found in other natural sources. The Willstatter technique is the foundation of the traditional approach for purifying carotenoids [180].
To prevent product degradation, this purification method uses salts (NaOH or KOH) as saponification agents at low temperatures (below 60 °C), followed by organic solvents (such as hexane or blends of ethanol, water, and dichloromethane) that will later be removed in order to obtain the desired carotenoid products. This time-consuming method of purification is presently being superseded by several chromatographic methods that have been compiled and thoroughly discussed in numerous publications [234, 235].
After the carotenoids have been extracted and purified, air, light, or heat may cause the final products to degrade [180]. The stability of standard solutions of carotenoids, such as lutein, lycopene, zeaxanthin, α and β-carotene, β-cryptoxanthin, and zeaxanthin, was examined after the extraction. With the exception of lycopene, these studies found that the carotenoid standard solutions (0.05–5 g mL−1) did not degrade and could be kept at − 70 °C for 6 months [236,237,238].
2.6 Application of some most representative primary carotenoids
Carotenoids are in very high demand in the healthcare and pharmaceutical industries because they are pro-vitamin A and have potent antioxidant properties (Table 5).
As an example, regular consumption of 9-(Z) neoxanthin may reduce the chance of developing lung cancer [241].
Violaxanthin and its derivatives have been shown to have potent lipid peroxidation inhibition characteristics and are reported in the literature to have antioxidant effects [242]. Additionally, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azobis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical tests were used to demonstrate the radical scavenger properties of violaxanthin derived from the microalgae Eustigmatos cf. polyphem [176].
In ophthalmological areas, zeaxanthin plays a large and vital function. It shields the visual organ from near UV rays and reduces the risk of age-related macular degeneration, and is naturally found in the central macula of the eye [243]. Its potential as a natural dye indicates its use in food, as antioxidant in cosmetics, and in the coloring of fish and fowl [244]. Zeaxanthin has been reported to be beneficial in treating cardiovascular issues and may help diabetic individuals from developing pancreatic and lung cancer [245]. This xanthophyll is helpful in adjuvant therapy because it can lead melanoma cells to apoptosis.
Antioxidant and photo-protective qualities are present in lycopene. It exhibits anti-cancerous properties in human colon, breast, prostate, liver, and lymphocyte cell lines [246]. It combats high blood pressure issues and early arthrosclerosis by enhancing endothelial function and reducing oxidative stress. Lycopene maintains blood cholesterol levels by acting as a hypolipidemic in a manner similar to statins [247]. Daily dosages of lycopene strengthen the skeletal system, improve intercellular connections of gap junctions, and maintain glucose homeostasis. According to [246], lycopene-induced tumor metastasis was inhibited as a result of regulated cell cycle progressions.
An in vivo study in high cholesterol-fed rats found that algal lycopene obtained from Chlorella marina displayed stronger antioxidant and anti-inflammatory effects compared to the lovastatin and trans-lycopene derived from tomatoes.
Although, at the industrial level, marigold is the primary source of lutein production, many microalgal strains are able to produce a considerable amount of lutein [248]. A crucial aspect to be considered is that, while lutein from marigolds is esterified, it is found in free forms in microalgae, which are more easily absorbed than esterified forms [249], with various benefits for human health: antioxidant, light-filtering and anti-inflammatory activity, eye protection, potential therapeutic benefits for many chronic diseases, lower risk of cancer.
The antioxidant lutein can protect eyes, brain, and skin from damage. Like zeaxanthin, it shields the eyes from harmful UV and blue light, earning the moniker "eye vitamin." It protects against age-related macular degeneration and cataract by lowering the amount of plasma factor D. It is also effective as a chemotherapeutic agent [250]. It is a chemical that actively fights prostate, breast, and colon cancers. Consuming lutein can also reduce the risk of developing lung cancer and early atherosclerosis. It lessens the consequences of neurodegenerative diseases brought on by inflammation. It is extensively used in chicken as a feather colorant, a yolk color enhancer, and as an additive in baby food, drugs, and cosmetics.
It is widely known that β-carotene has anti-inflammatory, antioxidant, immune, dermo-, hepato-, and retino-protective properties [251]. This pigment is widely used in the cosmetic and food coloring industries [241]. It has been claimed that this pigment plays a protective function from malignancies of the breast, colon, lungs, liver, and skin.
Apoptosis, cell differentiation, and proliferation may all be maintained by β-carotene, which can also improve gap junction intercellular communications and lessen the detrimental effects of H2O2 on them [244]. Its frequent inclusion in the diet guards against liver fibrosis and night blindness.
2.7 Market
The global market for carotenoids was estimated to be worth $1.24 billion in 2016 and $1.53 billion in 2021 [252]. Their worldwide economic value is determined by consumer demand and the cost of extraction.
Feed, food, and beverage pharmaceutical, cosmetic, aquaculture, and nutraceutical industries are significant carotenoid market segments [253]. At global level, a market of 1.5 billion USD was predicted; however, due to strong demand, it is anticipated to increase to 2 billion USD by 2022, with a compound annual growth rate (CAGR) of 5.7%. Due to the variety of uses, β-carotene, lutein, fucoxanthin, and zeaxanthin dominate the market. Currently, the main commercial source of lutein is marigold flowers, however, microalgae biomass contains 3 to 6 times as much lutein per unit of dry matter. As reported by [170], β-carotene accounts for 246.2 million USD (17% of total), lutein for 225 million USD (15.6% of total), lycopene for 107 million USD (7.4% of total), according to a report by BCC Research in the context of the global carotenoid market.
Because of its demand, it has been estimated that by 2022, the market value of lutein will increase to 357.7 million USD, from 263.8 million US dollars in 2017 (6.3% CAGR from 2016). Instead, the price of β-carotene on the market has been evaluated around 300–1500 USD/kg, depending on demand and carotenoid purity [254].
By 2023, the feed sector will reach the biggest market share within the global carotenoid market, due to fewer restrictive regulations and also considering the growth in the production of meat, poultry, and dairy products that will hasten the expansion of the feed business.
Concerning the carotenoid production on a large scale in European countries, Ambati et al. [252] explained that the expansion of the European carotenoid market may benefit from the investment and participation of well-known cosmetic companies as Hindustan Unilever, L' Oreal, Henkel, and Beiersdorf.
The bottleneck for a sustainable and economically advantageous carotenoids production with microalgae consists in the lack of raw materials, effective instruments, and techniques for the upstream (extraction) and downstream (purification) processes all contribute to high monetary value [255]. Moreover, the market for carotenoids is constrained by laws and tight regulations relating to health care, the environment, and animals. However, due to the increasing demand for natural goods and the depletion of conventional resources, major corporations are investing heavily in carotenoid manufacturing to increase their competitiveness in the carotenoid market. In Table 6 some of the main companies involved in carotenoids commercialization and production are reported.
3 Microalgae as fucoxanthin source
More than one billion people around the world will be living with obesity by 2030 [256]. The reasons for this disastrous trend are attributed to the global tendency towards the reduction in exercise and physical activity and the increased dietary intake of fats, sugars and calories. To prevent life-style-related diseases, like metabolic syndrome (MS), researchers are focusing on many functional ingredients in foods which may be useful for the prevention and treatment of life-style-related diseases [257], among these Fucoxanthin (hereinafter shortened as Fx), a characteristic carotenoid present in edible brown seaweeds and several species of microalgae. Fx is the major carotenoid pigment in marine ecosystem, representing more than 10% of total carotenoid production [258]. This yellow-orange pigment is produced by an algae group composed of brown and golden-brown macro and microalgae such as diatoms, haptophytes, dinophytes and brown seaweeds. Fx masks the color of chlorophyll and gives the characteristic brown color to the algae. In diatoms, it was proven that Fx enables the absorption of photons in a wide blue-green spectral range of visible light, which is the most available range in a water column [259]. Indeed, there is a lack of red light availability for chlorophylls at certain depths as only blue light can reach further to the cells. Therefore, along with light harvesting by chlorophylls, Fx increases the efficiency in using different light wavelength for carbon fixation.
It is widely reported in literature that Fx has various potential benefits for human health [260]. This paragraph aims to give an overall view, although not exhaustive, on microalgae-derived Fx in terms of the main species as sources of Fx, their cultivation strategies and conditions favoring Fx accumulation, the bioactivities of the pigment, and its potential application in farma-, nutra-, and cosmeceutical industries.
3.1 Structural characteristics of fucoxanthin
Fx, whose molecular structure was fully described in [261], is part of the carotenoid family under the denomination of xanthophylls, whose chemical structure is distinct from the second carotenoid family, the carotenes, as only the former contain oxygen. Moreover, the oxygenic functional groups consisting of epoxy, hydroxyl, carboxyl, and carbonyl groups, exceptionally express the superiority of Fx over other carotenoids. This particularity, along with an epoxide group and a conjugated carbonyl group, confer anti-oxidant properties to xanthophylls, which makes them very demanded in cosmetics and pharmacology [262]. The diverse health-promoting effects [263,264,265,266] of Fx are attributed to its unique chemical structure, presenting an acetyl group, an allenic bond, and a conjugated carbonyl, along with 5,6-monoepoxide (Fig. 10).
As other carotenoids, Fx is highly unsaturated, thus very reactive and sensitive to oxidation, readily vulnerable to a high number of factors, including light, heat, oxygen, enzymes, unsaturated lipids, and pro-oxidant molecules. Fx possesses four isomers, all-trans Fx, which is the most represented isomer in the natural environment, 9’-cis Fx, 13-cis Fx and 13’-cis Fx (Fig. 11). The trans-form of Fx is closely related to Fucoxanthin’s pharmacological activities, as evidenced by several studies. Degradation of all-trans isomers of Fx, with subsequent increase in 13-cis and 13′-cis isomers, was observed when the pigment is exposed to light and air or to high temperature [267]. According to Nakazawa et al. [268], cis forms of Fx demonstrated to have better antiproliferative effects against leukemia cells colon cancer cells. In a study conducted by Kawee-ai et al. [267] the antioxidant activity decreased with the increase of cis-isomers formation.
3.2 Producing species
Fucoxanthin is found in the Haptophyta, the Fx-containing Dinophyta, the Chrysophyta and in the Bacillariophyta. The latter two belong to a group referred to as the Heterokontophyta.
Currently Fx is produced mainly from the waste parts of brown seaweeds (macroalgae) such as Laminaria japonica, Eisenia bicyclis, Undaria pinnatifida, and Hizikia fusiformis. However, these macroalgae are mostly harvested for food in Asia and they contain very low concentrations of Fx [269], with content varying from 0.02 to 5 mg g−1 in fresh biomass and 0.01–2.1 mg g−1 in dried biomass [270]. Microalgae can be considered as a more promising source of Fx for commercial production, as the concentration of Fx in microalgae is much higher than in macroalgae, they are fast-growing microorganisms and the techniques of industrial production of microalgae are developing. Despite the abundance and diversity of Fx producing microalgae, only a few species were studied for commercial production of Fx [271, 272], diatoms and Haptophytes being the most promising groups [269].
The microalgae that have been characterized for Fx synthesis in the last decade are summarized in Table 7. The Fx content of most species ranges from 1 to 10 mg g−1 of dry biomass, but some elite species accumulate more than 20 mg g−1 of Fx. Of particular interest among them, Tisochrysis lutea accumulates up to 80 mg g−1 Fx in dry biomass, one the highest known [273]. The marine microalga Pavlova spp. (Haptophyta) is considered another good candidate: it has a satisfactory Fx content (20.9 mg g−1 in pilot scale) and a very thin cell wall, a favorable characteristic for Fx extraction [274]. Phaeodactylum tricornutum is a model diatom due to its high growth rate, ease of cultivation, availability of genome sequence and genetic manipulation, which has been used to enhance the synthesis of Fx [275]. Different strains of P. tricornutum exhibit various performances in Fx production with a Fx yield ranging from 2 to 60 mg g−1 under different culture conditions [276]. Cylindrotheca closterium is considered as a suitable candidate for Fx production due to its high Fx content (up to 25.5 mg g−1), resistance to grazers, relatively high growth rate and rapid sedimentation under static conditions [277]. Aside from the marine species, Fx can also be produced by freshwater species like Sellaphora minima (7.5 mg g−1), Nitzschia palea (5.5 mg g−1) [278] and Mallomonas sp. (26.6 mg g−1) [269].
3.3 Biological function and biosynthesis in microalgae
As a primary carotenoid, Fx is intricately organized with chlorophyll, antenna proteins and diadinoxanthin and diatoxanthin, forming a Fucoxanthin-chlorophyll a/c-protein (FCP) complex, a light harvesting complex which captures photons and transfers them to photosynthetic reaction centers [285, 286]. FCP complexes are embedded in the thylakoid membrane and associated with both photosystems II and I. Due to their particularity of absorbing in the blue-green spectrum, the light-harvesting complexes are capable of a wider light spectrum absorption thanks to Fx, between 390 and 580 nm [259]. This ability is useful and advantageous for algal cells when there is a lack of red light availability for chlorophylls at certain depths, where only blue light can reach further to the cells [259]. Several studies reported that diatoms contain different Fx molecules that according to their photon absorption capacity are classified as “Fx-blue” (λmax 463 nm), “Fx-green” (λmax 492 nm) and “Fx-red” (λmax 500–550 nm) [270].
Fx biosynthesis is part of the xanthophyll cycle, closely related to their photoprotection role. Biosynthesis of Fx is mainly regulated by light. The diatoms respond to variations in the quantity and quality of light, modulating the metabolic pathway of the photosynthetic pigments and thus changing their concentration. These changes in pigmentation lead to variations in photosynthetic responses as well as in microalgae growth rate [287].
The biosynthesis of Fx in microalgae is currently still being studied. A hypothetical Fx biosynthetic pathway proposed in P. tricornutum is reported in detail by Wang et al. [276]. The biosynthesis process model is comprised of the 2-C-methyl-erythritol 4-phosphate (MEP) pathway, along with formation of geranylgeranyl diphosphate (GGPP) and carotenoid synthesis. Xanthophyll cycle-involved pigments (zeaxanthin, antheraxanthin, and violaxanthin) are synthesized after the formation of β-carotene. The cycle is mediated by light intensity, i.e., under low light, zeaxanthin is converted into violaxanthin, via antheraxanthin, while under high light the process may be reverted by violaxanthin de-epoxidase (VDE). The transforming steps between violaxanthin and Fx have not yet been completely elucidated. Figure 12, shows two possible hypotheses. According to Lohr & Wilhelm [288], Fx is synthesized from violaxanthin via diadinoxanthin; on the other hand, Dambek et al. argue that violaxanthin is converted to neoxanthin, at which stage the metabolic flux diverges to produce Fx and diadinoxanthin separately [289]. In contrast with extraplastidial accumulation typical of other carotenoids, the overproduction of Fx taking place inside the plastids can require a concurrent increase in the other constituents of the FCP, and also a strategy to regulate Fx sequestration and/or degradation after its de-novo synthesis [276].
3.4 Effect of culture conditions and environmental factors on growth and fucoxanthin production
Different factors, including strains, cultivation systems, nutrient availability, light intensity and quality and harvest stage affect growth rate and pigment accumulation in microalgae [290]. Although the main carotenoids of commercial interest (astaxanthin and β-carotene) accumulate in microalgae under stress conditions, for Fx-producing microalgae the conditions that favor Fx synthesis appear quite different. In general, the biomass productivity and the Fx content are the two parameters directly responsible for Fx production in microalgae.
One of the most important parameters for Fx production and productivity is the light. Various studies have demonstrated the influence of light wavelength, intensity and the light-darkness cycle on the accumulation of Fx. Low light intensity (less than 100 µmol m−2 s−1) promotes the Fx synthesis [291, 292], while high light intensity (starting from 150 µmol m−2 s−1) can damage the photosystems, therefore, activates the production of photo-protective pigments (diadinoxanthin and diatoxanthin) [293]. The increase of Fx in the low light regime is ascribed to the compensation of low light irradiance, since Fx is a part of the light-harvesting antenna that promotes the photon capture for photosynthesis. Moreover, as more photons are available, cells do not need to capture more photons than necessary due to photon saturation and hence do not produce more chlorophylls and Fx.
As biomass production increases under high light an improved Fx productivity results from a compromise between high and low light intensity. This statement is confirmed by the study of Gao et al. [292] in cultures of Tisochrysis. lutea that obtained the highest Fx productivity (9.81 mg L−1 d−1) at 300 µmol m−2 s−1, while the highest Fx content (5.24 mg g−1) was achieved at 150 µmol m−2 s−1. Similar trend was shown in cultures of Isochrysis zhangjiajiensis as reported by Li et al. [294]. Fx content in P. tricornutum is lower under high light intensity (9.9 mg g−1 at 210 µmol m−2 s−1) than under low light intensity (42.8 mg g−1 at 100 µmol m−2 s−1) [295].
Fx absorbs blue-green light and thus has shown, in diatoms, to be accumulated under blue light even at high intensity, while red light favors the production of other pigments such as diadinoxanthin [295]. Furthermore, in the diatom Cylindrotheca closterium, red light and green light decreased the Fx content because of the lack of blue light [277]. In T. lutea, the highest Fx content was found in blue-green light while the highest Fx productivity was found in red-blue-green light (which is closer to white light) [292]. The accumulation of Fx in blue-green light was expected, as this spectrum is prevalent in the natural habitat of brown algae [259]. Indeed, the absorbance spectrum of Fx ranges from 450 to 540 nm in solution even thought is range is wider (390–580 nm), once the Fx is bound to a FCP.
