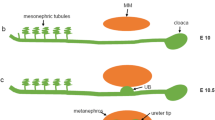
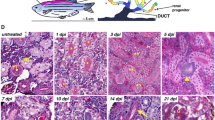
Abstract
A number of genes involved in kidney development are reactivated in the adult after acute kidney injury (AKI). This has led to the belief that tissue repair mechanisms recapitulate pathways involved in embryonic development after AKI. We will discuss evidence to support this hypothesis by comparing the mechanisms of development with common pathways known to regulate post-AKI repair, or that we identified as cell-specific candidates based on public datasets from recent AKI translational profiling studies. We will argue that while many of these developmental pathways are reactivated after AKI, this is not associated with general cellular reprogramming to an embryonic state. We will show that reactivation of these developmental genes is often associated with expression in cells that are not normally involved in mediating parallel responses in the embryo, and that depending on the cellular context, these responses can have beneficial or detrimental effects on injury and repair after AKI.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R (2013) Acute kidney injury: an increasing global concern. Lancet 382:170–179. Excellent AKI review
Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18:1292–1298
Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M (2012) Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 60:402–408
Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd (2012) Perkins, RM: increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81:477–485
Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, Wu PC, Chao CT, Wang JY, Kao TW, Young GH, Tsai PR, Tsai HB, Wang CL, Wu MS, Chiang WC, Tsai IJ, Hu FC, Lin SL, Chen YM, Tsai TJ, Ko WJ, Wu KD (2011) Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80:1222–1230
• Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81:442–448. An important review that describes the clinical association between AKI and CKD
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Investig 121:4210–4221
Sutton TA, Hato T, Mai E, Yoshimoto M, Kuehl S, Anderson M, Mang H, Plotkin Z, Chan RJ, Dagher PC (2013) p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol 24:113–124
Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC (2003) P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol 14:128–138
Rosen S, Stillman IE (2008) Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol 19:871–875
Stillman IE, Lima EQ, Burdmann EA (2008) Renal biopsies in acute kidney injury: who are we missing? Clin J Am Soc Nephrol 3:647–648
• Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS (2013) Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187:509–517. A large post mortem series of kidney histologies from patients dying of sepsis
Solez K, Morel-Maroger L, Sraer JD (1979) The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine 58:362–376
Solez K, Racusen LC, Marcussen N, Slatnik I, Keown P, Burdick JF, Olsen S (1993) Morphology of ischemic acute renal failure, normal function, and cyclosporine toxicity in cyclosporine-treated renal allograft recipients. Kidney Int 43:1058–1067
Villanueva S, Cespedes C, Vio CP (2006) Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol 290:R861–R870
Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, Tohda S, Sakano S, Sasaki S (2008) Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int 73:1240–1250
Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M, Sasaki S (2003) Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14:1223–1233
Simon M, Maresh JG, Harris SE, Hernandez JD, Arar M, Olson MS, Abboud HE (1999) Expression of bone morphogenetic protein-7 mRNA in normal and ischemic adult rat kidney. Am J Physiol 276:F382–F389
Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipila P, West KA, McMahon AP, Humphreys BD (2012) Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180:1441–1453
Cirio MC, de Groh ED, de Caestecker MP, Davidson AJ, Hukriede NA (2014) Kidney regeneration: common themes from the embryo to the adult. Pediatric Nephrol 29:553–564
•• Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, Grgic I, Kumar S, Humphreys BD, Hide WA, McMahon AP (2014) Cell-specific translational profiling in acute kidney injury. J Clin Invest 124:1242–1254. Key paper that uses Cre-activated TRAP technology to provide comprehensive translational profiling on endothelial cells, interstitial fibroblasts and renal tubular epithelial cells 24 hours after IR-AKI. Data from these studies was used to generate Tables 1 and 2 in this review
Kiesslich T, Berr F, Alinger B, Kemmerling R, Pichler M, Ocker M, Neureiter D (2012) Current status of therapeutic targeting of developmental signalling pathways in oncology. Curr Pharm Biotechnol 13:2184–2220
Sharfuddin AA, Molitoris BA (2011) Pathophysiology of ischemic acute kidney injury. Nature Rev Nephrol 7:189–200
Fligny C, Duffield JS (2013) Activation of pericytes: recent insights into kidney fibrosis and microvascular rarefaction. Curr Opin Rheumatol 25:78–86
Huen SC, Cantley LG (2014) Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol 30:199–209
Kramann R, Humphreys BD (2014) Kidney pericytes: roles in regeneration and fibrosis. Semin Nephrol 34:374–383
