Abstract
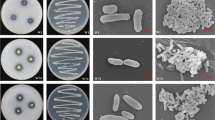
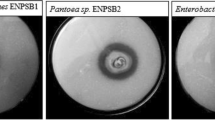
The current research as aimed (i) to isolate and select the purple nonsulfur bacteria (PNSB) possessing the potassium-solubilizing ability from acid paddy fields and (ii) to evaluate the ability to release the plant growth-promoting substances (PGPS) of selected PNSB. A total of 35 acid sulfate (AS) soil samples were collected in An Giang province, Vietnam. Then, 70 PNSB strains were isolated from the AS soil samples. In the current study, the isolated strains were screened and selected according to their tolerability to acidic conditions, ability to solubilize potassium, and characteristics of a plant growth promoter on basic isolation media with various incubation conditions. Therein, three strains, TT07.4, AN05.1, and AC04.1, presented the highest potassium solubilization under the microaerobic light (11.8–17.7 mg L−1) and aerobic dark (16.4–24.7 mg L−1) conditions and stresses from Al3+, Fe2+, and Mn2+ toxicity. The selected strains were identified as Rhodopseudomonas pentothenatexigens by the 16S rDNA sequence, with 99% similarity. The selected acidic-resistant strains possessed the traits of biofertilizers under both microaerobic light and aerobic dark conditions, with abilities to fix nitrogen (0.17–6.24; 7.93–11.2 mg L−1); solubilize phosphorus from insoluble compounds with 3.22–49.9 and 9.49–11.2 mg L−1 for Al-P, 21.9–25.8 and 20.2–25.1 mg L−1 for Ca-P, and 10.1–29.8 and 18.9–23.2 mg L−1 for Fe-P; produce 5-aminolevulinic acid (0.63–3.01; 1.19–6.39 mg L−1), exopolymeric substances (0.14–0.76; 0.21–0.86 mg L−1), indole-3-acetic acid (12.9–32.6; 13.6–17.8 mg L−1), and siderophores (28.4–30.3; 6.15–10.3%). The selected potassium-solubilizing strains have a great potential to apply in liquid form into rice seed and solid form in AS soils to supply nutrients and PGPS for enhancing rice growth and grain yield.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Minh LQ, Tuong TP, Van Mensvoort MEF, Bouma J (1997) Contamination of surface water as affected by land use in acid sulfate soils in the Mekong River Delta, Vietnam. Agric Ecosyst Environ 61(1):19–27. https://doi.org/10.1016/S0167-8809(96)01084-5
Nystrand MI, Österholm P, Yu C, Åström M (2016) Distribution and speciation of metals, phosphorus, sulfate and organic material in brackish estuary water affected by acid sulfate soils. Appl Geochem 66:264–274. https://doi.org/10.1016/j.apgeochem.2016.01.003
Mayakaduwage S, Mosley LM, Marschner P (2020) Threshold for labile phosphate in a sandy acid sulfate soil. Geoderma 371:114359. https://doi.org/10.1016/j.geoderma.2020.114359
Keene A, Melville MD, BCT M (2004) Using potassium potentials to examine nutrient availability in an acid sulfate soil landscape, northern Australia. In: Super Soil 2004: Proceedings of the Third Australian and New Zealand Soils Conference [CD-ROM]. Regional Institute Ltd., Sydney, Australia
Pandey D, Kehri HK, Zoomi I, Singh U, Chaudhri KL, Akhtar O (2020) Potassium solubilizing microbes: diversity, ecological significances and biotechnological applications. In: Yadav A, Singh J, Rastegari A, Yadav N (ed) Plant microbiomes for sustainable agriculture. Sustainable development and biodiversity. Springer, Cham, 25:263-286. https://doi.org/10.1007/978-3-030-38453-1_9
Sparks DL, Huang PM (1985) Physical chemistry of soil potassium. In: Munson RD (ed) Potassium in agriculture. ASA-CSSA-SSS, pp 201–276. https://doi.org/10.2134/1985.potassium.c9
Li T, Liang J, Chen X, Wang H, Zhang S, Pu Y, Xu X, Li H, Xu J, Wu X, Liu X (2021) The interacting roles and relative importance of climate, topography, soil properties and mineralogical composition on soil potassium variations at a national scale in China. Catena 196:104875. https://doi.org/10.1016/j.catena.2020.104875