In conclusion, high light intensity and extra-long lightening are favorable for biomass but adverse for Fx accumulation due to the photoprotection function of Fx; the preference of blue light, with regards to Fx accumulation, can arise from the distinctive absorption of blue-green light by Fx. This conflict needs to be solved to ensure concurrent biomass and Fx accumulation and to achieve a successful Fx production.
Nutrients play an important role in microalgae growth as they are required to synthesize bio-compounds essential for algal cells. Nitrate, phosphate, iron, and silicate (for diatoms) have been identified in various studies as nutrients that have an impact on cells growth and on metabolite accumulation [296]. Nitrogen is a major macronutrient required by microalgae to grow as it is essential in protein synthesis. The importance of nitrogen sources in producing Fx has been widely illustrated. For instance, two phases, nitrogen supply and nitrogen starvation were studied in batch cultivation of T. lutea. Under nitrogen supply phase the Fx content almost doubled and then decreased significantly under nitrogen deprivation phase [292]. The enrichment of nitrate in medium (ten-fold higher than f/2) improves the Fx content of P. tricornutum to 59.2 mg g−1 in seven days [295]. Sufficient nitrogen supply stimulates the biosynthesis of Fx (6.01 mg L−1 d−1) in Odontella aurita, more than fourfold higher than it does in nitrogen-deplete condition [297].
Compared to nitrogen, phosphorus is the main limiting nutrient for microalgae in the environment. However, the impact of phosphorus concentration was less conspicuous than nitrogen concentration on the growth and Fx accumulation of microalgae as shown in the work of Sun et al. [298].
In diatoms, another important nutrient is silicate which is required for cell growth as the cell wall contains silicon. High silicate level promotes the accumulation of both biomass and Fx in diatoms. The study of Mao et al. [299] showed that a high silicate concentration in medium promotes Fx production in Nitzschia laevis but at a low level it suppresses the Fx production. Higher silicate concentration avoids the adverse impact of high light (> 250 μmol m−2 s−1) on Fx accumulation in P. tricornutum [300]. Available evidence supports the understanding that rich nutrients favor Fx accumulation and protect microalgae from stress while deplete nutrients lead to reduced Fx in microalgae.
Optimum salinity is highly dependent on the microalgal species. The marine Isochrysis galbana showed a greater growth and Fx content (12 mg g−1) under a salinity of 35‰ than 20‰ [301]. Meanwhile, the growth (1.49 g L−1 d−1) and Fx content (7.4 mg g−1) of P. tricornutum were in favor of a 20‰ salinity level. C. fusiformis, grew better under 30‰ by producing the highest biomass concentration whereas the greatest Fx content (5.8 mg g−1) was generated under a salinity of 10‰ [302].
The responses of Fx synthesis to changes in temperature and pH have been poorly discussed as in most of the studies microalgae are grown in a temperature and pH-controlled environment, at values generally considered optimum for growth. Like salinity, each Fx-producing microalga has its optimum temperature to yield the maximum growth and Fx content. An optimization study has shown that the optimum temperature for T. lutea to generate the greatest Fx content was 25 °C [303]. Depending on cultivated species, the optimal temperature can vary in the mesophilic range [292, 302].
The effect of trophic modes, including photoautotrophy, heterotrophy and mixotrophy have been studies in several Fx-producing microalgae. As photoautotroph microorganisms, all the species may be cultivated under autotrophic conditions by capturing light and incorporating CO2 [276] [Table 1]. However, several species as N. laevis and Cyclotella cryptica are able to be grown heterotrophically in the dark using organic C sources, even showing an increased Fx content than autotrophic culture [304, 305].
By taking advantage of both photoautotrophic and heterotrophic modes, mixotrophic cultures are more effective in utilizing resources, achieving high biomass production and, as consequence of cell’s self-shading, even an enhanced Fx production. Alkhamis et al. [306] reported more than twice of Fx content in the mixotrophic culture of T. lutea compared with the phototrophic culture (11.5 vs 4.8 mg g−1). In a mixotrophic culture of the diatom N. laevis, the Fx content reached 15.6 mg g−1 while in heterotrophic culture it reached 10 mg g−1 [305]. Mixotrophic cultures of P. tricornutum have been widely investigated and have shown promising results for both biomass and Fx production [287, 307]. Yang et al. [174, 286] reported a maximum Fx content of 16.3 mg g–1, the highest level of ever reported so far.
The above results highlighted that ideal culture conditions to achieve desirable growth and Fx content are strongly specie-specific and would have to be optimized individually. Moreover, there is evidence that the mixotrophic cultivation strategy for P. tricornutum could be more suitable for both biomass and Fx accumulation. At an industrial scale, one limit of the mixotrophy culture could be the cost of the organic carbon sources, although in a circular economy perspective, waste products from industrial processes could be used.
3.5 Microalgae cultivation for fucoxanthin production
Fucoxanthin-producing microalgae are capable of growing in photoautotrophic and some strains also in mixotrophic conditions, both indoor and outdoor, in open and closed systems, in batch, semicontinuous, or continuous mode. The main advantage of using microalgae as a source of Fx is that they can be grown in close photobioreactors (PBRs) providing an available, stable and sustainable supply of biomass all year round. Besides, as microalgae can grow in PBRs, their growing conditions can be optimized for maximizing the production of a specific compound. Many PBR designs have been proposed [308], however, mass cultivation of Haptophytes and diatoms was widely performed in tubular, flat panel, vertical columns and bags.
The production of Fx from O. aurita is very attractive, with the maximum yield of 7.96 mg L−1 d−1 achieved indoors in a bubble column PBR. A comparable yield obtained in the scale-up flat plate PBR confirmed the technical feasibility and scalability of O. aurita-based Fx production on a large scale [271]. In the same microalga cultivated in a 240 L well-controlled PBR internally illuminated by LEDs lamps, red lighting was more suitable for cell growth and Fx production than blue light and white light. However, the biomass and Fx productivities were further promoted by optimizing the ratios of red and blue light, reaching the highest Fx productivity of 9.41 mg L−1 d−1 with a mixed ratio of red light and blue light at 8:2. These findings demonstrated a Fx over-production in O. aurita by using pilot scale PBRs under optimum light spectrum and also demonstrated its promising feasibility in commercial Fx production [309]. Blue light-emitting diode at 100 μmol m−2 s−1 and 18/6 L/D cycle induced maximum Fx productivity in C. closterium and minimized energy consumption. The Fx content and productivity in 20-L bag PBRs with continuous illumination reached 25.5 mg g−1 and 1.4 mg L−1 d−1, respectively. In P. tricornutum, Fx productivity in flat panel PBRs with f/2 medium was found to be 0.44–0.72 mg L−1 d−1 [295], which is significantly lower than the productivity of C. closterium.
Whereas many studies related to Fx production by microalgae have been done under indoor conditions, less data are available on outdoor cultivation where the fluctuating light intensities has a direct impact on biomass and bioproducts production. Outdoor pilot-scale cultivation has been only performed for few Fx producer species. P. tricornutum is the species widely cultivated in outdoor installations and pilot scale to produce biofuels, Fx and other valuable products. Cultures were grown outdoor on a batch mode in 4 bubble column PBRs, with working volume of 200 L each. Results indicate a Fx content of 8.6 mg g−1, leading to an estimated annual yield of 0.18 tons of Fx [310].
Gao et al. [311] grow T. lutea and P. tricornutum in outdoor flat panel PBRs 40 L in volume (Green Wall Panel®-III; [312] and biomass productivities of approximately 130 mg L−1 d−1 and 158 mg L−1d−1 as well as Fx productivity of 2.1 mg L−1 d−1 and 1.7 mg L−1 d−1 were attained, when a biomass concentration of 0.4 g L−1 was maintained in the semi-continuous cultivation [311]. Using 40-L GWP®-III (Green Wall Panel) PBRs, P. tricornutum Utex 640 was cultivated outdoors under different nitrogen regimes, and growth and fatty acid content and productivity were evaluated. The GWP-III used in this work was entirely made of stainless steel, and as in the previous designs, the external metal structure encloses a plastic culture chamber. Nitrogen replete cultures achieved the highest productivity of biomass (about 18 g m−2 d−1) and of valuable bioproducts such as EPA- eicosapentaenoic acid (about 0.35 g m−2 d−1) [313]. In the same location, same season, and with the same strain, Benavides et al. [313] attained land areal productivities of 11.7 and 13.1 g m−2 d−1 in circular ponds and a horizontal tubular photobioreactor, respectively, adopting a daily dilution regime. Noteworthy is the fact that the highest pool of the xanthophyll diadino/diatoxanthin was found in the PBR grown cultures, while the highest amount of the main carotenoid Fx was found in the pond cultures, most likely as a result of extensive low-light acclimation of this culture [313].
Outdoor cultivation of Pavlova sp. OPMS 30,543 was performed in acrylic pipe PBRs (5-mm thickness and different outer diameters), a plastic bag (0.1-mm thickness, 450-mm outer diameter), a 200-L polycarbonate open tank, and a 500-L raceway pond. Under optimized culture conditions and using the 60-mm diameter acrylic pipe PBR, a Fx content of about 21 mg g−1 and Fx productivity of 4.9 mg L−1 d−1 was obtained. This study demonstrated the usefulness of Pavlova sp. OPMS 30543 for Fx production in outdoor cultivation [274].
T. lutea and P. tricornutum were also cultivated at industrial scale in a 5000 L Camargue tubular PBRs [314] at Microphyt production plant (Baillargues, France) and under greenhouse. This PBR design ensures a co-circulation of the liquid medium and CO2-enriched air in order to optimize gas exchanges and are developed to minimize mechanical stress, which guarantees the preservation of the most fragile microalgal specie. Fx content was 19 mg g−1 and 13.3 mg g−1 in T. lutea and P. tricornutum, respectively [315].
The first commercial report on the production of microalgal Fx was in 2018 by Algatechnologies Ltd., a microalgal biotechnology company located in Israel, which introduced a registered patent of natural 3% Fx oleoresin with the trademark Fucovital® from P. tricornutum grown in an industrial-scale plant based on vertically arranged, fully controlled tubular PBRs exposed to sunlight [316].
Shear stress, an important factor in microalgal cultivation, especially for large-scale installations, is diverse and complex in different types of PBRs, as it sources from mixing device, air-bubbling and pumping. Generally, Fx producers (diatoms and haptophytes) are more susceptible to shear stress than green algae and cyanobacteria and therefore shear stress, should be carefully minimized in a specific PBR to avoid the detrimental effect on Fx productivity. It can be concluded that a PBR characterized by a low shear stress, high surface area to volume ratio as well as effective temperature and biofilm control capacity, favors outdoor microalgal Fx production.
3.6 Metabolism and potential benefits of fucoxanthin in human health
Elucidating the mechanisms of absorption and the metabolism of Fx is important to understand its physiological effects. Both absorption and metabolism are closely related to the molecule’s bioavailability. In mammals, Fucoxanthinol and Amarouciaxanthin A are known as the major Fx metabolites (Fig. 13). Once ingested, dietary Fx is hydrolyzed to Fucoxanthinol in the gastrointestinal tract by digestive enzymes such as lipase and cholesterol esterase. Once deacetylated, Fx becomes a non-polar molecule of greater bioavailability ready to be incorporated into chylomicrons, making it possible for Fucoxanthinol to reach different systemic organs [317, 318]. In murine liver cells, Fucoxanthinol was converted into Amarouciaxanthin A which was predominantly shown in liver microsomes of mice and in HepG2 cells [319]. The in vitro study by Hashimoto et al. [320] demonstrated that Fucoxanthinol and Amarouciaxanthin A were detected in many tissues (plasma, erythrocytes, liver, kidneys, heart, spleen for Fucoxanthinol, and adipose tissue for Amarouciaxanthin A), while Fx was found in traces, therefore almost completely metabolized. Fx metabolites accumulate in abdominal adipose tissue at a higher rate than in plasma and other tissues, suggesting that adipose tissue is a main target of Fx metabolites.
The use of Fx has risen in the two last decades due to its potential bioactivities. Valuable properties of Fx such as anti-oxidant, anti-cancer, anti-obesity, anti-diabetic, anti-inflammatory, anti-angiogenic, anti-malarial, etc. have been shown in many studies. It can also be used as a protective agent for dermal, ophthalmic, bone, cerebrovascular, and cardiovascular disorders [260, 266, 319, 321]. Over the last few years, an increasing number of researches on Fx with pharmacological properties, either used in its purified form or extracted from marine algae, have been published [260, 318]. Here, a schematic view of the most relevant results regarding microalgae will be given.
3.6.1 Antioxidant activity
Fucoxanthin has shown strong antioxidant properties which can help reduce oxidative stress and prevent a variety of diseases [260, 322]. Compared to other carotenoids, Fx has the highest antioxidant activity under anoxic conditions [323] and the antioxidant properties of Fx and its metabolite Fucoxanthinol include scavenging of free radicals and quenching singlet oxygen in vitro [322].
The purified Fx from O. aurita was identified as all-trans-Fx, which exhibited strong antioxidant properties, with an effective concentration for 50% scavenging (EC50) of DPPH radical and ABTS radical being 0.14 and 0.03 mg mL−1, respectively [271]. According to the study of Foo et al. [324] that used four antioxidant assays, Chaetoceros calcitrans and I. galbana displayed the highest antioxidant activity, followed by Odontella sinensis and Skeletonema costatum which showed moderate bioactivities. P. tricornutum and Saccharina japonica exhibited the lowest antioxidant activities amongst the algal species examined. Purified Fx obtained by P. tricornutum exhibited strong free-radical scavenging activity in vitro, obtaining values of 0.14 mg mL−1 using DPPH assay and 0.05 mg mL−1 with ABTS assay. Furthermore, it has been reported that the anticancer effects of Fx are closely related to its antioxidant activity or pro-oxidative development [325]. The antioxidant effects of Fx extracted from P. tricornutum do not significantly differ from that of astaxanthin and both showed a strong antioxidant effect compared to β-carotene [326].
3.6.2 Anti-obesity and antidiabetic activity
In general, nutritionally rich diets coupled with irrational eating habits could result in obesity and diabetes mellitus. Diabetes mellitus is usually caused by obesity because excessive energy intake and accumulation of lipids can elevate insulin resistance. Fx was proven to play an important role in reducing insulin resistance and blood glucose. The glycated hemoglobin (HbA1c) level is a risk indicator of glycemia and diabetic complications. The study of Woo et al. [327] suggested that supplementing 0.05% and 0.2% Fx significantly reduced the blood HbA1c and plasma insulin level compared with the control group. Studies on mice and rats have shown that Fx supplementation could play a beneficial role in preventing obesity through various pathways: by directly affecting weight loss and lipid metabolism via a reduction of plasmatic and hepatic triglyceride concentrations in the liver [319, 328], or reducing mRNA levels of the enzyme fatty acid synthase (FAS) and inducing uncoupling protein-1 (UCP1) in abdominal white adipose tissue (WAT) which leads to the oxidation of fatty acids and heat production [319, 329, 330]. Mayer et al. [331] in “in vivo” trials showed that P. tricornutum might be a promising marine source of novel food ingredients for the prevention of Metabolic Syndrome (MS) and obesity. Body weight, fat mass, inflammatory markers and insulinemia decreased in high-fat diet (HF) rats supplemented with 12% of P. tricornutum biomass versus the HF group without the microalga. Total plasmatic cholesterol, triacylglycerols and leptine diminished in HF-Phaeo rats, while HDL-cholesterol increased. In similar experiments in which rats were submitted to a standard diet or high-fat diet (HF), groups supplemented with 12% of T. lutea (HF-Tiso) or 12% of D. lutheri evidenced a difference against control groups, demonstrating these microalgae being effective for the prevention of obesity and weight gain and for the improvement of lipid and glucose metabolism [281, 282].
3.6.3 Anti-cancer activity
Numerous studies have shown the antitumor and cancer preventive properties of Fucoxanthin and Fucoxanthinol and this potential activity has also been explored against various cancer types [259, 332,333,334] The proposed mechanisms show how Fx and its metabolite Fucoxanthinol can inhibit tumor cell proliferation inducing cell cycle arrest and apoptosis as well as inhibiting angiogenesis [332,333,334]. The inhibition of cell proliferation by Fx is due to cell growth arrest at G0/G1 or G1 phase of the cell cycle [335]. The apoptosis-inducing activity of Fucoxanthinol was found to be more potent than that of Fx. The study by Yamamoto et al. [336] showed that Fx and Fucoxanthinol induced cell cycle arrest during G1 phase and caspase-dependent apoptosis in primary effusion lymphoma cells. Smaller doses of Fx induce G1 cell cycle arrest in different cancer cells including colon carcinoma, hepatocarcinoma, gastric adenocarcinoma, Burkitt’s and Hodgkin’s lymphoma, and prostate cancer [335], see [Table 2]. The antiproliferative effect of Fx was confirmed “in vivo” by studies showing tumor growth arrest in the presence of Fx with several types of cancer [335] see [Table 3]. Oral or intraperitoneal administration of Fx inhibited the growth of lymphoma, melanoma and cervical cancer and caused apoptosis in sarcoma [336,337,338,339]. Fx extracted from P. tricornutum did not have anti-inflammatory properties. but it is able to induce apoptosis in different cancer [326].
The molecular mechanism that supports the observed G0/G1 phase arrest appears to be dependent on the cancer cell type but mainly involves cyclin D1 and/or D2 and CDK4 downregulation. Fx has been highlighted to induce autophagy and apoptosis in cancer cell lines and several pathways have been studied and identified to be involved in those cytotoxic effects [335].
3.6.4 Anti-inflammatory activity
Fucoxanthin has been shown to have marked anti-inflammatory effects in different “in vitro” experimental models. In this line, Fx suppressed the inflammatory mediators including nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor-α (TNFα), interleukin-(IL-)1β, IL-6, and inflammatory cytokines such as cyclooxygenase (COX) and inducible nitric oxide synthase (iNOS). Fx and its derivatives, significantly inhibited NO production in lipopolysaccharide (LPD)-stimulated murine macrophage RWA 264.7 cells [337, 340]. Fx reduced the concentrations of NO, prostaglandin E2, TNF-α, IL-1β, and IL-6 through the inhibition of nuclear factor κB activation and the phosphorylation of mitogen-activated protein kinases [337]. In a study using Fx extracted from P. tricornutum, Neumann et al. [326] found no effects of Fx on the NO production of LPS-stimulated RAW 264.7 cells nor on the mRNA-expression of pro-inflammatory cytokines in human PBMCs. It is noteworthy that all mentioned studies utilized Fx extracted from seaweeds, therefore, it can be assumed that the carotenoid from P. tricornutum might have different effects.
3.6.5 Skin protective effect
Studies carried out “in vitro” and “in vivo” with Fx have demonstrated its positive effect in reducing the damage caused by UV. When tested in “in vitro” assay, Fx decreased the production of intracellular reactive oxygen species (ROS) caused by exposure of dermal fibroblasts to ultraviolet radiation, with an effect comparable to that of N-acetylcysteine (NAC) and other strong antioxidant compounds. “In vivo” results showed that Fx promotes synthesis of the structural protein filaggrin, which generates a protective barrier in case of burns induced by radiation [341]. Oral administration of Fx produced a suppression of transcription of the melanogenesis factor, by inhibiting dermal mRNA expression related to this disease [342]. In human fibroblast cells, Fx photoprotective role is also linked to its ability to remediate DNA damage and to its high antioxidant activity [343]. As a conclusion, administering Fx either as food supplement or a drug can reduce or even prevent the appearance of melanomas or otherwise mitigate the harmful effects of exposure to UV radiation.
3.6.6 Neuroprotective effect
Fucoxanthin extracted from the brown alga Hizikia fusiformis, widely consumed in Asia, has been demonstrated to inhibit the expression of N-myc, a proto-oncogene protein, as well as the proliferation of GOTO cells, a cell line responsible for human neuroblastoma. An amount of Fx equivalent to 1–10 g mL−1 is enough to inhibit the growth rate of such a cell line (GOTO) at 38%, decreasing the rate of progression of this neurodegenerative disease. Fx has been also demonstrated to be implied in the activation of the Nrf2 pathway and its related genes, such as those for the cytoprotective enzymes NQO1 and HO-1, showing that it may protect the brain against ischemia/reperfusion (I/R) injury. The study of Hu et al. indicated that treatment with Fx effectively reduced middle cerebral artery occlusion-induced cerebral I/R injury [344].
3.7 Safety and stability
Humans have been eating seaweed since ancient times and it seems that the Fucoxanthin in the form of eating seaweed does not provide a direct risk to human health. Relatively to the ingestion through food supplements and their relative dose, reference can be made to work of Beppu et al. [257] in which several doses of Fx administrated to living male and female mice. Both in the single dose study (1000 and 2000 mg kg−1) and in a separate repeated dose study (500 and 1000 mg kg−1 d−1for 30 days) no increased mortality or abnormal growth were detected.
Although Fx is a suitable ingredient in medical and nutritional applications, its chemical stability and bioavailability can be reduced in real condition due to lipophilic and rich electron structure as well as poor water solubility and high melting point. Therefore, Fx is degraded during extraction, purification, storage, and utilization processes as a result of the acids, light, heat, oxygen, enzymes, metals, and pro-oxidant molecules. Fx stability is decreased in low and high pH values that caused degradation and trans–cis isomerization [345]. High temperatures, air and light exposure may induce the double bonds breakage, oxidation and isomerization in the Fx molecule and consequently pigment degradation [345, 346]. Among the potential solutions suggested for improving the stability of Fx against external factors, encapsulation and emulsion systems are promising approaches [291].
3.8 Current market and regulatory issues
In 2020, the global Fucoxanthin market was valued at approximately US$ 600 million, growing at a compound annual growth rate (CAGR) of 6% to reach US$ 780 million by the end of 2025. The current price for pure Fx ranges from USD 40,000 to 80,000 per kilogram, depending on quality and concentration [316].
Several Fx-based products are available in the market, many of which are slimming food supplements, mostly coming from seaweeds and rarely containing pure Fx. Brown seaweeds such as Laminaria sp., Fucus sp., Sargassum sp., Hizikia fusiformis and Undaria pinnatifida are the commercial sources of Fx for food [148]. They are usually sold in Asia as powder or oil in capsule or cachet, distributed by several major Asia companies, such as Yangling Ciyuan Biotech Co. and Agrochemi Co., and authorized in Europe by the Novel Food Regulation [148].
Fx from microalgae is utilized as supplement to whole feed or food, as it could enhance its functional and nutritional value. Several Fx products derived from microalgae are authorized for the market in the US, i.e., Fucovital™ by Algatechnologies Ltd, Israel (from P. tricornutum; https://www.algatech.com/algatech-product/fucovital, accessed on 10 November 2022) which have obtained a “new dietary ingredient notification” (NDIN1048-2017) by the US-FDA; NutriXanthin™ and DermaXanthin™ by Alga-health, Israel, that could be used as ingredients for food supplements and cosmetics products (https://alga-health.com/products, accessed on 10 november 2022) and BrainPhyt™ and PhaeoSOL™ by Microphyt, France (https://microphyt.eu/, accessed on 10 november 2022). BrainPhyt™ is a microalgal extract with 2% Fx, advertised to improve cognitive performance (https://www.brainphyt.com/). PhaeoSOL™ obtained in 2019 the status of New Dietary Ingredient from the US-FDA. Fx as nutraceutical is approved by the Food Standards Australia and New Zealand (FSAN), and currently is being investigated by the United State Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). As reported previously, two microalgal products have obtained approval as new dietary ingredient from the US-FDA. However, European regulation appears much more restrictive. Concerning food applications, the Novel Food Regulation in EU authorized the consumption of the Fx-producing seaweeds which, as used as food prior to 15th of May 1997, are listed as “not novel” in the EU Novel Foods Catalogue, whereas only one microalga, the diatom O. aurita, was authorized in 2005 (European Union, 2005; Regulation EU2015/2283; https://www.algae-novel-food.com) [148]. P. tricornutum, based on the lack of a safe history of use in the food chain and limited knowledge on its potential production of bioactive compounds with possible toxic effects, currently cannot be recommended for the list of qualified presumption of safety (QPS) [347]. On the other hand, Fx is not reported in the EU Register on nutrition and health claims (EU Register on nutrition and health claim;https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home), nor in the European Pharmacopoeia (EP10), United States Pharmacopoeia National Formulary or Japanese Pharmacopoeia.
3.8.1 Concluding remarks
Except for the fundamental role in microalgal photosynthesis, Fucoxanthin has attracted more and more attentions from different industrial sectors. Towards the eventual commercialization of microalgal Fx, significant scientific and technical progresses have been made in last decade at each step of production, although several bottlenecks, such as screening of Fx-producers, conflict between biomass and Fx accumulation under high light condition, unclear steps in biosynthesis pathway and limited evaluation of outdoor scale-up cultivation and extraction, still exist. Several microalgal species can be considered as candidate producers for Fx to meet global market demands. The species, trophic mode, and bioreactor types which contribute to high biomass and enhanced productivities are still being developed and improved for commercial production. To achieve increased economic benefits, breeding, culturing, harvesting, and co-extraction should be cautiously considered. Fx as natural product may represent novel and functional ingredient for the treatment and/or prophylaxis of diseases. However, the application of Fx from microalgae in humans remains at an early stage of development and it is necessary to continue with the studies, to reach the successive clinical phases, more in vivo studies and, subsequently, in humans.
Data availability
All data supporting the findings of this study are available on request from the corresponding author.
Abbreviations
- Amax :
-
Absorption maximum
- APC:
-
Allophycocyanin
- CAGR:
-
Compound annual growth rate
- DMAPP:
-
Dimethylallyl diphosphate
- EFSA:
-
European food safety authority
- FCP:
-
Fucoxanthin-chlorophyll a/c-protein complex
- FDA:
-
Food and drug administration
- Fx:
-
Fucoxanthin
- GGPP:
-
Geranylgeranyl pyrophosphate
- IPP:
-
Isopentenyl diphosphate
- MEP:
-
Methylerythritol phosphate
- PBPs:
-
Phycobiliproteins
- PBR:
-
Photobioreactor
- PC:
-
Phycocyanin
- PE:
-
Phycoerythrin
References
Pagels, F., Guedes, A. C., Amaro, H. M., Kijjoa, A., & Vasconcelos, V. (2019). Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnology Advances, 37(3), 422–443. https://doi.org/10.1016/j.biotechadv.2019.02.010
Boussiba, S., & Richmond, A. E. (1980). C-phycocyanin as a storage protein in the blue-green alga Spirulina platensis. Archives of Microbiology, 125(1), 143–147. https://doi.org/10.1007/BF00403211
Kannaujiya, V. K., Sundaram, S., & Sinha, R. P. (2017). Phycobiliproteins: recent developments and future applications (p. 147). Singapore: Springer. https://doi.org/10.1007/978-981-10-6460-9
Marx, A., & Adir, N. (2013). X-Ray Crystal Structure of Allophycocyanin from Synechococcus elongatus PCC 7942. DOI: https://doi.org/10.2210/pdb4F0U/pdb
Marx, A., & Adir, N. (2013). Allophycocyanin and phycocyanin crystal structures reveal facets of phycobilisome assembly. Biochimica et Biophysica Acta Bioenergetics, 1827(3), 311–318. https://doi.org/10.1016/j.bbabio.2012.11.006
Marx, A., & Adir, N. (2013). X-Ray Crystal Structure of Phycocyanin from Synechococcus elongatus PCC 7942. DOI: https://doi.org/10.2210/pdb4H0M/pdb
Tanaka, Y., Gai, Z., & Kishimura, H. (2016). Crystal structure of phycoerythrin. DOI: https://doi.org/10.2210/pdb5b13/pdb
Miyabe, Y., Furuta, T., Takeda, T., Kanno, G., Shimizu, T., Tanaka, Y., & Kishimura, H. (2017). Structural properties of phycoerythrin from dulse Palmaria palmata. Journal of Food Biochemistry, 41(1), e12301. https://doi.org/10.1111/jfbc.12301
MacColl, R. (1998). Cyanobacterial phycobilisomes. Journal of Structural Biology, 124(2–3), 311–334. https://doi.org/10.1006/jsbi.1998.4062
Sui, S.F., Ma, J.F., You, X., & Sun, S. (2019). Structure of the phycobilisome from the red alga Porphyridium purpureum. DOI: https://doi.org/10.2210/pdb6KGX/pdb
Ma, J., You, X., Sun, S., Wang, X., Qin, S., & Sui, S. F. (2020). Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature, 579(7797), 146–151. https://doi.org/10.1038/s41586-020-2020-7
Gantt, E. (1990). Pigmentation and photoacclimation Biology of the red algae (pp. 203–221). Cambridge University Press.
Manirafasha, E., Ndikubwimana, T., Zeng, X., Lu, Y., & Jing, K. (2016). Phycobiliprotein: potential microalgae derived pharmaceutical and biological reagent. Biochemical Engineering Journal, 109, 282–296. https://doi.org/10.1016/j.bej.2016.01.025
de Morais, M. G., da Fontoura Prates, D., Moreira, J. B., Duarte, J. H., & Costa, J. A. V. (2018). Phycocyanin from microalgae: properties, extraction and purification, with some recent applications. Industrial Biotechnology, 14(1), 30–37. https://doi.org/10.1089/ind.2017.0009
Morocho-Jácome, A. L., Ruscinc, N., Martinez, R. M., de Carvalho, J. C. M., Santos de Almeida, T., Rosado, C., & Baby, A. R. (2020). (Bio) Technological aspects of microalgae pigments for cosmetics. Applied Microbiology and Biotechnology, 104(22), 9513–9522. https://doi.org/10.1007/s00253-020-10936-x
Prado, J. M., Veggi, P. C., Náthia-Neves, G., & Meireles, M. A. A. (2020). Extraction methods for obtaining natural blue colorants. Current Analytical Chemistry, 16(5), 504–532. https://doi.org/10.2174/1573411014666181115125740
Tan, H. T., Yusoff, F. M., Khaw, Y. S., Ahmad, S. A., & Shaharuddin, N. A. (2021). Uncovering research trends of phycobiliproteins using bibliometric approach. Plants, 10(11), 2358. https://doi.org/10.3390/plants10112358
Chen, H., Qi, H., & Xiong, P. (2022). Phycobiliproteins—A family of algae-derived biliproteins: productions, characterization and pharmaceutical potentials. Marine Drugs, 20(7), 450. https://doi.org/10.3390/md20070450
Dagnino-Leone, J., Figueroa, C. P., Castañeda, M. L., Youlton, A. D., Vallejos-Almirall, A., Agurto-Muñoz, A., & Agurto-Muñoz, C. (2022). Phycobiliproteins: structural aspects, functional characteristics, and biotechnological perspectives. Computational and Structural Biotechnology Journal. https://doi.org/10.1016/j.csbj.2022.02.016
Nowruzi, B., Konur, O., & Anvar, S. A. A. (2022). The stability of the phycobiliproteins in the adverse environmental conditions relevant to the food storage. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02855-8
Patel, S. N., Sonani, R. R., Roy, D., Singh, N. K., Subudhi, S., Pabbi, S., & Madamwar, D. (2022). Exploring the structural aspects and therapeutic perspectives of cyanobacterial phycobiliproteins. Biotech, 12(9), 1–15. https://doi.org/10.1007/s13205-022-03284-2
Meticulous Research (2020). Phycocyanin market, global forecast to 2027. https://www.meticulousresearch.com/pressrelease/30/phycocyanin-market-2027
Madamwar, D., Patel, D. K., Desai, S. N., Upadhyay, K. K., & Devkar, R. V. (2015). Apoptotic potential of C-phycoerythrin from Phormidium sp. A27DM and Halomicronema sp. A32DM on human lung carcinoma cells. EXCLI journal, 14, 527. https://doi.org/10.17179/excli2014-696
Deniz, I., Ozen, M. O., & Yesil-Celiktas, O. (2016). Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. The Journal of Supercritical Fluids, 108, 13–18. https://doi.org/10.1016/j.supflu.2015.10.015
Liu, Q., Huang, Y., Zhang, R., Cai, T., & Cai, Y. (2016). Medical application of Spirulina platensis derived C-phycocyanin. Evidence-Based Complementary and Alternative Medicine. https://doi.org/10.1155/2016/7803846
Dranseikienė, D., Balčiūnaitė-Murzienė, G., Karosienė, J., Morudov, D., Juodžiukynienė, N., Hudz, N., ... & Savickienė, N. (2022). Cyano-Phycocyanin: Mechanisms of Action on Human Skin and Future Perspectives in Medicine. Plants, 11(9), 1249. https://doi.org/10.3390/plants11091249
Mahendran, S., Sankaralingam, S., Maheswari, P., Annalakshmi, P., Pandiarajan, J., Seethapathy, P., & Venkatesh, S. (2022). Isolation and purification of phycocyanin pigments from Spirulina sp. biomass and evaluation of its anticancer and antioxidant potential. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02765-x
Sala, L., Moraes, C. C., & Kalil, S. J. (2018). Cell pretreatment with ethylenediaminetetraacetic acid for selective extraction of C-phycocyanin with food grade purity. Biotechnology Progress, 34(5), 1261–1268. https://doi.org/10.1002/btpr.2713
Lauceri, R., Zittelli, G. C., & Torzillo, G. (2019). A simple method for rapid purification of phycobiliproteins from Arthrospira platensis and Porphyridium cruentum biomass. Algal Research, 44, 101685. https://doi.org/10.1016/j.algal.2019.101685
García-López, D. A., Olguín, E. J., González-Portela, R. E., Sánchez-Galván, G., De Philippis, R., Lovitt, R. W., & Saldívar, R. P. (2020). A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresource Technology, 298, 122548. https://doi.org/10.1016/j.biortech.2019.122548
Chaiklahan, R., Chirasuwan, N., Srinorasing, T., Attasat, S., Nopharatana, A., & Bunnag, B. (2022). Enhanced biomass and phycocyanin production of Arthrospira (Spirulina) platensis by a cultivation management strategy: Light intensity and cell concentration. Bioresource Technology, 343, 126077. https://doi.org/10.1016/j.biortech.2021.126077
Chini Zittelli, G., Mugnai, G., Milia, M., Cicchi, B., Benavides, A. S., Angioni, A., & Torzillo, G. (2022). Effects of blue, orange, and white lights on growth, chlorophyll fluorescence, and phycocyanin production of Arthrospira platensis cultures. Algal Research, 61, 102583. https://doi.org/10.1016/j.algal.2021.102583
Fratelli, C., Burck, M., Amarante, M. C. A., & Braga, A. R. C. (2021). Antioxidant potential of nature’s “something blue”: something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends in Food Science & Technology, 107, 309–323. https://doi.org/10.1016/j.tifs.2020.10.043
Jaeschke, D. P., Teixeira, I. R., Marczak, L. D. F., & Mercali, G. D. (2021). Phycocyanin from Spirulina: a review of extraction methods and stability. Food Research International, 143, 110314. https://doi.org/10.1016/j.foodres.2021.110314
Fabre, J. F., Niangoran, N. U. F., Gaignard, C., Buso, D., Mouloungui, Z., & Valentin, R. (2022). Extraction, purification, and stability of C-phycocyanin from Arthrospira platensis. European Food Research and Technology. https://doi.org/10.1007/s00217-022-03987-z
Gomes-Diaz, J. S., Teixeira, J. A., & Rocha, C. M. (2022). Recent advances in the valorization of algae polysaccharides for food and nutraceutical applications: a review on the role of green processing technologies. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-022-02812-5