Ahmad M, Nadeem SM, Naveed M, Zahir ZA (2016) Potassium-solubilizing bacteria and their application in agriculture. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 293–313. https://doi.org/10.1007/978-81-322-2776-2_21
Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 187–201. https://doi.org/10.1007/978-81-322-2776-2_14
Meena VS, Maurya BR, Verma JP (2014) Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res 169(5-6):337–347. https://doi.org/10.1016/j.micres.2013.09.003
Xiao Y, Wang X, Chen W, Huang Q (2017) Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J 34(10):873–880. https://doi.org/10.1080/01490451.2017.1286416
Liu Z, Rong Q, Zhou W, Liang G (2017) Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS One 12(3):e0172767. https://doi.org/10.1371/journal.pone.0172767
Khuong NQ, Kantachote D, Onthong J, Xuan LNT, Sukhoom A (2018) Enhancement of rice growth and yield in actual acid sulfate soils by potent acid-resistant Rhodopseudomonas palustris strains for producing safe rice. Plant Soil 429(1):483–501. https://doi.org/10.1007/s11104-018-3705-7
Sakpirom J, Nunkaew T, Khan E, Kantachote D (2021) Optimization of carriers and packaging for effective biofertilizers to enhance Oryza sativa L. growth in paddy soil. Rhizosphere 19:100383. https://doi.org/10.1016/j.rhisph.2021.100383
Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016) Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic plains of India. Biocatal Agric Biotechnol 7:202–209. https://doi.org/10.1016/j.bcab.2016.06.007
Bakhshandeh E, Pirdashti H, Lendeh KS (2017) Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol Eng 103:164–169. https://doi.org/10.1016/j.ecoleng.2017.03.008
Khati P, Mishra PK, Parihar M, Kumari A, Joshi S, Bisht JK, Pattanayak A (2020) Potassium solubilization and mobilization: functional impact on plant growth for sustainable agriculture. In: Yadav A, Rastegari A, Yadav N, Kour D (eds) Advances in plant microbiome and sustainable agriculture: functional annotation and future challenges. Springer, Singapore, pp 21–40. https://doi.org/10.1007/978-981-15-3204-7_2
Sun F, Ou Q, Wang N, Xuan Guo Z, Ou Y, Li N, Peng C (2020) Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob Ecol Conserv 23:e01141. https://doi.org/10.1016/j.gecco.2020.e01141
Ali AM, Awad MY, Hegab SA, Gawad AMAE, Eissa MA (2021) Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J Plant Nutr 44(3):411–420. https://doi.org/10.1080/01904167.2020.1822399
Chen YH, Yang XZ, Zhuang LI, An XH, Li YQ, Cheng CG (2020) Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J Integr Agric 19(10):2458–2469. https://doi.org/10.1016/S2095-3119(20)63303-2
Maurya BR, Meena VS, Meena OP (2014) Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos 27(1):181–187. https://doi.org/10.5958/j.2229-4473.27.1.028
Dotaniya ML, Meena VD, Basak BB, Meena RS (2016) Potassium uptake by crops as well as microorganisms. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 267–280. https://doi.org/10.1007/978-81-322-2776-2_19
Etesami H, Emami S, Alikhani HA (2017) Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects: a review. J Soil Sci Plant Nutr 17(4):897–911. https://doi.org/10.4067/S0718-95162017000400005
Rashwan BR, Shaheen AEA (2021) The impact of potassium sources and bio fertilizer on corn plant and potassium availability in calcareous soil. Asian J Soil Sci 5(1):27–37. https://doi.org/10.9734/asrj/2021/v5i130099
Khuong NQ, Kantachote D, Onthong J, Sukhoom A (2017) The potential of acid-resistant purple nonsulfur bacteria isolated from acid sulfate soils for reducing toxicity of Al3+ and Fe2+ using biosorption for agricultural application. Biocatal Agric Biotechnol 12:329–340. https://doi.org/10.1016/j.bcab.2017.10.022
Nookongbut P, Kantachote D, Khuong NQ, Sukhoom A, Tantirungkij M, Limtong S (2019) Selection of acid-resistant purple nonsulfur bacteria from peat swamp forests to apply as biofertilizers and biocontrol agents. J Soil Sci Plant Nutr 19(3):488–500. https://doi.org/10.1007/s42729-019-00044-9
Khuong NQ, Kantachote D, Nookongbut P, Onthong J, Xuan LNT, Sukhoom A (2020a) Mechanisms of acid-resistant Rhodopseudomonas palustris strains to ameliorate acidic stress and promote plant growth. Biocatal Agric Biotechnol 24:101520. https://doi.org/10.1016/j.bcab.2020.101520
Khuong NQ, Kantachote D, Nookongbut P, Xuan LNT, Nhan TC, Xuan NTT, Tantirungkij M (2020) Potential of Mn2+-resistant purple nonsulfur bacteria isolated from acid sulfate soils to act as bioremediators and plant growth promoters via mechanisms of resistance. J Soil Sci Plant Nutr 20(4):2364–2378. https://doi.org/10.1007/s42729-020-00303-0
Khuong NQ, Huu TN, Thuc LV, Thu LTM, Xuan DT, Quang LT, Nhan TC, Tran HN, Tien PD, Xuan LNT, Kantachote D (2021) Two strains of Luteovulum sphaeroides (purple nonsulfur bacteria) promote rice cultivation in saline soils by increasing available phosphorus. Rhizosphere 20:100456. https://doi.org/10.1016/j.rhisph.2021.100456
Khuong NQ, Kantachote D, Dung NTT, Huu TN, Thuc LV, Thu LTM, Quang LT, Xuan DT, Nhan TC, Tien PD, Xuan LNT (2023) Potential of potent purple nonsulfur bacteria isolated from rice-shrimp systems to ameliorate rice (Oryza sativa L.) growth and yield in saline acid sulfate soil. J Plant Nutr 46(3):473–494. https://doi.org/10.1080/01904167.2022.2087089
Ge H, Zhang F (2019) Growth - promoting ability of Rhodopseudomonas palustris G5 and its effect on induced resistance in cucumber against salt stress. J Plant Growth Regul 8:180–188. https://doi.org/10.1007/s00344-018-9825-8
Ge H, Liu Z (2020) Alleviation of tetrabromobisphenol A toxicity in soybean seedlings by Rhodopseudomonas palustris RP1n1. Arch Microbiol 202(4):895–903. https://doi.org/10.1007/s00203-019-01797-8
Giller KE, Witter E, McGrath SP (1998) Toxicity of heavy metals to microorganisms and microbial process in agricultural soils: a review. Soil Biol Biochem 30:1389–1414. https://doi.org/10.1016/S0038-0717(97)00270-8
Chaudri AM, McGrath SP, Giller KE, Rietz E, Sauerbeck DR (1993) Enumeration of indigenous Rhizobium leguminosarum biovar trifolii in soils previously treated with metal-contaminated sewage sludge. Soil Biol Biochem 25:301–309. https://doi.org/10.1016/0038-0717(93)90128-X
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412. https://doi.org/10.1016/j.soilbio.2004.08.030
Khan MS, Zaidi A, Wani PA, Oves M (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:1–19. https://doi.org/10.1007/s10311-008-0155-0
Coleman NT, Craig D (1961) The spontaneous alteration of hydrogen clay. Soil Sci 91(1):14–18
Robinson GW (1922) A new method for the mechanical analysis of soils and other dispersions. J Agric Sci 12(3):306–321. https://doi.org/10.1017/S0021859600005360
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Brown JW (2013) Enrichment and isolation of purple non-sulfur bacteria. Department of Biological Sciences. College of Sciences, North Carolina State University. http://www.mbio.ncsu.edu/mb452/purplenonsulfurs/purples.html. Accessed 15 Nov 2022
Kantachote D, Torpee S, Umsakul K (2005) The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electron. J Biotechnol 8(3). https://doi.org/10.2225/vol8-issue3-fulltext-8
Nelson DW (1983) Determination of ammonium in KCl extracts of soils by the salicylate method. Commun Soil Sci Plant Anal 14(11):1051–1062. https://doi.org/10.1080/00103628309367431
Murphy J, Riley J (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Glickman E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61(12):793–796. https://doi.org/10.1128/aem.61.2.793-796.1995
Burnham BF (1970) δ-Aminolevulinic acid synthase (from Rhodopseudomonas sphaeroides). Methods Enzymol 17:195–204
Eboigbodin KE, Biggs CA (2008) Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromolecules. 9(2):686–695. https://doi.org/10.1021/bm701043c