İlter, I., Akyıl, S., Demirel, Z., Koç, M., Conk-Dalay, M., & Kaymak-Ertekin, F. (2018). Optimization of phycocyanin extraction from Spirulina platensis using different techniques. Journal of Food Composition and Analysis, 70, 78–88. https://doi.org/10.1016/j.jfca.2018.04.007
Bachchhav, M. B., Kulkarni, M. V., & Ingale, A. G. (2020). Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Separation Science and Technology, 55(5), 932–944. https://doi.org/10.1080/01496395.2019.1580293
Sommer, M. C., Balazinski, M., Rataj, R., Wenske, S., Kolb, J. F., & Zocher, K. (2021). Assessment of phycocyanin extraction from Cyanidium caldarium by spark discharges, compared to freeze-thaw cycles, sonication, and pulsed electric fields. Microorganisms, 9(7), 1452. https://doi.org/10.3390/microorganisms9071452
Yong, S. X. M., Song, C. P., & Choo, W. S. (2021). Impact of high-pressure homogenization on the extractability and stability of phytochemicals. Frontiers in Sustainable Food Systems, 4, 593259. https://doi.org/10.3389/fsufs.2020.593259
Carlos, V., Adrielly, T., dos Santos, K. M., & de Cássia, E. F. (2021). Pressurized extraction of phycobiliproteins from Arthrospira platensis and evaluation of its effect on antioxidant and anticancer activities of these biomolecules. Journal of Applied Phycology, 33(2), 929–938. https://doi.org/10.1007/s10811-020-02358-z
Matulis, D. (2016). Selective precipitation of proteins. Current Protocols in Protein Science, 83(1), 4–5. https://doi.org/10.1002/0471140864.ps0405s83
Cai, C., Li, C., Wu, S., Wang, Q., Guo, Z., & He, P. (2012). Large scale preparation of phycobiliproteins from Porphyra yezoensis using co-precipitation with ammonium sulfate. Natural Science. https://doi.org/10.4236/ns.2012.48071
Martins, M., Soares, B. P., Santos, J. H., Bharmoria, P., Torres Acosta, M. A., Dias, A. C., & Ventura, S. P. (2021). Sustainable strategy based on induced precipitation for the purification of phycobiliproteins. ACS Sustainable Chemistry & Engineering, 9(10), 3942–3954. https://doi.org/10.1021/acssuschemeng.0c09218
Lauceri, R., Zittelli, G. C., Maserti, B., & Torzillo, G. (2018). Purification of phycocyanin from Arthrospira platensis by hydrophobic interaction membrane chromatography. Algal Research, 35, 333–340. https://doi.org/10.1016/j.algal.2018.09.003
Zang, F., Qin, S., Ma, C., Li, W., & Lin, J. (2020). Preparation of high-purity R-phycoerythrin and R-phycocyanin from Pyropia yezoensis in membrane chromatography. Journal of Applied Phycology, 32(5), 3411–3418. https://doi.org/10.1007/s10811-020-02208-y
Rathore, A. S., Kumar, D., & Kateja, N. (2018). Recent developments in chromatographic purification of biopharmaceuticals. Biotechnology letters, 40(6), 895–905. https://doi.org/10.1007/s10529-018-2552-1
Minkova, K., Tchorbadjieva, M., Tchernov, A., Stojanova, M., Gigova, L., & Busheva, M. (2007). Improved procedure for separation and purification of arthronema africanum phycobiliproteins. Biotechnology letters, 29(4), 647–651. https://doi.org/10.1007/s10529-006-9274-5
Rito-Palomares, M., Nunez, L., & Amador, D. (2001). Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. Journal of Chemical Technology & Biotechnology, 76(12), 1273–1280. https://doi.org/10.1002/jctb.507
Luo, X., Smith, P., Raston, C. L., & Zhang, W. (2016). Vortex fluidic device-intensified aqueous two phase extraction of C-phycocyanin from Spirulina maxima. ACS Sustainable Chemistry & Engineering, 4(7), 3905–3911. https://doi.org/10.1021/acssuschemeng.6b00756
Park, J., Lee, H., Dinh, T. B., Choi, S., De Saeger, J., Depuydt, S., & Han, T. (2022). Commercial potential of the cyanobacterium Arthrospira maxima: physiological and biochemical traits and the purification of phycocyanin. Biology, 11(5), 628. https://doi.org/10.3390/biology11050628
Nisticò, D. M., Piro, A., Oliva, D., Osso, V., Mazzuca, S., Fagà, F. A., & Cassano, A. (2022). A combination of aqueous extraction and ultrafiltration for the purification of phycocyanin from Arthrospira maxima. Microorganisms, 10(2), 308. https://doi.org/10.3390/microorganisms10020308
Boussiba, S., & Richmond, A. E. (1979). Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Archives of Microbiology, 120(2), 155–159. https://doi.org/10.1007/BF00409102
Patil, G., Chethana, S., Sridevi, A. S., & Raghavarao, K. S. M. S. (2006). Method to obtain C-phycocyanin of high purity. Journal of chromatography A, 1127(1–2), 76–81. https://doi.org/10.1016/j.chroma.2006.05.073
Niu, J.-F., Wang, G.-C., Lin, X.-Z., & Zhou, B.-C. (2007). Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. Journal of Chromatography B, 850(1–2), 267–276. https://doi.org/10.1016/j.jchromb.2006.11.043
Patil, G., Chethana, S., Madhusudhan, M. C., & Raghavarao, K. S. M. S. (2008). Fractionation and purification of the phycobiliproteins from Spirulina platensis. Bioresource Technology, 99(15), 7393–7396. https://doi.org/10.1016/j.biortech.2008.01.028
Moraes, C. C., & Kalil, S. J. (2009). Strategy for a protein purification design using C-phycocyanin extract. Bioresource technology, 100(21), 5312–5317. https://doi.org/10.1016/j.biortech.2009.05.026
Su, H. N., Xie, B. B., Chen, X. L., Wang, J. X., Zhang, X. Y., Zhou, B. C., & Zhang, Y. Z. (2010). Efficient separation and purification of allophycocyanin from Spirulina (Arthrospira) platensis. Journal of applied phycology, 22(1), 65–70. https://doi.org/10.1007/s10811-009-9427-8
Yan, S. G., Zhu, L. P., Su, H. N., Zhang, X. Y., Chen, X. L., Zhou, B. C., & Zhang, Y. Z. (2011). Single-step chromatography for simultaneous purification of C-phycocyanin and allophycocyanin with high purity and recovery from Spirulina (Arthrospira) platensis. Journal of Applied Phycology, 23(1), 1–6. https://doi.org/10.1007/s10811-010-9525-7
Liao, X., Zhang, B., Wang, X., Yan, H., & Zhang, X. (2011). Purification of C-phycocyanin from Spirulina platensis by single-step ion-exchange chromatography. Chromatographia, 73(3), 291–296. https://doi.org/10.1007/s10337-010-1874-5
Bermejo, R., & Ramos, A. (2012). Pilot scale recovery of phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. Chromatographia, 75(5), 195–204. https://doi.org/10.1007/s10337-012-2200-1
Zhao, L., Peng, Y. L., Gao, J. M., & Cai, W. M. (2014). Bioprocess intensification: An aqueous two-phase process for the purification of C-phycocyanin from dry Spirulina platensis. European Food Research and Technology, 238(3), 451–457. https://doi.org/10.1007/s00217-013-2124-5
Moraes, C. C., Sala, L., Ores, J. D. C., Braga, A. R. C., Costa, J. A. V., & Kalil, S. J. (2015). Expanded and fixed bed ion exchange chromatography for the recovery of C-phycocyanin in a single step by using lysed cells. The Canadian Journal of Chemical Engineering, 93(1), 111–115. https://doi.org/10.1002/cjce.22098
Wang, F., Liu, Y. H., Ma, Y., Cui, Z. G., & Shao, L. L. (2017). Application of TMA-PEG to promote C-phycocyanin extraction from S. platensis in the PEG ATPS. Process Biochemistry, 52, 283–294. https://doi.org/10.1016/j.procbio.2016.11.006
Böcker, L., Hostettler, T., Diener, M., Eder, S., Demuth, T., Adamcik, J., & Mathys, A. (2020). Time-temperature-resolved functional and structural changes of phycocyanin extracted from Arthrospira platensis/Spirulina. Food chemistry, 316, 126374. https://doi.org/10.1016/j.foodchem.2020.126374
Choi, Y. H., Mah, S. K., Ng, Y. S., & Lee, S. Y. (2021). Fractionation of phycocyanin and carbohydrate from Spirulina platensis using ionic liquid-based aqueous two-phase system. In IOP Conference Series: Materials Science and Engineering (Vol. 1195, No. 1, p. 012039). IOP Publishing. Doi:https://doi.org/10.1088/1757-899X/1195/1/012039
İlter, I., Koç, M., Demirel, Z., Conk Dalay, M., & Kaymak Ertekin, F. (2021). Improving the stability of phycocyanin by spray dried microencapsulation. Journal of Food Processing and Preservation, 45(7), e15646. https://doi.org/10.1111/jfpp.15646
Scorza, L. C., Simon, U., Wear, M., Zouliatis, A., Dimartino, S., & McCormick, A. J. (2021). Evaluation of novel 3D-printed monolithic adsorbers against conventional chromatography columns for the purification of c-phycocyanin from Spirulina. Algal Research, 55, 102253. https://doi.org/10.1016/j.algal.2021.102253
Wan, M., Zhao, H., Guo, J., Yan, L., Zhang, D., Bai, W., & Li, Y. (2021). Comparison of C-phycocyanin from extremophilic Galdieria sulphuraria and Spirulina platensis on stability and antioxidant capacity. Algal Research, 58, 102391. https://doi.org/10.1016/j.algal.2021.102391
Berrouane, N. E. H., Attal, F. S., Benchabane, A., Saghour, I., Bitam, A., Gachovska, T., & Amiali, M. (2022). Freeze–thaw-, enzyme-, ultrasound-and pulsed electric field-assisted extractions of C-phycocyanin from Spirulina platensis dry biomass. Journal of Food Measurement and Characterization, 16(2), 1625–1635. https://doi.org/10.1007/s11694-021-01264-3
Huo, Y., Hou, X., Yu, Y., Wen, X., Ding, Y., Li, Y., & Wang, Z. (2022). Improving the thermal and oxidative stability of food-grade phycocyanin from Arthrospira platensis by Addition of Saccharides and Sugar Alcohols. Foods, 11(12), 1752. https://doi.org/10.3390/foods11121752
Patel, A., Mishra, S., Pawar, R., & Ghosh, P. K. (2005). Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expression and Purification, 40(2), 248–255. https://doi.org/10.1016/j.pep.2004.10.028
Chaiklahan, R., Chirasuwan, N., Loha, V., Tia, S., & Bunnag, B. (2018). Stepwise extraction of high-value chemicals from Arthrospira (Spirulina) and an economic feasibility study. Biotechnology Reports, 20, e00280. https://doi.org/10.1016/j.foodchem.2020.126374
Rossano, R., Ungaro, N., D’Ambrosio, A., Liuzzi, G. M., & Riccio, P. (2003). Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongata Ellis & Solander. Journal of biotechnology, 101(3), 289–293. https://doi.org/10.1016/S0168-1656(03)00002-6
Sørensen, L., Hantke, A., & Eriksen, N. T. (2013). Purification of the photosynthetic pigment C-phycocyanin from heterotrophic Galdieria sulphuraria. Journal of the Science of Food and Agriculture, 93(12), 2933–2938. https://doi.org/10.1002/jsfa.6116
Avci, S., & Haznedaroglu, B. Z. (2022). Pretreatment of algal and cyanobacterial biomass for high quality phycocyanin extraction. Journal of Applied Phycology, 2, 1–12. https://doi.org/10.1007/s10811-022-02770-7
Carfagna, S., Landi, V., Coraggio, F., Salbitani, G., Vona, V., Pinto, G., & Ciniglia, C. (2018). Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Research, 31, 406–412. https://doi.org/10.1016/j.algal.2018.02.030
Imbimbo, P., Romanucci, V., Pollio, A., Fontanarosa, C., Amoresano, A., Zarrelli, A., & Monti, D. M. (2019). A cascade extraction of active phycocyanin and fatty acids from Galdieria phlegrea. Applied Microbiology and Biotechnology, 103(23), 9455–9464. https://doi.org/10.1007/s00253-019-10154-0
Ferraro, G., Imbimbo, P., Marseglia, A., Illiano, A., Fontanarosa, C., Amoresano, A., & Merlino, A. (2020). A thermophilic C-phycocyanin with unprecedented biophysical and biochemical properties. International journal of biological macromolecules, 150, 38–51. https://doi.org/10.1016/j.ijbiomac.2020.02.045
Sathuvan, M., Thangam, R., Venkateshbabu, G., Cheong, K. L., Kang, H., & Liu, Y. (2022). Single-step purified R-phycoerythrin transmits cellular imaging functionalities in vitro. International Journal of Biological Macromolecules, 194, 563–570. https://doi.org/10.1016/j.ijbiomac.2021.11.099
Denis, C., Massé, A., Fleurence, J., & Jaouen, P. (2009). Concentration and pre-purification with ultrafiltration of a R-phycoerythrin solution extracted from macro-algae Grateloupia turuturu: Process definition and up-scaling. Separation and Purification Technology, 69(1), 37–42. https://doi.org/10.1016/j.seppur.2009.06.017
Sonani, R. R., Singh, N. K., Kumar, J., Thakar, D., & Madamwar, D. (2014). Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochemistry, 49(10), 1757–1766. https://doi.org/10.1016/j.procbio.2014.06.022
Ghosh, T., Chatterjee, S., Bhayani, K., & Mishra, S. (2020). A natural cyanobacterial protein C-phycoerythrin as an Hg 2+ selective fluorescent probe in aqueous systems. New Journal of Chemistry, 44(16), 6601–6609. https://doi.org/10.1039/D0NJ01059F
Johnson, E. M., Kumar, K., & Das, D. (2014). Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresource Technology, 166, 541–547. https://doi.org/10.1016/j.biortech.2014.05.097
Soni, B., Trivedi, U., & Madamwar, D. (2008). A novel method of single step hydrophobic interaction chromatography for the purification of phycocyanin from Phormidium fragile and its characterization for antioxidant property. Bioresource Technology, 99(1), 188–194. https://doi.org/10.1016/j.biortech.2006.11.010
Roy, D., & Pabbi, S. (2022). A simple improved protocol for purification of C-phycocyanin from overproducing cyanobacteria and its characterization. Journal of Applied Phycology, 34(2), 799–810. https://doi.org/10.1007/s10811-021-02649-z
Xu, Y., Wang, Q., & Hou, Y. (2020). Efficient purification of R-phycoerythrin from marine algae (Porphyra yezoensis) based on a deep eutectic solvents aqueous two-phase system. Marine drugs, 18(12), 618. https://doi.org/10.3390/md18120618
Wang, Q., Zhang, Q., Zhang, A., & Hou, Y. (2022). Three-phase partitioning, a recyclable method for the purification of R-phycoerythrin from a red algae Porphyra yezoensis. Preparative Biochemistry & Biotechnology, 2, 1–8. https://doi.org/10.1080/10826068.2022.2065685
Bermejo, R., Acién, F. G., Ibáñez, M. J., Fernández, J. M., Molina, E., & Alvarez-Pez, J. M. (2003). Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. Journal of Chromatography B, 790(1–2), 317–325. https://doi.org/10.1016/S1570-0232(03)00168-5
Benavides, J., & Rito-Palomares, M. (2006). Simplified two-stage method to B-phycoerythrin recovery from Porphyridium cruentum. Journal of Chromatography B, 844(1), 39–44. https://doi.org/10.1016/j.jchromb.2006.06.029
Hernandez-Mireles, T., & Rito-Palomares, M. (2006). Improved recovery of B-phycoerythrin produced by the red microalga Porphyridium cruentum. Journal of Chemical Technology & Biotechnology: International Research in Process, Environmental & Clean Technology, 81(6), 989–996. https://doi.org/10.1002/jctb.1503
Ruiz-Ruiz, F., Benavides, J., & Rito-Palomares, M. (2013). Scaling-up of a B-phycoerythrin production and purification bioprocess involving aqueous two-phase systems: Practical experiences. Process Biochemistry, 48(4), 738–745. https://doi.org/10.1016/j.procbio.2013.02.010
Gonzalez-Ramirez, E., Andujar-Sanchez, M., Ortiz-Salmeron, E., Bacarizo, J., Cuadri, C., Mazzuca-Sobczuk, T., & Martinez-Rodriguez, S. (2014). Thermal and pH stability of the B-phycoerythrin from the red algae Porphyridium cruentum. Food Biophysics, 9(2), 184–192. https://doi.org/10.1007/s11483-014-9331-x
Marcati, A., Ursu, A. V., Laroche, C., Soanen, N., Marchal, L., Jubeau, S., & Michaud, P. (2014). Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Research, 5, 258–263. https://doi.org/10.1016/j.algal.2014.03.006
Ibáñez-González, M. J., Mazzuca-Sobczuk, T., Redondo-Miranda, R. M., Molina-Grima, E., & Cooney, C. L. (2016). A novel vortex flow reactor for the purification of B-phycoerythrin from Porphyridium cruentum. Chemical Engineering Research and Design, 111, 24–33. https://doi.org/10.1016/j.cherd.2016.03.032
Tang, Z., Ju, B., Li, W., Wen, S., Pu, Y., & Qin, S. (2016). One-step chromatographic procedure for purification of B-phycoerythrin from Porphyridium cruentum. Protein Expression and Purification, 123, 70–74. https://doi.org/10.1016/j.pep.2016.01.018
Huang, Z., Guo, S., Guo, Z., He, Y., & Chen, B. (2022). Integrated green one-step strategy for concurrent recovery of phycobiliproteins and polyunsaturated fatty acids from wet Porphyridium biomass. Food Chemistry, 389, 133103. https://doi.org/10.1016/j.foodchem.2022.133103
Pu, Y., Dong, S., Li, M., Dong, K., Zhao, H., Tang, Z., & Li, W. (2022). Efficient purification and characterization of high-purity phycoerythrin 545 from Rhodomonas sp. https://doi.org/10.21203/rs.3.rs-1947300/v1
Abalde, J., Betancourt, L., Torres, E., Cid, A., & Barwell, C. (1998). Purification and characterization of phycocyanin from the marine cyanobacterium Synechococcus sp. IO9201. Plant Science, 136(1), 109–120. https://doi.org/10.1016/S0168-9452(98)00113-7
Sonani, R. R., Patel, S., Bhastana, B., Jakharia, K., Chaubey, M. G., Singh, N. K., & Madamwar, D. (2017). Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresource technology, 245, 325–331. https://doi.org/10.1016/j.biortech.2017.08.129
Antecka, A., Klepacz-Smółka, A., Szeląg, R., Pietrzyk, D., & Ledakowicz, S. (2022). Comparison of three methods for thermostable C-phycocyanin separation and purification. Chemical Engineering and Processing-Process Intensification, 171, 108563. https://doi.org/10.1016/j.cep.2021.108563
Ramos, A., Acien, F. G., Fernandez-Sevilla, J. M., Gonzalez, C. V., & Bermejo, R. (2011). Development of a process for large-scale purification of C-phycocyanin from Synechocystis aquatilis using expanded bed adsorption chromatography. Journal of Chromatography B, 879(7–8), 511–519. https://doi.org/10.1016/j.jchromb.2011.01.013
Puzorjov, A., Unal, S. M., Wear, M. A., & McCormick, A. J. (2022). Pilot scale production, extraction and purification of a thermostable phycocyanin from Synechocystis sp PCC 6803. Bioresource Technology, 345, 126459. https://doi.org/10.1016/j.biortech.2021.126459
U.S. Food and Drug Administration, in: Color Additive Inventories - Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices, 2016, pp. 1–20 (accessed November 10, 2022), https://www.fda.gov/industry/color-additive-inventories/summary-color-additives-use-united-states-foods-drugs-cosmetics-and-medical-devices
European Commission, Guidance notes on the classification of food extracts with coloring properties, 2013. https://publications.jrc.ec.europa.eu/repository/handle/JRC96974
Vonshak, A., & Richmond, A. (1988). Mass production of the blue green alga Spirulina: an overview. Biomass, 15, 233–247.