Ferreira ML, Casabuono AC, Stacchiotti ST, Couto AS, Ramirez SA, Vullo DL (2017) Chemical characterization of Pseudomonas veronii 2E soluble exopolymer as Cd (II) ligand for the biotreatment of electroplating wastes. Int Biodeterior Biodegrad 119:605–613. https://doi.org/10.1016/j.ibiod.2016.10.013
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 28(8):751–759. https://doi.org/10.1016/0003-2697(87)90612-9
Attanandana T, Vacharotayan S (1986) Acid sulfate soils: their characteristics. genesis, amelioration and utilization. Jpn J Southeast Asian Stud 24(2):154–180. https://doi.org/10.20495/tak.24.2_154
Upjohn B, Fenton G, Conyers M (2005) Soil acidity and liming. Agfact AC.19, 3rd edition. New South Wales Department of Primary Industries
Anjos L, Gaistardo C, Deckers J, Dondeyne S, Eberhardt E, Gerasimova M, Harms B, Jones A, Krasilnikov P, Reinsch T, Vargas R, Zhang G, authors. Schad P, Van Huyssteen C, Micheli E, editors (2015) World reference base for soil resources 2014 International soil classification system for naming soils and creating legends for soil maps. Rome (Italy): FAO; 2015. JRC91947
Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour, PN, Tabatabai MA, Johnston CT, Sumner ME (ed) (1996) Methods of soil analysis. Part 3-Chemical Methods. SSSA Book Ser. 5.3. SSSA, ASA, Madison, W.I. https://doi.org/10.2136/sssabookser5.3
Suzuki Y, Kelly SD, Kemner KM, Banfield JF (2003) Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment. Appl Environ Microbiol 69:1337–1346. https://doi.org/10.1128/AEM.69.3.1337-1346.2003
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA 6: molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 30(12):2725-2729. 10.1093/molbev/mst197
Horneck DA, Sullivan DM, Owen JS, Hart JM (2011) Soil test interpretation guide. Technical Report, Oregon State University, Extension Service
Khuong NQ, Kantachote D, Thuc LV, Huu TN, Nhan TC, Nguyen PC, Thu LTM, Van TTB, Xuan NTT, Xuan LNT, Xuan DT (2022) Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res 221:105393. https://doi.org/10.1016/j.still.2022.105393
Nookongbut P, Kantachote D, Megharaj M, Naidu R (2018) Reduction in arsenic toxicity and uptake in rice (Oryza sativa L.) by As-resistant purple nonsulfur bacteria. Environ Sci Pollut Res 25(36):36530–36544. https://doi.org/10.1007/s11356-018-3568-8
Nguyen KQ, Kantachote D, Onthong J, Sukhoom A (2018) Al3+ and Fe2+ toxicity reduction potential by acid-resistant strains of Rhodopseudomonas palustris isolated from acid sulfate soils under acidic conditions. Ann Microbiol 68(4):217–228
Sakpirom J, Kantachote D, Nunkaew T, Khan E (2017) Characterizations of purple non-sulfur bacteria isolated from paddy fields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metal bioremediation. Res Microbiol 168(3):266–275. https://doi.org/10.1016/j.resmic.2016.12.001
Kang Z, Ding W, Gong X, Liu Q, Du G, Chen J (2017) Recent advances in production of 5-aminolevulinic acid using biological strategies. World J Microbiol Biotechnol 33(11):200. https://doi.org/10.1007/s11274-017-2366-7
Mounir AM, Osman YM, Khalil OA (2020) Impact of potassium solubilizing bacteria on growth and yield of garlic. Plant Arch 20(2):8374–8388
Batista BD, Dourado MN, Figueredo EF, Hortencio RO, Marques JPR, Piotto FA, Bonatelli ML, Settles ML, Azevedo JL, Quecine MC (2021) The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom). Arch Microbiol 203(7):3869–3882. https://doi.org/10.1007/s00203-021-02361-z
Hsu SH, Shen MW, Chen JC, Lur HS, Liu CT (2021) The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front Plant Sci 12:573634. https://doi.org/10.3389/fpls.2021.573634
Feng K, Cai Z, Ding T, Yan H, Liu X, Zhang Z (2019) Effects of potassium-solubulizing and photosynthetic bacteria on tolerance to salt stress in maize. J Appl Microbiol 126(5):1530–1540. https://doi.org/10.1111/jam.14220
Zhang C, Kong F (2014) Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol 82:18–25. https://doi.org/10.1016/j.apsoil.2014.05.002