Wicaksono, H. A., Satyantini, W. H., & Masithah, E. D. (2019). The spectrum of light and nutrients required to increase the production of phycocyanin Spirulina platensis. IOP Conference Series Earth and Environmental Science. https://doi.org/10.1088/1755-1315/236/1/012008
Luimstra, V. M., Schuurmans, J. M., Verschoor, A. M., Hellingwerf, K. J., Huisman, J., & Matthijs, H. C. P. (2018). Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynthesis Research, 138, 177–189. https://doi.org/10.1007/s11120-018-0561-5
Fujita, Y. (1997). A study on the dynamics features of photosystem stoichiometry: Accomplishments and problems for future studies. Photosynthesis Research, 53, 83–93. https://doi.org/10.1023/a:1005870301868
Myers, J., Graham, J. R., & Wang, R. T. (1980). Light harvesting in Anaystis nidulans studied in pigment mutants. Plant Physiology, 66, 1144–1149. https://doi.org/10.1104/pp.66.6.1144
Mullineaux, C. W. (2008). Phycobilisome-reaction centre interaction in cyanobacteria. Photosynthesis Research, 95, 175–182. https://doi.org/10.1007/s11120-007-9249-y
García-López, D. A., Olguín, E. J., González-Portela, R. E., Sánchez-Galván, R., Lowitt, R. W., Llewllyn, C. A., Fuentes, G. C., & Parra Saldívar, R. (2020). A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresource Technology, 298, 122548.
Figueroa, F. L., Aguilera, J., & Niell, F. X. (1995). Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). European Journal of Phycology, 30, 11–18. https://doi.org/10.1080/09670269500650761
Milia, M., Corrias, F., Piero, A. P., Chini Zittelli, G., Cicchi, B., Torzillo, G., Andreotti, V., & Angioni, A. (2022). Influence of different light sources on the biochemical composition of Arthrospira spp., grown in model systems. Food, 11(3), 399. https://doi.org/10.3390/foods11030399
Park, J., & Dinh, T. B. (2019). Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresource Technology, 291, 121846. https://doi.org/10.1016/j.biortech.2019.121846
Manirafasha, E., Murwanashyaka, T., Ndikubwimana, T., Ahmed, N. R., Liu, J., Lu, Y., Zeng, X., Ling, K., & Jing, K. (2018). Enhancement of cell growth and phycocyanin production in Arthrospira (Spirulina) platensis by metabolic stress and nitrate fed-batch. Bioresource Technology, 255, 293–301. https://doi.org/10.1016/j.biortech.2017.12.068
Mousavi, M., Mehrzad, J., Najafi, M. F., Zhaiani, R., & Shamsian, S. A. A. (2022). Nitrate and ammonia: Two key nitrogen sources for biomass and phycocyanin production by Arhrospira (Spirulina) platensis. Journal Applied Phycology, 34, 2271–2281. https://doi.org/10.1007/s10811-021-02664-0
Boussiba, S., & Gibson, J. (1991). Ammonia traslocation in of ammonia toxicity cyanobacteria. FEMS Microbiology Review, 88, 1–14. https://doi.org/10.1016/0378-1097(91)90692-4
Drath, M., Knoft, N., Batshauer, A., Marin, K., Novak, J., & Forchhammer, K. (2008). Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiology, 147(1), 206–215. https://doi.org/10.1104/pp.108.117218
Markou, G., & Muylaert, K. (2016). Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis and Chlorella vulgaris. Bioresource Technology, 216, 453–461. https://doi.org/10.1016/j.biortech.2016.05.094
Markou, G., Vandamme, D., & Muylaert, K. (2014). Ammonia inhibition on Arthrospira platensis in relation to the initial biomass density and pH. Bioresource Technology, 156, 259–265. https://doi.org/10.1016/j.biortech.2014.05.040
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilizing products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003.
Borowitska, M. A., & Vonshak, A. (2017). Scaling up microalgal cultures to commercial scale. European Journal Phycology, 4, 407–418. https://doi.org/10.1080/09670262.2017.1365177
Mendoza, J. L., Granados, M. R., de Godos, I., Acién, F. G., Molina, E., Heaven, S., & Banks, C. J. (2013). Oxygen transfer and evolution in microalgal culture in open raceways. Bioresource Technology, 137, 188–195. https://doi.org/10.1016/j.biortech.2013.03.127
Torzillo, G., Giovannetti, L., Bocci, F., & Materassi, R. (1984). Effect of oxygen concentration on the protein content of Spirulina biomass. Biotechnology Bioengineering, 26, 1134–1135. https://doi.org/10.1002/bit.260260920
Torzillo, G., Pushparaj, B., Bocci, F., Balloni, W., Materassi, R., & Florenzano, G. (1986). Production of Spirulina biomass in closed photobioreactors. Biomass, 11, 61–64. https://doi.org/10.1016/0144-4565(86)90021-1
Hidasi, N., & Belay, A. (2018). Diurnal variation of various culture and biochemical parameters of Arthrospira platensis in large-scale outdoor ponds. Algal Research, 29, 212–129. https://doi.org/10.1016/0144-4565(86)90021-1
Torzillo, G., Sacchi, A., & Materassi, R. (1991). Temperature as an important factor affecting productivity and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. Bioresource Technology, 28, 95–100. https://doi.org/10.1016/0960-8524(91)90137-9
Borowitska, M. A. (2016). Algal physiology and largescale outdoor cultures of microalgae. In M. A. Borowitzka, J. Beardall, & J. A. Raven (Eds.), The Physiology of Microalgae, Developments in Applied PHYCOLOGY (pp. 601–652). Springer International Publishing.
Tomaselli, L., Torzillo, G., Giovannetti, L., Pushparaj, B., Bocci, F., Tredici, M. R., Papuzzo, T., Balloni, W., & Materassi, R. (1987). Recent research on Spirulina in Italy. Hydrobiologia, 151(152), 79–82.
Kumar, M., Kulshreshtha, J., & Singh, G. P. (2011). Growth and pigment accumulation of cyanobacterium Spirulina platensis at different light intensities and temperature. Brazilian Journal of Microbiology, 42, 1128–1135. https://doi.org/10.1590/S1517-838220110003000034
Maurya, S. S., Maurya, J. N., & Pandey, V. D. (2014). Factors regulating phycobiliprotein production in cyanobacteria. International. Journal Current. Microbiology and Applied Science, 3, 764–771.
De Oliveira, M. A. C. L., Monteiro, M. P. C., Robbs, P. G., & Leite, S. G. F. (1999). Growth and biochemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquaculture International, 7, 261–275. https://doi.org/10.1023/A:1009233230706
Belay, A. (1997). Mass culture of Spirulina outdoors—the Earthrise Farm experience. In A. Vonshak (Ed.), Spirulina platensis (Arthrospira): Physiology, Cell-biology and Biotechnology (pp. 131–158). Taylor and Francis.
Sakamoto, T., & Bryant, D. A. (1998). Growth at low temperature causes nitrogen limitation in the cyanobacterium Synechococcus sp. PCC 7002. Archives of Microbiology, 169, 10–19. https://doi.org/10.1007/s002030050535
Vonshak, A., Torzillo, G., Masojídek, J., & Boussiba, S. (2001). Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environment, 24, 1113–1118. https://doi.org/10.1046/j.0016-8025.2001.00759.x
Torzillo, G., Bernardini, P., & Masojidek, J. (1998). On-line monitoring of chlorophyll fluorescence to assess the extent of photoinhibition of photosynthesis induced by high oxygen concentration and low temperature and its effect on the productivity of outdoor cultures of Spirulina platensis (Cyanobacteria). Journal of Phycology, 34, 504–510. https://doi.org/10.1046/j.1529-8817.1998.340504.x
Gupta, A., Mohan, D., Saxena, R. K., & Singh, S. (2018). Phototrophic cultivation of NaCl tolerant of Spirulina platensis for enhanced C phycocyanin production under optimized culture conditions and its dynamic modeling. Journal of Phycology, 54, 44–55. https://doi.org/10.1111/jpy.12597
Teng Tan, H., Yusoff, M. F., Khaw, Y. S., Nazarudin, M. F., Noor Mazli, N. A. I., Ahmad, S. A., Shaharuddin, N. A., & Toda, T. (2022). Characterisation and selection of freshwater cyanobacteria for phycobiliprotein contents. Aquaculture International. https://doi.org/10.1007/s10499-022-00985-6
Foy, R. H. (1980). The influence of surface to volume ratio on the growth rates of planktonic blue-green algae. British Phycology Journal, 15, 279–289. https://doi.org/10.1080/00071618000650281
Aouir, A., Amiali, M., Bitam, A., Benchabane, A., & Raghavan, V. G. (2017). Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. Food Measure, 11, 913–923. https://doi.org/10.1007/s11694-016-9463-4
Tredici, M. R., & Chini Zittelli, G. (1997). Cultivation of Spirulina (Arthrospira) platensis in flat plate reactor. In A. Vonshak (Ed.), Spirulina platensis (Arthrospira) Physiology cell biology and Biotechnology (pp. 117–130). Taylor & Francis.
Pinto, G., Ciniglia, C., Cascone, C., & Pollio, A. (2007). Species composition of Cyanidiales assemblages in Pisciarelli (Campi Flegrei, Italy) and description of Galdieria phlegrea sp Nov. In J. Seckbach (Ed.), Algae and Cyanobacteria in Extreme Environments Cellular Origin, Life in Extreme Habitats and Astrobiology (pp. 489–502). Springer. https://doi.org/10.1007/978-1-4020-6112-7_26
Carfagna, S., Napolitano, G., Barone, D., Pinto, G., Pollio, A., & Venditti, P. (2015). Dietary supplementation with the microalga Galdieria sulphuraria (Rhodophyta) reduces prolonged exercise-induced oxidative stress in rat tissue. Oxidative Medicine and Cell Longevity. https://doi.org/10.1155/2015/732090
Abiusi, F., Trompetter, E., Henink, H., Wijffels, R. H., & Janssen, M. (2021). Autotrophic and mixotrophic biomass production of the acidophilic Galdieria sulpuraria ACUF 64. Algal Research, 60, 102513. https://doi.org/10.1016/j.algal.2021.102513
Abiusi, F., Moñino, P. F., Canziani, S., Janssen, M., Wijffels, R. H., & Barbosa, M. (2022). Mixotrophic cultivation of Gadieria sulphuraria for C-phycocyanin and protein production. Algal Research, 31, 102603. https://doi.org/10.1016/j.algal.2021.102603
Perez Saura, P., Chabi, M., Corato, A., Cardol, P., & Remacle, C. (2022). Cell adaptability of the extremophilic red microalga Galdieria suphuraria to the availability of carbon sources. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2022.978246
European Union. (2015). Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods. Official Journal of the European Union, L327/1 of 11 December 201, pp 1–22. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri5CELEX:32015R2283&from5EN
Eriksen, N. (2008). Production of phycocyanin- a pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology Biotechnology, 80, 1–14. https://doi.org/10.1007/s00253-008-1542-y
European Commission Regulation (EC) No 133/208 of the European Parliament and of the Council of 16 December 2008 on food additives. Official Journal European Union, 51(2008), 16–34.
Wan, M., Wang, Z., Zhang, Z., Wang, J., Yu, A., & Li, Y. (2015). A novel paradigm for the high.efficient production of phycocyanin from Galdieria sulphuraria. Bioresource Technology, 218, 272–278. https://doi.org/10.1016/j.biortech.2016.06.045
Rahman, D. Y., Sarian, F. D., van Wijk, A., Martinez Garcia, M., & van der Maarel, M. J. E. C. (2017). Thermostable phycocyanin from red alga Cyanidioschyzon merolae, a new natural blue food colorant. Journal Applied Phycology, 29, 1233–1239. https://doi.org/10.1007/s108111-016.1007-0
Barbier, G., Oesterhelt, C., Larson, M. D., Halgren, R. G., Wilkerson, C., Garavito, R. M., Benning, C., & Weber, A. P. M. (2005). Comparative genomics of two closely related unicellular thermo-acidophilic red algae, Galdieria sulphuraria and Cyanidioschyzon merolae, reveals the molecular basis of the metabolic flexibility of Galdieria sulphuraria and significant differences in carbohydrate metabolism of both algae. Plant Physiology, 137(2), 460–474. https://doi.org/10.1104/pp.104.051169
Faieta, M., Neri, L., Sacchetti, G., Di Michele, A., & Pittia, P. (2020). Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Research International, 132, 109093. https://doi.org/10.1016/foodres.2020.109093
Adjali, A., Clarot, I., Chen, Z., Marchioni, E., & Boudier, A. (2022). Physicochemical degradation of phycocyanin and means to improve its stability: A short review. Journal of Pharmaceutical Analysis, 12, 406–414. https://doi.org/10.1016/j.jpha.2021.12.005
Tan, H. T., Yusoff, F., Khaw, Y. S., Nazarudin, M. F., Mazli, N. A. I. N., Ahmad, S. A., Shaharuddin, N. A., & Toda, T. (2022). Characterization and selection of freshwater cyanobacteria for phycobiliprotein contents. Aquaculture International. https://doi.org/10.1007/s10499-022-00985-6
Chaiklahan, R., Chirasuwan, N., & Bunnag, B. (2012). Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochemistry, 47, 659–664. https://doi.org/10.1016/j.procbio.2012.01.010
Wu, H.-L., Wang, G.-H., Xiang, W.-Z., Li, T., & He, H. (2016). Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. International Journal of Food Properties, 19(10), 2349–2362. https://doi.org/10.1080/10942912.2015.1038564
Böcker, L., Ortmann, S., Surber, J., Leeb, E., Reineke, K., & Mathys, A. (2019). Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innovative Food Science and Emerging Technologies, 52, 116–121. https://doi.org/10.1016/j.ifset.2018.11.007
Wan, M., Zhao, H., Yan, L., Zhang, D., & Bai, W. (2021). Comparison of C-phycocyanin from Galdieria sulphuraria and Spirulina platensis on stability and antioxidant capacity. Algal Research, 58, 102391. https://doi.org/10.1016/j.algal.2021.102391
Choi, W. Y., & Lee, H. Y. (2018). Kinetic analysis of stabilizing C-phycocyanin in the Spirulina platensis extracts from ultrasonic process associated with effects of light and temperature. Applied Sciences, 8, 1662. https://doi.org/10.3390/app8091662
Pérez-Rico, D., Alarcón-Jiménez, J. L., González-Morales, E., & Guerra- Álvarez, L. F. (2020). Phycocyanin thermo-photostability: An accelerated life-test analysis. Journal Mexican Chemistry Society, 64(3), 218–229.
Miskra, S. K., Shrivastav, A., & Mishra, S. (2008). Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochemistry, 43(4), 339–345. https://doi.org/10.1016/j.procbio.2007.12.012
Insights, F. M. (2018). Phycocyanin Market: Food & Beverage: Global Industry Opportunity Assessment (2018–2028). [WWW Document]. Futur. Mark. Insights. https://www.futuremarketinsights.com/reports/phycocyanin-market
Chen, K. H., Wang, S. S. S., Show, P. L., Lin, G. T., & Chang, Y. K. (2018). A rapid and efficient technique for direct extraction of C-phycocyanin from highly turbid Spirulina platensis algae using hydrophobic interaction chromatography in stirred fluidized bed. Biochemical Engineering Journal, 140, 47–56. https://doi.org/10.1016/j.bej.2018.09.005
Chang, Y. K., Show, P. L., Lan, J. C. W., Tsai, J. C., & Huang, C. R. (2018). Isolation of C-phycocyanin from Spirulina platensis microalga using Ionic liquid based aqueous two-phase system. Bioresource Technology, 270, 320–327. https://doi.org/10.1016/j.biortech.2018.07.138
Hilali, S., Wils, L., Chevalley, A., Clément-Larosière, B., & Boudesocque-Delaye, L. (2022). Glycerol-based NaDES as green solvents for ultrasound-assisted extraction of phycocyanin from Arthrospira platensis—RSM optimization and ANN modelling. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-021-02263-6
Simon, U., Scorza, L. C., Teworte, S., McCormick, A. J., & Dimartino, S. (2021). Demonstration of protein capture and separation using three-dimensional printed anion exchange monoliths fabricated in one-step. Journal of Separation Science, 44(6), 1078–1088. https://doi.org/10.1002/jssc.202000722
Lauceri, R., Cavone, C., Zittelli, G. C., Kamburska, L., Musazzi, S., & Torzillo, G. (2022). High purity grade phycocyanin recovery by decupling cell lysis from the pigment extraction–an innovative approach. https://doi.org/10.21203/rs.3.rs-2060629/v1
Gupta, A. K., Seth, K., Maheshwari, K., Baroliya, P. K., Meena, M., Kumar, A., Vinayak, V., & Harish,. (2021). Biosynthesis and extraction of high-value carotenoid from algae. Frontiers in Bioscience-Landmark, 6, 171–190. https://doi.org/10.52586/4932
Ren, Y., Sun, H., Deng, J., Huang, J., & Chen, F. (2021). Carotenoid production from microalgae: Biosynthesis, salinity responses and novel biotechnologies. Marine Drugs, 19, 713. https://doi.org/10.1016/j.foodchem.2018.10.066
Dautermann, O., Lyska, D., Andersen-Ranberg, J., Becker, M., Fröhlich-Nowoisky, J., Gartmann, H., et al. (2020). An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Science Advances. https://doi.org/10.1126/sciadv.aaw9183
Di Lena, G., Casini, I., Lucarini, M., & Lombardi-Boccia, G. (2019). Carotenoid profiling of five microalgae species from large-scale production. Food Research International, 120, 810–818. https://doi.org/10.1016/j.foodres.2018.11.043
Yang, R., & Wei, D. (2020). Improving fucoxanthin production in mixotrophic culture of marine diatom Phaeodactylum tricornutum by LED light shift and nitrogen supplementation. Frontiers in Bioengineering and Biotechnology, 8, 820. https://doi.org/10.3389/fbioe.2020.00820
Gómez-Loredo, A., Benavides, J., & Rito-Palomares, M. (2015). Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. Journal of Applied Phycology, 28, 849–860. https://doi.org/10.1007/s10811-015-0635-0
Wang, F., Huang, L., Gao, B., & Zhang, C. (2018). Optimum production conditions, purification, identification, and antioxidant activity of violaxanthin from Microalga Eustigmatos cf. polyphem (Eustigmatophyceae). Marine Drugs, 16(6), 190–203. https://doi.org/10.3390/md16060190
Faraloni, C., & Torzillo, G. (2010). Phenotypic characterization and hydrogen production in Chlamydomonas reinhardtii QB binding D1 protein mutants under sulfur starvation: Changes in chlorophyll fluorescence and pigment composition. Journal of Phycology, 46(4), 788–799. https://doi.org/10.1111/j.1529-8817.2010.00857.x
Lacour, T., Marcel Babin, M., & Lavaud, J. (2020). Diversity in xanthophyll cycle pigments content and related nonphotochemical quenching (NPQ) among microalgae: Implications for growth strategy and ecology. Journal of Phycology, 56(2), 245–263. https://doi.org/10.1111/jpy.12944
Ma, R., Zhang, Z., Ho, S. H., Ruan, C., Li, J., Xie, Y., Shi, X., Liu, L., & Jianfeng, C. J. (2020). Two-stage bioprocess for hyper-production of lutein from microalga Chlorella sorokiniana FZU60: Effects of temperature, light intensity, and operation strategies. Algal Research, 52, 102119. https://doi.org/10.1016/j.algal.2020.102119
Gong, M., & Bassi, A. (2016). Carotenoids from microalgae: A review of recent developments. Biotechnology Advances, 34, 1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005
McClure, D. D., Nightingale, J. K., Luiz, A., Black, S., Zhu, J., & Kavanagh, J. M. (2019). Pilot-scale production of lutein using Chlorella vulgaris. Algal Research, 44, 101707. https://doi.org/10.1016/j.algal.2019.101707
Přibyl, P., Pilný, J., Cepák, V., & Kaštánek, P. (2016). The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Research, 16, 69–75. https://doi.org/10.1016/j.algal.2016.02.028
Wang, X., Zhang, M. M., Sun, Z., Liu, S. F., Qin, Z. H., Mou, J. H., Zhou, Z. G., & Lin, C. (2020). Sustainable lipid and lutein production from Chlorella mixotrophic fermentation by food waste hydrolysate. Journal of Hazardous Materials, 400, 123258. https://doi.org/10.1016/j.jhazmat.2020.123258
Chen, J. H., Chen, C. Y., Hasunuma, T., Kondo, A., Chang, C. H., Ng, I. S., & Chang, J. S. (2019). Enhancing lutein production with mixotrophic cultivation of Chlorella sorokiniana MB-1-M12 using different bioprocess operation strategies. Bioresource Technology, 278, 17–25. https://doi.org/10.1016/j.biortech.2019.01.041
Sathasivam, R., & Ki, J. (2018). A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs, 16, 26. https://doi.org/10.3390/md16010026
Soontornchaiboon, W., Joo, S. S., & Kim, S. M. (2012). Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biological and Pharmaceutical Bulletin, 35(7), 1137–1144. https://doi.org/10.1248/bpb.b12-00187
Kim, J., Lee, S., Baek, K., & Jin, E. (2020). Site-specific gene knock-out and on-site heterologous gene overexpression in Chlamydomonas reinhardtii via a CRISPR-Cas9-mediated knock-in method. Frontiers in Plant Science, 11, 306. https://doi.org/10.3389/fpls.2020.00306
Rammuni, M., Ariyadasa, T. U., Nimarshana, P., & Attalage, R. (2018). Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chemistry, 277, 128–134. https://doi.org/10.1016/j.foodchem.2018.10.066
Mitra, M., & Mishra, S. (2019). A comparative analysis of different extraction solvent systems on the extractability of eicosapentaenoic acid from the marine eustigmatophyte Nannochloropsis oceanica. Algal Research, 38, 101387. https://doi.org/10.1016/j.algal.2018.101387
Pushpalatha, S., Sangeetha, R., Ariraman, S., Ashokkumar, B., & Varalakshmi, P. (2020). Photocatalyst (TiO2) as an enhancer: An at-tempt to enhance the production of carotenoids and lipids with the combined oxidative stresses in Coelastrella sp. Clean Technologies and Environmental Policy, 23, 41–53. https://doi.org/10.1007/s10098-020-01879-y
Koo, S. Y., Cha, K. H., Song, D. G., Chung, D., & Pan, C. H. (2011). Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. Environmental Biology Fishes, 24, 725–730. https://doi.org/10.1007/s10811-011-9691-2
Singh, D., Barrow, C. J., Mathur, A. S., Tuli, D. K., & Puri, M. (2015). Optimization of zeaxanthin and β-carotene extraction from Chlorella saccharophila isolated from New Zealand marine waters. Biocatalysis and Agricultural Biotechnology, 4(2), 166–173. https://doi.org/10.1016/j.bcab.2015.02.001
Raposo, M., de Morais, A., de Morais, R., Raposo, M. F. J., De Morais, A. M. M. B., & De Morais, R. M. S. C. (2015). Carotenoids from marine microalgae: a valuable natural source for the prevention of chronic diseases. Marine Drugs, 13, 5128–5155. https://doi.org/10.3390/md13085128
Kuczyńska, P., & Jemiola-Rzeminska, M. (2017). Isolation and purification of all-trans diadinoxanthin and all-trans diatoxanthin from diatom Phaeodactylum tricornutum. Journal of Applied Phycology, 29, 79–87. https://doi.org/10.1007/s10811-016-0961-x
Pulz, O., & Gross, W. (2004). Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65(6), 635–648. https://doi.org/10.1007/s00253-004-1647-x
Liaqat, F., Khazi, M. I., Bahadar, A., He, L., Aslam, A., Liaquat, R., Agathos, S. N., & Li, J. (2022). Mixotrophic cultivation of microalgae for carotenoid production. Reviews in Aquaculture, 2, 1–27. https://doi.org/10.1111/raq.12700
Serra, A. T., Silva, S. D., Pleno de Gouveia, L., Alexandre, A. M. R. C., Pereira, C. V., Pereira, A. B., Partidário, C., Silva, N. E., Bohn, T., Gonçalves, V. S., et al. (2021). A single dose of marine Chlorella vulgaris increases plasma concentrations of lutein, β-carotene and zeaxanthin in healthy male volunteers. Antioxidants, 10, 1164. https://doi.org/10.3390/antiox10081164
Hu, J., Nagarajan, D., Zhanga, Q., Chang, J. S., & Lee, D. J. (2018). Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnology Advances, 36(1), 54–67. https://doi.org/10.1016/j.biotechadv.2017.09.009
Chen, J. H., Chen, C. Y., & Chang, J. S. (2017). Lutein production with wild-type and mutant strains of Chlorella sorokiniana MB-1 under mixotrophic growth. Journal of the Taiwan Institute of Chemical Engeeners, 79, 66–73. https://doi.org/10.1016/j.jtice.2017.04.022
Shi, X. M. (2002). High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnology Progress, 18, 723–727. https://doi.org/10.1021/bp0101987
Singh, D. P., Khattar, J. S., Rajput, A., Chaudhary, R., & Singh, R. (2019). High production of carotenoids by the green microalga Asterarcys quadricellulare PUMCC 5.1.1 under optimized culture conditions. PLoS ONE, 14(9), e0221930. https://doi.org/10.1371/journal.pone.0221930
Aluç, Y., Kök, O., & Tüzün, I. (2022). Profiling the carotenoids of microalga (Scenedesmus obliquus) extract by HPLC and its antioxidant capacity. Journal of Applied Biological Sciences, 16(2), 206–219. https://doi.org/10.5281/zenodo.6590217
Faraloni, C., Di Lorenzo, T., & Bonetti, A. (2021). Impact of light stress on the synthesis of both antioxidants polyphenols and carotenoids, as fast photoprotective response in Chlamydomonas reinhardtii: New prospective for biotechnological potential of this microalga. Symmetry, 213(11), 2. https://doi.org/10.3390/sym13112220
Huang, K., Su, Z., He, M., Wu, Y., & Wang, M. (2022). Simultaneous accumulation of astaxanthin and β-carotene in Chlamydomonas reinhardtii by the introduction of foreign β-carotene hydroxylase gene in response to high light stress. Biotechnological Letters, 44(2), 321–331. https://doi.org/10.1007/s10529-022-03230-5
Fernández-Sevilla, J. M., Acién- Fernández, F. G., & Molina-Grima, E. (2010). Biotechnological production of lutein and its applications. Applied Microbiology and Biotechnology, 86, 27–40. https://doi.org/10.1007/s00253-009-2420-y
Kleinegris, D. M. M., Janssen, M., Brandeburg, W. A., & Wijffels, R. H. (2011). Continuous production of carotenoids from Dunaliella salina. Enzyme Microbial Biotechnology, 85, 289–295. https://doi.org/10.1016/j.enzmictec.2010.11.005
Schüler, L. M., Santos, T., Pereira, H., Duarte, P., Gangadhar, N. K., Florindo, C., Schulze, P. S. C., Barreira, L., & Varel, J. C. S. (2019). Improved production of lutein and β-carotene by thermal and light intensity upshifts in the marine microalga Tetraselmis sp CTP4. Algal Research, 45, 101732. https://doi.org/10.1016/j.algal.2019.101732
Touloupakis, E., Faraloni, C., Silva Benavides, A. M., & Torzillo, G. (2021). Recent achievements in microalgal photobiological hydrogen production. Energies, 14, 7170. https://doi.org/10.3390/en14217170
Forján, E., Garbayo, I., Casal, C., & Vílchez, C. (2022). Enhancement of carotenoid production in Nannochloropsis by phosphate and sulphur limitation. In A. Méndez-Vilas (Ed.), Communicating Current Research and Educational Topics and Trends in Applied Microbiology (pp. 356–364). Microbiology Book Series.
Lamers, P. P., Janssen, M., De Vos, R. C., Bino, R. J., & Wijffels, R. H. (2012). Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. Journal of Biotechnology, 162(1), 21–27. https://doi.org/10.1016/j.jbiotec.2012.04.018
Rabbani, S., Beyer, P., Lintig, J., Hugueney, P., & Kleinig, H. (1998). Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiology, 116, 1239–1248. https://doi.org/10.1104/pp.116.4.1239
Belotti, G., de Caprariis, B., De Filippis, P., Scarsella, M., & Verdone, N. (2014). Effect of Chlorella vulgaris growing conditions on bio-oil production via fast pyrolysis. Biomass and Bioenergy, 6, 187–195. https://doi.org/10.1016/j.biombioe.2013.12.011
Barghbani, R., Rezaei, K., & Javanshir, A. (2012). Investigating the effects of several parameters on the growth of Chlorella vulgaris using Taguchi’s experimental approach. International Journal of Biotechnology for Wellness Industries, 1(2), 128–133. https://doi.org/10.6000/1927-3037/2012.01.02.04
Lasmarito, T. C., Widianingsih, W., & Endrawati, H. (2022). Kandungan Lutein Mikroalga Chlorella vulgaris dengan Salinitas Berbeda pada Media Kultur. Journal of Marine Research, 11(2), 320–326. https://doi.org/10.14710/jmr.v11i2.33819
Li, J., Zhao, X., Chang, J. S., & Miao, X. A. (2022). Two-stage culture strategy for scenedesmus sp FSP3 for CO2 fixation and the simultaneous production of lutein under light and salt stress. Molecules, 27, 7497. https://doi.org/10.3390/molecules2721749
Elloumi, W., Jebal, A., Maalej, A., Chamkha, M., & Sayadi, S. (2020). Effect of mild salinity stress on the growth, fatty acid and carotenoid compositions, and biological activities of the thermal freshwater microalgae Scenedesmus sp. Biomolecules, 10, 1515. https://doi.org/10.3390/biom10111515
Benavente-Valdésa, J. R., Aguilara, C., Contreras-Esquivela, J. C., Méndez-Zavalab, A., & Montañez, J. (2016). Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnology Reports, 10, 117–125. https://doi.org/10.1016/j.btre.2016.04.001
Jaime, L., Rodríguez-Meizoso, I., Cifuentes, A., Santoyo, S., Suarez, S., Ibáñez, E., et al. (2010). Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT Food Science and Technology, 43, 105–112. https://doi.org/10.1016/j.lwt.2009.06.023
Pasquet, V., Chérouvrier, J., Farhat, F., Thiéry, V., Piot, J., Bérard, J., et al. (2011). Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochemistry, 46, 59–67. https://doi.org/10.1016/j.procbio.2010.07.009
Khoo, K. S., Chew, K. W., Yew, G. Y., Manickam, S., & Ooi, C. W. (2020). Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrasonics Sonochemistry, 67, 105052. https://doi.org/10.1016/j.ultsonch.2020.105052
Chen, C., Jesisca Hsieh, C., Lee, D., Chang, C., & Chang, J. (2016). Production, extraction and stabilization of lutein from microalga Chlorella sorokiniana MB-1. Bioresource Technology, 200, 500–505. https://doi.org/10.1016/j.biortech.2015.10.071
Luengo, E., & Raso, J. (2017). Pulsed electric field-assisted extraction of pigments from Chlorella vulgaris. Handbook of Electroporation., 34, 2939–2954. https://doi.org/10.1007/978-3-319-32886-7_219
Molino, A., Mehariya, S., Iovine, A., Larocca, V., Di Sanzo, G., Martino, M., et al. (2018). Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Marine Drugs, 16, 432. https://doi.org/10.3390/md16110432
Kapoore, R. V., Butler, T. O., Pandhal, J., & Vaidyanathan, S. (2018). Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology, 7, 18. https://doi.org/10.3390/biology7010018
Fabrowska, J., Messyasz, B., Szyling, J., Walkowiak, J., & Łęska, B. (2018). Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycological Research, 66, 52–57. https://doi.org/10.1111/pre.12191
Chronopoulou, L., Dal Bosco, C., Di Caprio, F., Prosini, L., Gentili, A., Pagnanelli, F., et al. (2019). Extraction of carotenoids and fat soluble vitamins from Tetradesmus obliquus microalgae: An optimized approach by using supercritical CO2. Molecules, 24, 2581. https://doi.org/10.3390/molecules24142581
Şahin, S., Nasir, N. T. B., Erken, I., Çakmak, Z.-E., & Çakmak, T. (2019). Antioxidant composite films with chitosan and carotenoid extract from Chlorella vulgaris: optimization of ultrasonic-assisted extraction of carotenoids and surface characterization of chitosan films. Material Research Express, 6, 95404. https://doi.org/10.1088/2053-1591/ab2def
Jaeschke, D. P., Merlo, E. A., Mercali, G. D., Rech, R., & Marczak, L. D. F. (2019). The effect of temperature and moderate electric field pre-treatment on carotenoid extraction from Heterochlorella lu-189 teoviridis. International Journal of Food Science & Technology, 54, 396–402. https://doi.org/10.1111/ijfs.13950
Derwenskus, F., Metz, F., Gille, A., Schmid-Staiger, U., Briviba, K., Schließmann, U., et al. (2018). Pressurized extraction of unsaturated fatty acids and carotenoids from wet C. vulgaris and P. tricornutum biomass using subcritical liquids. GCB Bioenergy, 11, 35–344. https://doi.org/10.1111/gcbb.12563
Castro-Puyana, M., Pérez-Sánchez, A., Valdés, A., Ibrahim, O. H. M., Suarez-Álvarez, S., Ferragut, J. A., et al. (2017). Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Research International, 99, 1048–1055. https://doi.org/10.1016/j.foodres.2016.05.021
Cha, K. H., Lee, H. J., Koo, S. Y., Song, D., Lee, D., & Pan, C. (2010). Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. Journal of Agricultural and Food Chemistry, 58, 793–797. https://doi.org/10.1021/jf902628j
Guedes, A. C., Gião, M. S., Matias, A. A., Nunes, A. V. M., Pintado, M. E., Duarte, C. M. M., et al. (2013). Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. Journal of Food Engineering, 116, 478–482. https://doi.org/10.1016/j.jfoodeng.2012.12.015
Lafarga, T., Clemente, I., & Garcia-Vaquero, M. (2020). Carotenoids from microalgae. In M. G. Charis (Ed.), Carotenoids: Properties, Processing and Applications (pp. 149–187). Academic Press.