Trueman AM, McLaughlin MJ, Mosley LM, Fitzpatrick RW (2020) Composition and dissolution kinetics of jarosite-rich segregations extracted from an acid sulfate soil with sulfuric material. Chem Geol 543:119606. 10.1016/j.chemgeo.2020.119606
Das I, Pradhan M (2016) Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. In: Meena V, Maurya B, Verma J, Meena R (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 281–291. https://doi.org/10.1007/978-81-322-2776-2_20
Bahadur I, Maurya R, Roy P, Kumar A (2019) Potassium-solubilizing bacteria (KSB): a microbial tool for K-solubility, cycling, and availability to plants. In: Kumar A, Meena V (ed) Plant growth promoting rhizobacteria for agricultural sustainability: From theory to practices, Springer, Singapore, pp 257-265. https://doi.org/10.1007/978-981-13-7553-8_13. In:
Pramanik P, Goswami AJ, Ghosh S, Kalita C (2019) An indigenous strain of potassium-solubilizing bacteria Bacillus pseudomycoides enhanced potassium uptake in tea plants by increasing potassium availability in the mica waste-treated soil of North-east India. J Appl Microbiol 126(1):215–222. https://doi.org/10.1111/jam.14130
Kaur T, Devi R, Kour D, Yadav A, Yadav AN (2021) Plant growth promotion of barley (Hordeum vulgare L.) by potassium solubilizing bacteria with multifarious plant growth promoting attributes. Plant Sci Today 8(sp1):17-24. https://doi.org/10.14719/pst.1377
Chen Y, Ye J, Kong Q (2020) Potassium-solubilizing activity of Bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests 11(12):1348. https://doi.org/10.3390/f11121348
Kantachote D, Nunkaew T, Kantha T, Chaiprapat S (2016) Biofertilizers from Rhodopseudomonas palustris strains to enhance rice yields and reduce methane emissions. Appl Soil Ecol 100:154–161. https://doi.org/10.1016/j.apsoil.2015.12.015
Khuong NQ, Kantachote D, Thuc LV, Huu TN, Nhan TC, Nguyen PC, Thu LTM, Van TTB, Xuan NTT, Xuan LNT, Xuan DT (2022) Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res 221:105393. https://doi.org/10.1016/j.still.2022.105393
Nookongbut P, Jingjit N, Kantachote D, Sukhoom A, Tantirungkij M (2020) Selection of acid tolerant purple nonsulfur bacteria for application in agriculture. Chiang Mai Univ J Nat Sci 19(4):774–790. https://doi.org/10.12982/CMUJNS.2020.0049
Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ (2015) Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym 115:334–341. https://doi.org/10.1016/j.carbpol.2014.08.099
Acknowledgements
We thank Prof. Dr. Duangporn Kantachote for her assistance with the English language.
Funding
The research leading to these results received funding from Can Tho University under Grant Agreement No T2021-94.
Author information
Authors and Affiliations
Contributions
Conceptualization: Nguyen Quoc Khuong, Jakkapan Sakpirom; methodology: Nguyen Quoc Khuong, Jakkapan Sakpirom; formal analysis and investigation: Truong Oanh Oanh, Le Vinh Thuc, Le Thi My Thu, Do Thi Xuan, Le Thanh Quang, Ly Ngoc Thanh Xuan; writing—original draft preparation: Nguyen Quoc Khuong; writing—review and editing: Nguyen Quoc Khuong, Jakkapan Sakpirom; funding acquisition: Nguyen Quoc Khuong; resources: Nguyen Quoc Khuong, Jakkapan Sakpirom; supervision: Jakkapan Sakpirom.
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all individual participants included in the study. The participant has consented to the submission of the case report to the journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lucy Seldin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khuong, N.Q., Sakpirom, J., Oanh, T.O. et al. Isolation and characterization of novel potassium-solubilizing purple nonsulfur bacteria from acidic paddy soils using culture-dependent and culture-independent techniques. Braz J Microbiol 54, 2333–2348 (2023). https://doi.org/10.1007/s42770-023-01069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01069-0