Mercadante, A. Z., Rodrigues, D. B., Petry, F. C., & Mariutti, L. R. B. (2017). Carotenoid esters in foods—A review and practical directions on analysis and occurrence. Food Research International, 99, 830–850. https://doi.org/10.1016/j.foodres.2016.12.018
Saini, R. K., Nile, S. H., & Park, S. W. (2015). Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Research International, 76, 735–750. https://doi.org/10.1016/j.foodres.2015.07.047
Camacho-Rodríguez, J., Macías-Sánchez, M., Cerón-García, M., Alarcón, F., & Molina-Grima, E. (2018). Microalgae as a potential ingredient for partial fish meal replacement in aquafeeds: Nutrient stability under different storage conditions. Journal of Applied Phycology, 30, 1049–1059. https://doi.org/10.1007/s10811-017-1281-5
Ortiz, D., Ponrajan, A., Bonnet, J. P., Rocheford, T., & Ferruzzi, M. G. (2018). Carotenoid stability during dry milling, storage, and extrusion processing of biofortified maize genotypes. Journal of Agricultural and Food Chemistry, 66, 4683–4691. https://doi.org/10.1021/acs.jafc.7b05706
Safafar, H., Langvad, S., Møller, P., & Jacobsen, C. (2017). Storage conditions affect oxidative stability and nutritional composition of freeze-dried Nannochloropsis salina. European Journal of Lipid Science and Technology, 119, 1600477. https://doi.org/10.1002/ejlt.201600477
Guest, J., & Grant, R. (2016). Carotenoids and neurobiological health. Advances in Neurobiology, 12, 199–228. https://doi.org/10.1007/978-3-319-28383-8_11
Mullan, K., Willimas, M. A., Cardwell, C. R., McGuinness, B., Passmore, P., Silvestri, G., Woodside, J. V., & McKay, G. J. (2017). Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 3, 432–439. https://doi.org/10.1016/j.trci.2017.06.006
Saini, R. K., Moon, S. H., Gansukh, E., & Keum, Y. (2018). An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chemistry, 266, 56–65. https://doi.org/10.1016/j.foodchem.2018.05.104
Araki, M., Kaku, N., Harada, M., Ando, Y., Yamaguchi, R., & Shindo, K. (2016). Production of auroxanthins from violaxanthin and 9-cis-violaxanthin by acidic treatment and the antioxidant activities of violaxanthin, 9- cis-violaxanthin, and auroxanthins. Journal of Agricultural and Food Chemistry, 64(49), 93529355. https://doi.org/10.1021/acs.jafc.6b04506
Landrum, J. T., & Bone, R. A. (2001). Lutein, zeaxanthin, and the macular pigment. Archives of Biochemistry and Biophysics, 385, 28–40. https://doi.org/10.1006/abbi.2000.2171
Kim, J., Lee, W., Rhee, H. C., & Kim, S. (2016). Red paprika (Capsicum annuum L.) and its main carotenoids, capsanthin and β-carotene, prevent hydrogen peroxide-induced inhibition of gap-junction intercellular communication. Chemico-Biological Interactions, 254, 146–155. https://doi.org/10.1016/j.cbi.2016.05.004
Jansen, R. J., Robinson, D. P., Stolzenberg-Solomon, R. Z., Bamlet, W. R., de Andrade, M., Oberg, A. L., et al. (2013). Nutrients from fruit and vegetable consumption reduce the risk of pancreatic cancer. Journal of Gastrointestinal Cancer, 44, 152–161. https://doi.org/10.1007/s12029-012-9441-y
Viuda-Martos, M., Sanchez-Zapata, E., Sayas-Barberá, E., Sendra, E., Pérez-Álvarez, J. A., & Fernández-López, J. (2014). Tomato and tomato byproducts. Human health benefits of lycopene and its application to meat products: A review. Critical Reviews in Food Science and Nutrition, 54, 1032–1049. https://doi.org/10.1080/10408398.2011.623799
Alvi, S., Ansari, I. A., Khan, I., Iqbal, J., & Khan, M. S. (2017). Potential role of lycopene in targeting proprotein convertase subtilisin/kexin type-9 to combat hypercholesterolemia. Free Radical Biology & Medicine, 108, 394–403. https://doi.org/10.1016/j.freeradbiomed.2017.04.012
Fábryová, T., Cheel, J., Kubáč, D., Hrouzeka, P., Vu, D. L., Tůmová, L., & Kopecký, J. (2019). Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Research, 41, 101574. https://doi.org/10.1016/j.algal.2019.101574
Saha, S. K., Ermis, H., & Murray, P. (2020). Marine microalgae for potential lutein production. Applied Science, 10(18), 6457. https://doi.org/10.3390/app10186457
Gong, X., Smith, J. R., Swanson, H. M., & Rubin, L. P. (2018). Carotenoid lutein selectively inhibits breast cancer cell growth and potentiates the effect of chemotherapeutic agents through ros-mediated mechanisms. Molecules, 23, 905. https://doi.org/10.3390/molecules23040905
Chidambara Murthy, K. N., Vanitha, A., Rajesha, J., Mahadeva Swamy, M., Sowmya, P. R., & Ravishankar, G. A. (2005). In vivo antioxidant activity of carotenoids from Dunaliella salina green microalga. Life Sciences, 76, 1381–1390. https://doi.org/10.1016/j.lfs.2004.10.015
Ambati, R. R., Gogisetty, D., Aswathanarayana, R. G., Ravi, S., Bikkina, P. N., Bo, L., & Yuepeng, S. (2019). Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Critical Reviews in Food Science and Nutrition, 59, 1880–1902. https://doi.org/10.1080/10408398.2018.143256
Lin, J., Lee, D., & Chang, J. (2015). Lutein production from biomass: Marigold flowers versus microalgae. Bioresource Technology, 184, 421–428. https://doi.org/10.1016/j.biortech.2014.09.099
Yaakob, Z., Ali, E., Zainal, A., Mohamad, M., & Takriff, M.,S. (20014). An overview: biomolecules from microalgae for animal feed and aquaculture. Journal of Biological Research, 21(6). https://doi.org/10.1186/2241-5793-21-6
Ventura, S. P. M., Nobre, B. P., Ertekin, F., Garcia-Vaquero, M., Viera, F., Koc, M., et al. (2017). Extraction of value added compounds from microalgae. In M. Gonzalez (Ed.), Microalgae-based biofuels and bioproducts From Feedstock cultivation to end products (pp. 461–483). Elsevier.
Lobstein, T., Brinsden, H., & Neveux, M. (2022). World Obesity Atlas 2022. World Obesity Federation 2022.
Beppu, F., Niwano, Y., Tsukui, T., Hosokawa, M., & Miyashita, K. (2009). Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. Journal of Toxicological Sciences, 34, 501–510.
Matsuno, T. (2001). Aquatic animal carotenoids. Fisheries Science, 67, 771–783. https://doi.org/10.1046/j.1444-2906.2001.00323
Wang, W., Yu, L.-J., Xu, C., Tomizaki, T., Zhao, S., Umena, Y., Chen, X., Qin, X., Xin, Y., Suga, M., Han, G., Kuan, T., & Shen, J.-R. (2019). Structural basis for blue-green light harvesting and energy dissipation in diatoms. Science, 363, 6427. https://doi.org/10.1126/science.aav0365
Mohibbullah, M., Haque, M. N., Sohag, A. A. M., Hossain, M. T., Zahan, M. S., Uddin, M. J., Hannan, M. A., Moon, I. S., & Choi, J.-S. (2022). A systematic review on marine algae-derived fucoxanthin: An update of pharmacological insights. Marine. Drugs, 20, 279. https://doi.org/10.3390/md20050279
Englert, G., Bjørnland, T., & Liaaen-Jensen, S. (1990). 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Magnetic Resonance in Chemistry, 28(6), 519–528. https://doi.org/10.1002/mrc.1260280610
Paiva, S. A. R., & Russell, R. M. (1999). β-carotene and other carotenoids as antioxidants. Journal of the American College of Nutrition, 18(5), 426–433. https://doi.org/10.1080/07315724.1999.10718880
Sathasivam, R., & Ki, J.-S. (2018). A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs, 16(1), 26. https://doi.org/10.3390/md16010026
Koo, S. Y., Hwang, J.-H., Yang, S.-H., Um, J.-I., Hong, K. W., Kang, K., Pan, C.-H., Hwang, K. T., & Kim, S. M. (2019). Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin. Marine Drugs, 17(5), 311. https://doi.org/10.3390/md17050311
Liu, M., Li, W., Chen, Y., Wan, X., & Wang, J. (2020). Fucoxanthin: a promising compound for human inflammation-related diseases. Life Sciences, 255, 117850. https://doi.org/10.1016/j.lfs.2020.117850
Zarekarizi, A., Hoffmann, L., & Burritt, D. (2019). Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. Journal of Applied Phycology, 31, 281–299. https://doi.org/10.1007/s10811-018-1558-3
Kawee-ai, A., Kuntiya, A., & Kim, S. M. (2013). Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Natural Product Communication, 8(10), 1381–1386.
Nakazawa, Y., Sashima, T., Hosokawa, M., & Miyashita, K. (2009). Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. Journal of Functional Foods, 1(1), 88–97. https://doi.org/10.1016/j.jff.2008.09.015
Petrushkina, M., Gusev, E., Sorokin, B., Zotko, N., Mamaeva, A., Filimonova, A., Kulikovskiy, M., Maltsev, Y., Yampolsky, I., Guglya, E., Vinokurov, V., Namsaraev, Z., & Kuzmin, D. (2017). Fucoxanthin production by heterokont microalgae. Algal Research, 24, 387–393. https://doi.org/10.1016/j.algal.2017.03.016
Pajot, A., Hao Huynh, G., Picot, L., Marchal, L., & Nicolau, E. (2022). Fucoxanthin from algae to human, an extraordinary bioresource: insights and advances in up and downstream processes. Marine Drugs, 20(4), 222. https://doi.org/10.3390/md20040222
Xia, S., Wang, K., Wan, L., Aifen Li, A., Hu, Q., & Zhang, C. (2013). Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Marine Drugs, 11(7), 2667–2681. https://doi.org/10.3390/md11072667
Min Kim, S. M., Kang, S.-W., Kwon, O.-N., Chung, D., & Pan, C.-H. (2012). Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. Journal of the Korean Society for Applied Biological Chemistry, 55, 477–483. https://doi.org/10.1007/s13765-012-2108-3
Mohamadnia, S., Tavakoli, O., & Faramarzi, M. A. (2021). Enhancing production of fucoxanthin by the optimization of culture media of the microalga Tisochrysis lutea. Aquaculture, 533, 736074. https://doi.org/10.1016/j.aquaculture.2020.736074
Kanamoto, A., Kato, Y., Yoshida, E., Hasunuma, T., & Kondo, A. (2021). Development of a method for fucoxanthin production using the haptophyte marine microalga Pavlova sp. OPMS 30543. Marine Biotechnology, 23(2), 331–341. https://doi.org/10.1007/s10126-021-10028-5
Eilers, U., Bikoulis, A., Breitenbach, J., Büchel, C., & Sandmann, G. (2016). Limitations in the biosynthesis of fucoxanthin as targets for genetic engineering in Phaeodactylum tricornutum. Journal Applied Phycology, 28(1), 123–129. https://doi.org/10.1007/s10811-015-0583-8
Wang, S., Wu, S., Yang, G., Pan, K., Wang, L., & Hu, Z. (2021). A review on the progress, challenges and prospects in commercializing microalgal fucoxanthin. Biotechnology Advances, 53, 107865. https://doi.org/10.1016/j.biotechadv.2021.107865
Wang, S., Verma, S. K., Said, I. H., Thomsen, L., Ullrich, M. S., & Kuhnert, N. (2018). Changes in the fucoxanthin production and protein profiles in in response to blue light-emitting diode light. Microbial Cell Factories, 17(110), 1–13. https://doi.org/10.1186/s12934-018-0957-0
Gèrin, S., Delhez, T., Corato, A., Remacle, C., & Franck, F. (2020). A novel culture medium for freshwater diatoms promotes efficient photoautotrophic batch production of biomass, fucoxanthin, and eicosapentaenoic acid. Journal Applied Phycology, 32(3), 1581–1596. https://doi.org/10.1007/s10811-020-02097-1
Zarekarizi, A., Hoffmann, L., & Burritt, D. (2019). Approaches for the sustainable production of fucoxanthin, a xanthophyll with potential health benefits. Journal Applied Phycology, 31, 281–299. https://doi.org/10.1007/s10811-018-1558-3
Martin, C., Côme, M., Ulmann, L., Chini Zittelli, G., Faraloni, C., Nazih, H., Ouguerram, K., Chénais, B., & Mimouni, V. (2019). Preventive effects of the marine microalga Phaeodactylum tricornutum, used as a food supplement, on risk factors associated with metabolic syndrome in Wistar rats. Nutrients, 11, 1069. https://doi.org/10.3390/nu11051069
Mayer, C., Richard, L., Côme, M., Ulmann, L., Nazih, H., Chénais, B., Ouguerram, K., & Mimouni, V. (2021). The marine microalga, Tisochrysis lutea, protects against metabolic disorders associated with metabolic syndrome and obesity. Nutrients, 13, 430. https://doi.org/10.3390/nu13020430
Mayer, C., Côme, M., Ulmann, L., Martin, I., Chini Zittelli, G., Faraloni, C., Ouguerram, K., Chénais, B., & Mimouni, V. (2022). The potential of the marine microalga Diacronema lutheri in the prevention of obesity and metabolic syndrome in high-fat-fed wistar rats. Molecules, 27, 4246. https://doi.org/10.3390/molecules27134246
Wan-Loy, C., & Siew-Moi, P. (2016). Marine algae as a potential source for anti-obesity agents. Marine Drugs, 14, 222. https://doi.org/10.3390/md14120222
Din, N. A. S., Mohd Alayudin, A. S., Sofian-Seng, N.-S., Rahman, H. A., Mohd Razali, N. S., Lim, S. J., & Wan Mustapha, W. A. (2022). Brown algae as functional food source of fucoxanthin: A review. Foods, 11, 2235. https://doi.org/10.3390/foods11152235
Huang, J. J., Lin, S., Xu, W., & Cheung, P. C. K. (2017). Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnology Advances, 35, 597–618. https://doi.org/10.1016/j.biotechadv.2017.05.001
Yang, R., Wei, D., & Xie, J. (2020). Diatoms as cell factories for high-value products: Chrysolaminarin, eicosapentaenoic acid, and fucoxanthin. Critical Reviews in Biotechnology, 40(7), 993–1009. https://doi.org/10.1080/07388551.2020.1805402
Bauer, C., Schmitz, C., Corrêa, R., Herrera, C., Ramlov, F., Oliveira, E., Pizzato, A., & Maraschin, M. (2019). In Vitro Fucoxanthin Production by the Phaeodactylum tricornutum Diatom. In Studies in Natural Products Chemistry (pp. 211–242). Elsevier.
Lohr, M., & Wilhelm, C. (2001). Xanthophyll synthesis in diatoms: Quantification of putative intermediates and comparison of pigment conversion kinetics with rate constants derived from a model. Planta, 212(3), 382–391. https://doi.org/10.1007/s004250000403
Dambek, M., Eilers, U., Breitenbach, J., Steiger, S., Büchel, C., & Sandmann, G. (2012). Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. Journal of Experimental Botany, 63(15), 5607–5612. https://doi.org/10.1093/jxb/ers211
Begum, H., Yusoff, F. M. D., Banerjee, S., Khatoon, H., & Shariff, M. (2016). Availability and utilization of pigments from microalgae. Critical Reviews in Food Science and Nutrition, 56, 2209–2222. https://doi.org/10.1080/10408398.2013.764841
Mohamadnia, S., Tavakoli, O., Faramarzi, M. A., & Shamsollahi, Z. (2020). Production of fucoxanthin by the microalga Tisochrysis lutea: a review of recent developments. Aquaculture, 516, 734637. https://doi.org/10.1016/j.aquaculture.2019.734637
Gao, F., Teles, I., Wijffels, R. H., & Barbosa, M. J. (2020). Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresource Technology, 315, 123894. https://doi.org/10.1016/j.biortech.2020.123894
Lavaud, J., Rousseau, B., van Gorkom, H. J., & Etienne, A.-L. (2002). Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiology, 129, 1398–1406. https://doi.org/10.1104/pp.002014
Li, Y., Sun, H., Wang, Y., Yang, S., Wang, J., Wu, T., Lu, X., Chu, Y., & Chen, F. (2021). Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement. Bioresource Technology, 347, 126401. https://doi.org/10.1016/j.biortech.2021.126401
McClure, D. D., Luiz, A., Gerber, B., Barton, G. W., & Kavanagh, J. M. (2018). An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Research, 29, 41–48. https://doi.org/10.1016/j.algal.2017.11.015
Grobbelaar, J. U. (2013). Inorganic algal nutrition. In A. Richmond & H. Qiang (Eds.), Handbook of Microalgal Culture: Applied Phycology and Biotechnology (2nd ed., pp. 123–133). Wiley. https://doi.org/10.1002/9781118567166
Xia, S., Gao, B., Fu, J., Xiong, J., & Zhang, C. (2018). Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. Journal of Bioscience and Bioengineering, 126, 723–729. https://doi.org/10.1016/j.jbiosc.2018.06.002
Sun, Z., Wang, X., & Liu, J. (2019). Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Research, 41, 101545. https://doi.org/10.1016/j.algal.2019.101545
Mao, X., Chen, S. H. Y., Lu, X., Yu, J., & Liu, B. (2020). High silicate concentration facilitates fucoxanthin and eicosapentaenoic acid (EPA) production under heterotrophic condition in the marine diatom Nitzschia laevis. Algal Research, 52, 102086. https://doi.org/10.1016/j.algal.2020.102086
Yi, Z., Su, Y., Cherek, P., Nelson, D. R., Lin, J., Rolfsson, O., Wu, H., Salehi-Ashtiani, K., Brynjolfsson, S., & Fu, W. (2019). Combined artificial high-silicate medium and LED illumination promote carotenoid accumulation in the marine diatom Phaeodactylum tricornutum. Microbial Cell Factories, 18(1), 1–11. https://doi.org/10.1186/s12934-019-1263-1
Mousavi Nadushan, R., & Hosseinzade, I. (2020). Optimization of production and antioxidant activity of fucoxanthin from marine haptophyte algae, Isochrysis galbana. Iranian Journal of Fisheries Sciences, 19(6), 2901–2908. https://doi.org/10.22092/ijfs.2020.122837
Wang, H., Zhang, Y., Chen, L., Cheng, W., & Liu, T. (2018). Combined production of fucoxanthin and EPA from two diatom strains Phaeodactylum tricornutum and Cylindrotheca fusiformis cultures. Bioprocess Biosystems Engineering, 41(7), 1061–1071. https://doi.org/10.1007/s00449-018-1935-y
Beuzenberg, V., Goodwin, E. O., Puddick, J., Romanazzi, D., Adams, S. L., & Packer, M. A. (2017). Optimising conditions for growth and xanthophyll production in continuous culture of Tisochrysis lutea using photobioreactor arrays and central composite design experiments. New Zealand Journal of Botany, 55(1), 64–78. https://doi.org/10.1080/0028825X.2016.1238398
Guo, B., Liu, B., Yang, B., Sun, P., Lu, X., Liu, J., & Chen, F. (2016). Screening of diatom strains and characterization of Cyclotella cryptica as a potential fucoxanthin producer. Marine Drugs, 14(7), 125. https://doi.org/10.3390/md14070125
Lu, X., Sun, H., Zhao, W., Cheng, K. W., Chen, F., & Liu, B. (2018). A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Marine Drugs, 16(7), 219. https://doi.org/10.3390/md16070219
Alkhamis, Y., & Qin, J. G. (2015). Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. Journal Applied Phycology, 28, 35–42. https://doi.org/10.1007/s10811-015-0599-0
Garcia, M. C. C., Miron, A. S., Sevilla, J. M. F., Grima, E. M., & Camacho, F. G. (2005). Mixotrophic growth of the microalga Phaeodactylum tricornutum—Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochemistry, 40(1), 297–305. https://doi.org/10.1016/j.procbio.2004.01.016
Chini Zittelli, G., Biondi, N., Rodolfi, L., & Tredici, M. R. (2013). Photobioreactors for mass production of microalgae. In A. Richmond & Q. Hu (Eds.), Handbook of microalgal culture: applied phycology and biotechnology (2nd ed., pp. 225–266). Wiley.
Zhang, H., Gong, P., Cai, Q., Zhang, C., & Gao, B. (2022). Maximizing fucoxanthin production in Odontella aurita by optimizing the ratio of red and blue light-emitting diodes in an auto-controlled internally illuminated photobioreactor. Bioresource Technology, 344, 126260. https://doi.org/10.1016/j.biortech.2021.126260
Branco-Vieira, M., San Martin, S., Agurto, C., Freitas, M.-A.V., Martins, A. A., & Mata, M. (2020). Biotechnological potential of Phaeodactylum tricornutum for biorefinery processes. Fuel, 268, 117357. https://doi.org/10.1016/j.fuel.2020.117357
Gao, F., Sá, M., Teles, I., Wijffels, R. H., & Barbosa, M. J. (2021). Production and monitoring of biomass and fucoxanthin with brown microalgae under outdoor conditions. Biotechnology Bioengineering, 118(3), 1355–1365. https://doi.org/10.1002/bit.27657
Rodolfi, L., Biondi, N., Guccione, A., Bassi, N., Dottavio, M., Arganaraz, G., & Tredici, M. R. (2017). Oil and eicosapentaenoic acid production by the diatom Phaeodactylum tricornutum cultivated outdoors in Green Wall Panel (GWP®) reactors. Biotechnology and Bioengineering, 114(10), 2204–2210. https://doi.org/10.1002/bit.26353
Benavides, A. M., Torzillo, G., Kopecký, J., & Masojídek, J. (2013). Productivity and biochemical composition of Phaeodactylum tricornutum (Bacillariophyceae) cultures grown outdoors in tubular photobioreactors and open ponds. Biomass and Bioenergy, 54, 115–122. https://doi.org/10.1016/j.biombioe.2013.03.016
Muller-Feuga, A., Lemar, M., Vermel, E., Pradelles, R., Rimbaud, L., & Valiorgue, P. (2012). Appraisal of a horizontal two-phase flow photobioreactor for industrial production of delicate microalgae species. Journal of Applied Phycology, 24, 349–355. https://doi.org/10.1007/s10811-012-9820-6
Delbrut, A., Albina, P., Lapierre, T., Pradelles, R., & Dubreucq, E. (2018). Fucoxanthin and polyunsaturated fatty acids co-extraction by a green process. Molecules, 23(4), 874. https://doi.org/10.3390/molecules23040874
Abu-Ghosh, S., Dubinsky, Z., Verdelho, V., & Iluz, D. (2021). Unconventional high-value products from microalgae: A review. Bioresource Technology, 329, 124895. https://doi.org/10.1016/j.biortech.2021.124895
Zhang, H., Tang, Y., Zhang, Y., Zhang, S., Qu, J., Wang, X., Kong, R., Han, C., & Liu, Z. (2015). Fucoxanthin: A promising medicinal and nutritional ingredient. Available online: https://www.hindawi.com/journals/ecam/2015/723515 (accessed on 8 November 2022).
Lourenço-Lopes, C., Fraga-Corral, M., Jimenez-Lopez, C., Carpena, M., Pereira, A. G., Garcia-Oliveira, P., Prieto, M. A., & Simal-Gandara, J. (2021). Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends in Food Science & Technology, 117, 163–181. https://doi.org/10.1016/j.tifs.2021.03.012
Gammone, M. A., & D’Orazio, N. (2015). Anti-obesity activity of the marine carotenoid fucoxanthin. Marine Drugs, 13(2196), 2214. https://doi.org/10.3390/md13042196
Hashimoto, T., Ozaki, Y., Taminato, M., Das, S. K., Mizuno, M., Yoshimura, K., Maoka, T., & Kanazawa, K. (2009). The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. British Journal of Nutrition, 102(2), 242–248. https://doi.org/10.1017/S0007114508199007
D’Orazio, N., Gemello, E., Gammone, M. A., De Girolamo, M., Ficoneri, C., & Riccioni, G. (2012). Fucoxantin: A treasure from the sea. Marine Drugs, 10, 604–616. https://doi.org/10.3390/md10030604
Sachindra, N. M., Sato, E., Maeda, H., Hosokawa, M., Niwano, Y., Kohno, M., & Miyashita, K. (2007). Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. Journal of Agricultural and Food Chemistry, 55(21), 8516–8522. https://doi.org/10.1021/jf071848a
Nomura, T., Kikuchi, M., Kubodera, A., & Kawakami, Y. (1997). Proton-donative antioxidant activity of fucoxanthin with 1,1-Diphenyl-2-Picrylhydrazyl (DPPH). Biochemistry And Molecular Biology International, 42(2), 361–370. https://doi.org/10.1080/15216549700202761
Foo, S. C., Yusoff, F., Ismail, M., Basri, M., Yau, S. K., Khong, N. M. H., & Chan, K. W. (2017). Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. Journal of Biotechnology, 241, 175–183. https://doi.org/10.1016/j.jbiotec.2016.11.026
Zhao, X., Gao, L., & Zhao, X. (2022). Rapid purification of fucoxanthin from phaeodactylum tricornutum. Molecules, 27(10), 3189. https://doi.org/10.3390/molecules27103189
Neumann, U., Derwenskus, F., Flister, V. F., Schmid-Staiger, U., Hirth, T., & Bischoff, S. C. (2019). Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants, 8(6), 183. https://doi.org/10.3390/antiox8060183
Woo, M. N., Jeon, S. M., Kim, H. J., et al. (2010). Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chemico Biological Interactions, 186(3), 316–322. https://doi.org/10.1016/j.cbi.2010.05.006
Muradian, K., Vaiserman, A., Min, K. J., & Fraifeld, V. E. (2015). Fucoxanthin and lipid metabolism: A minireview. Nutrition, Metabolism and Cardiovascular Diseases, 25(10), 891–897. https://doi.org/10.1016/j.numecd.2015.05.010
Maeda, H., Hosokawa, M., Sashima, T., Funayama, K., & Miyashita, K. (2005). Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochemical and Biophysical Research Communications, 332(2), 392–397. https://doi.org/10.1016/j.bbrc.2005.05.002
Maeda, H., Hosokawa, M., Sashima, T., Murakami-Funayama, K., & Miyashita, K. (2009). Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Molecular Medicine Reports, 2(6), 897–902.
Mayer, C., Côme, M., Ulmann, L., Chini Zittelli, G., Faraloni, C., Nazih, H., Ouguerram, K., Chénais, B., & Mimouni, V. (2019). Preventive effects of the marine microalga Phaeodactylum tricornutum, used as a food supplement, on risk factors associated with metabolic syndrome in Wistar rats. Nutrients, 11(5), 1–19. https://doi.org/10.3390/nu11051069
Martin, L. J. (2015). Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Marine Drugs, 13(8), 4784–4798. https://doi.org/10.3390/md13084784
Pádua, D., Rocha, E., Gargiulo, D., & Ramos, A. A. (2015). Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochemistry Letters, 14, 91–98. https://doi.org/10.1016/j.phytol.2015.09.007
Satomi, Y. (2017). Antitumor and cancer-preventative function of fucoxanthin: A marine carotenoid. Anticancer Research, 37(4), 1557–1562. https://doi.org/10.21873/anticanres.11484
Méresse, S., Fodil, M., Fleury, F., & Chénais, B. (2020). Fucoxanthin, a marine-derived carotenoid from brown seaweeds and microalgae: A promising bioactive compound for cancer therapy. International Journal of Molecular Sciences, 21, 9273. https://doi.org/10.3390/ijms21239273
Yamamoto, K., Ishikawa, C., Katano, H., Yasumoto, T., & Mori, N. (2011). Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Letters, 300(2), 225–234. https://doi.org/10.1016/j.canlet.2010.10.016
Kim, K. N., Heo, S. J., Yoon, W. J., Kang, S. M., Ahn, G., Yi, T. H., & Jeon, Y. (2010). Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW264.7 macrophages. European Journal of Pharmacology, 649(1–3), 369–375. https://doi.org/10.1016/j.ejphar.2010.09.032
Ye, G., Lu, Q., Zhao, W., Du, D., Jin, L., & Liu, Y. (2014). Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biology, 35, 11261–11267. https://doi.org/10.1007/s13277-014-2337-7
Wang, J., Chen, S., Xu, S., Yu, X., Ma, D., Hu, X., & Cao, X. (2012). In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-Rregulating STAT3/EGFR signaling in sarcoma180 (S180) xenografts-bearing mice. Marine Drugs, 10(9), 2055–2068. https://doi.org/10.3390/md10092055
Heo, S. J., Yoon, W. J., Kim, K. N., Ahn, G. N., Kang, S. M., Kang, D. H., Affan, A., Oh, C., Jung, W. K., & Jeon, Y. J. (2010). Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food and Chemical Toxicology, 48, 2045–2051. https://doi.org/10.1016/j.fct.2010.05.003
Matsui, M., Tanaka, K., Higashiguchi, N., Okawa, H., Yamada, Y., Tanaka, K., Taira, S., Aoyama, T., Takanishi, M., Natsume, C., Takakura, Y., Fujita, N., Hashimoto, T., & Fujita, T. (2016). Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. Journal of Pharmacological Sciences, 132(1), 55–64. https://doi.org/10.1016/j.jphs.2016.08.004
Shimoda, H., Tanaka, J., Shan, S. J., & Maoka, T. (2010). Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. Journal of Pharmacy and Pharmacology, 62, 1137–1145. https://doi.org/10.1111/j.2042-7158.2010.01139.x
Heo, S. J., & Jeon, Y. J. (2009). Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. Journal of Photochemistry and Photobiology B: Biology, 95(2), 101–107. https://doi.org/10.1016/j.jphotobiol.2008.11.011
Hu, L., Chen, W., Tian, F., Yuan, C., Wang, H., & Yue, H. (2018). Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomedicine & Pharmacotherapy, 106(1), 1484–1489. https://doi.org/10.1016/j.biopha.2018.07.088
Yip, W. H., Joe, L. S., Mustapha, W. A. W., Maskat, M. Y., & Said, M. (2014). Characterisation and stability of pigments extracted from Sargassum binderi obtained from semporna Sabah. Sains Malaysiana, 43(9), 1345–1354.
Zhao, D., Kim, S. M., Pan, C. H., & Chung, D. (2014). Effects of heating, aerial exposure and illumination on stability of fucoxanthin in canola oil. Food Chemistry, 145, 505–513. https://doi.org/10.1016/j.foodchem.2013.08.045
EFSA Panel on Biological Hazards (BIOHAZ). (2019). Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 10: Suitability of taxonomic units notified to EFSA until March 2019. European Food Safety Authority EFSA Journal, 17(7), 5753. https://doi.org/10.2903/j.efsa.2019.5753
Acknowledgements
We wish to thank Dr. Luciano Falqui and Dr. Mimmo Scollo from Plastica Alfa SpA for the information concerning the phycocyanin market and Dr. Bernardo Cicchi for English revision of the manuscript. G.T. wishes to thank prof A. Vonshak for the valuable discussion on Arthrospira strain selection section.
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. The work has been partially supported by the Project “NATURAL-BLU” Programma Amico—Programma Di Incentivo e Sostegno alle Attività di Applicazione, Miglioramento e Costruzione dei Trovati Brevettati—Bando MISE per il Cofinanziamento—Proof Of Concept (PoC) 2020.
Author information
Authors and Affiliations
Contributions
All authors declare that they have equally contributed to the manuscript in the following three parts: (1) the conception and design of the study, (2) drafting the article and revising it critically for important intellectual content and (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that might affect this work. All the authors confirmed authorship of the manuscript and agreed to submit it for peer review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chini Zittelli, G., Lauceri, R., Faraloni, C. et al. Valuable pigments from microalgae: phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem Photobiol Sci 22, 1733–1789 (2023). https://doi.org/10.1007/s43630-023-00407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00407-3