Kopan R, Chen S, Little M (2014) Nephron progenitor cells: shifting the balance of self-renewal and differentiation. Curr Top Dev Biol 107:293–331
Costantini F, Kopan R (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18:698–712
• Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97. This paper describes the use of FoxD1 Cre to label vascular pericytes in the kidney and demonstrate in a quantitative fashion that delamination of these cells from the vessel wall contributes to most, if not all, of the interstitial fibroblast expansion that occurs after injury. Findings contrast markedly with Le Bleu et al. Nat Med 2013 using different genetic markers
Yu J, Carroll TJ, McMahon AP (2002) Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129:5301–5312
Herzlinger D, Hurtado R (2014) Patterning the renal vascular bed. Semin Cell Dev Biol 36C:50–56
Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, Nagasawa Y, Hamano T, Matsui I, Kawada N, Imai E, Nagasawa T, Rakugi H, Isaka Y (2009) The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol 20:1714–1723
Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A, Mackay F, Schulz S, Stumm R (2012) CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney. PLoS One 7:e42814
Hum S, Rymer C, Schaefer C, Bushnell D, Sims-Lucas S (2014) Ablation of the renal stroma defines its critical role in nephron progenitor and vasculature patterning. PLoS One 9:e88400
Rymer C, Paredes J, Halt K, Schaefer C, Wiersch J, Zhang G, Potoka D, Vainio S, Gittes GK, Bates CM, Sims-Lucas S (2014) Renal blood flow and oxygenation drive nephron progenitor differentiation. Am J Physiol Renal Physiol 307:F337–F345
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455
Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH (2007) Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol 308:232–246
Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK (2010) Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298:F1078–F1094
Witzgall R, Brown D, Schwarz C, Bonventre JV (1994) Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Investig 93:2175–2188
•• Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD (2014) Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 111:1527–1532. An important paper that uses genetic labeling techniques to demonstrate conclusively that proliferating proximal tubular epithelial cells are derived from de-differentitated injury epithelial cells and not from a discrete population of intra-tubular stem cells
• Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV (2011) Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108:9226–9231. This paper uses sequential labeling with different thymidine analogues to show that tubular cell proliferation after AKI is a stochastic process that is unlikely to involve selective expansion of discrete intra-tubular or extra-tubular stem cells
Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291
•• Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, Lim X, Van-Amerongen R, Bowman A, Januszyk M, Pleniceanu O, Nusse R, Longaker MT, Weissman IL, Dekel B (2014) In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 7:1270–1283. This paper describes the use of genetic lineage tracing and clonal analysis to demonstrate that nephron segments are derived from lineage restricted nephron progenitor cells at different stages on embryonic development. The paper also demonstrates that once differentiated in the adult kidney, these cells contribute equally to proliferative repair after injury, providing further evidence against the existence of regenerative stem cells in the adult kidney
• Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ (2014) Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci USA 111:1533–1538. This paper using genetic labeling techniques to demonstrate that proliferating proximal tubular epithelial cells are derived from de-differentitated injury epithelial cells and not from a discrete population of intra-tubular stem cells. Findings support and are complementary to the studies in the Kusaba T. et al PNSA 2014 paper
Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P (2012) Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30:1714–1725
Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P (2007) Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18:3128–3138
• Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ (2013) Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229:645–659. This paper describes the appearance of CD24 positive, proliferating tubular epithelial cells after injury in rat and human kidneys
Kusaba T, Humphreys BD (2014) Controversies on the origin of proliferating epithelial cells after kidney injury. Pediatr Nephrol 29:673–679
Kusaba T, Humphreys BD (2014) Reply to Corbeil et al.: dedifferentiation and multipotency. Proc Natl Acad Sci USA 111:E1453
Basile DP, Donohoe D, Roethe K, Osborn JL (2001) Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281:F887–F899
Horbelt M, Lee SY, Mang HE, Knipe NL, Sado Y, Kribben A, Sutton TA (2007) Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiol Renal Physiol 293:F688–F695
Tanaka S, Tanaka T, Nangaku M (2014) Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol 00425:02014
Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA (2011) Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300:F721–F733
Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS (2014) The role of pericyte detachment in vascular rarefaction. J Vasc Res 51:247–258
Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS (2011) Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178:911–923
Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS (2013) EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24:559–572
• Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin SL, Davis GE, Gharib SA, Humphreys BD, Duffield JS (2012) Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23:868–883. Key paper describing the pathways regulating pericyte detachment from vessels in the kidney after injury
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170:1807–1816
• LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, Sugimoto H, Kalluri R (2013) Origin and function of myofibroblasts in kidney fibrosis. Nature Med 19:1047–1053. Extensive genetic analysis of the origin of renal fibroblasts after injury
Kinsey GR, Okusa MD (2012) Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care 16:214
Jang HR, Rabb H (2014) Immune cells in experimental acute kidney injury. Nature Rev Nephrol. doi:10.1038/nrneph.2014.180
Williams TM, Little MH, Ricardo SD (2010) Macrophages in renal development, injury, and repair. Semin Nephrol 30:255–267
• Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG (2014) GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. doi:10.1681/ASN.2014060612. This paper describes the cooperative role of renal tubular epithelium-derived GM-CSF (AKA CSF-2) and CSF-1 in promoting M2 polarization of renal macrophages after IR-AKI
• Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC (2012) CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122:4519–4532. This paper describes the role of tubular epithelium-derived CSF-1 in promoting selective expansion of alternate activated, regenerative M2 macrophages after AKI
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210
Chai OH, Song CH, Park SK, Kim W, Cho ES (2013) Molecular regulation of kidney development. Anat Cell Biol 46:19–31
Halt K, Vainio S (2014) Coordination of kidney organogenesis by Wnt signaling. Pediat Nephrol 29:737–744
Itaranta P, Chi L, Seppanen T, Niku M, Tuukkanen J, Peltoketo H, Vainio S (2006) Wnt-4 signaling is involved in the control of smooth muscle cell fate via Bmp-4 in the medullary stroma of the developing kidney. Dev Biol 293:473–483
Park JS, Valerius MT, McMahon AP (2007) Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134:2533–2539
Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP (2003) Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130:3175–3185
DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD (2013) Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol 24:1399–1412
Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS (2010) Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA 107:4194–4199
Yoshino K, Rubin JS, Higinbotham KG, Uren A, Anest V, Plisov SY, Perantoni AO (2001) Secreted Frizzled-related proteins can regulate metanephric development. Mech Dev 102:45–55
Barak H, Surendran K, Boyle SC (2012) The role of Notch signaling in kidney development and disease. Adv Exp Med Biol 727:99–113
Boyle SC, Liu Z, Kopan R (2014) Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141:346–354
Benedito R, Hellstrom M (2013) Notch as a hub for signaling in angiogenesis. Exp Cell Res 319:1281–1288
Chen J, Chen JK, Conway EM, Harris RC (2013) Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol 24:2023–2033
•• Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K (2010) Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest 120:4040–4054. An important paper demonstrating that persistent activation of Notch signaling promotes renal interstitial fibrosis
Huang R, Zhou Q, Veeraragoo P, Yu H, Xiao Z (2011) Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the gamma-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail 33:207–216
Sorensen-Zender I, Rong S, Susnik N, Zender S, Pennekamp P, Melk A, Haller H, Schmitt R (2014) Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury. Am J Physiol Renal Physiol 306:F907–F915
Gupta S, Li S, Abedin MJ, Wang L, Schneider E, Najafian B, Rosenberg M (2010) Effect of Notch activation on the regenerative response to acute renal failure. Am J Physiol Renal Physiol 298:F209–F215
Nishinakamura R, Sakaguchi M (2014) BMP signaling and its modifiers in kidney development. Pediatric Nephrol 29:681–686
Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L (2009) BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development 136:3557–3566
Ikeya M, Fukushima K, Kawada M, Onishi S, Furuta Y, Yonemura S, Kitamura T, Nosaka T, Sasai Y (2010) Cv2, functioning as a pro-BMP factor via twisted gastrulation, is required for early development of nephron precursors. Dev Biol 337:405–414
Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R (2007) Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134:2397–2405
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I (2000) Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Investig 105:863–873
Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D (2009) Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol 181:401–407
Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I (2008) Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol 19:685–694
Almanzar MM, Frazier KS, Dube PH, Piqueras AI, Jones WK, Charette MF, Paredes AL (1998) Osteogenic protein-1 mRNA expression is selectively modulated after acute ischemic renal injury. J Am Soc Nephrol 9:1456–1463
Vukicevic S, Basic V, Rogic D, Basic N, Shih MS, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H, Ryan S, Griffiths D, Maliakal J, Jelic M, Pastorcic M, Stavljenic A, Sampath TK (1998) Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Investig 102:202–214
Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W Jr, Bey P, Rusckowski M, Tampe B, Tampe D, Kanasaki K, Zeisberg M, Kalluri R (2012) Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nature Med 18:396–404
Larman BW, Karolak MJ, Adams DC, Oxburgh L (2009) Chordin-like 1 and twisted gastrulation 1 regulate BMP signaling following kidney injury. J Am Soc Nephrol 20:1020–1031
Cain JE, Islam E, Haxho F, Chen L, Bridgewater D, Nieuwenhuis E, Hui CC, Rosenblum ND (2009) GLI3 repressor controls nephron number via regulation of Wnt11 and Ret in ureteric tip cells. PLoS One 4:e7313
Cain JE, Rosenblum ND (2011) Control of mammalian kidney development by the Hedgehog signaling pathway. Pediat Nephrol 26:1365–1371
Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND (2006) GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development 133:569–578
Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M, Hou FF, Liu Y (2014) Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25:2187–2200
Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE (2003) Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Investig 111:707–716
Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C (1998) Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125:3313–3322
Leonard EC, Friedrich JL, Basile DP (2008) VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295:F1648–F1657
• Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, Quaggin SE (2011) Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 121:2278–2289. Key paper describing the role of Ang-1 in renal vascular development
Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, Thomas D, Tufro A (2009) Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development 136:3979–3989
• Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C (2010) Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137:283–292. Key paper describing the role of retinoic acid signaling during embryonic kidney development
Paroly SS, Wang F, Spraggon L, Merregaert J, Batourina E, Tycko B, Schmidt-Ott KM, Grimmond S, Little M, Mendelsohn C (2013) Stromal protein Ecm1 regulates ureteric bud patterning and branching. PLoS One 8:e84155
• Grgic I, Krautzberger AM, Hofmeister A, Lalli M, Dirocco DP, Fleig SV, Liu J, Duffield JS, McMahon AP, Aronow B, Humphreys BD (2014) Translational profiles of medullary myofibroblasts during kidney fibrosis. J Am Soc Nephrol 25:1979–1990. This paper describes the use of TRAP technology using a Collagen 1α1 promotor to identify renal fibroblast-specific translational profiles after ureteric ureteral obstruction. Findings are complementary to the FoxD1-specific translational profiling studies after IR-AKI described in Liu J et al. JCI 2014
Maden M (2002) Retinoic acid and limb regeneration–a personal view. Int J Dev Biol 46:883–886
Maden M (1983) The effect of vitamin A on the regenerating axolotl limb. J Embryol Exp Morphol 77:273–295
Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD (2011) Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 20:397–404
Blum N, Begemann G (2012) Retinoic acid signaling controls the formation, proliferation and survival of the blastema during adult zebrafish fin regeneration. Development 139:107–116
Mathew R, Huang J, Shah M, Patel K, Gewitz M, Sehgal PB (2004) Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 110:1499–1506
Oster SK, Ho CS, Soucie EL, Penn LZ (2002) The myc oncogene: marvelouslY Complex. Adv Cancer Res 84:81–154
Mugrauer G, Ekblom P (1991) Contrasting expression patterns of three members of the myc family of protooncogenes in the developing and adult mouse kidney. J Cell Biol 112:13–25
Couillard M, Trudel M (2009) C-myc as a modulator of renal stem/progenitor cell population. Dev Dyn 238:405–414
Bates CM, Kharzai S, Erwin T, Rossant J, Parada LF (2000) Role of N-myc in the developing mouse kidney. Dev Biol 222:317–325
Mah SP, Saueressig H, Goulding M, Kintner C, Dressler GR (2000) Kidney development in cadherin-6 mutants: delayed mesenchyme-to-epithelial conversion and loss of nephrons. Dev Biol 223:38–53
Reginensi A, Clarkson M, Neirijnck Y, Lu B, Ohyama T, Groves AK, Sock E, Wegner M, Costantini F, Chaboissier MC, Schedl A (2011) SOX9 controls epithelial branching by activating RET effector genes during kidney development. Hum Mol Genet 20:1143–1153
Yosypiv IV (2014) Renin-angiotensin system in ureteric bud branching morphogenesis: implications for kidney disease. Pediatr Nephrol 29:609–620
Acknowledgments
We would like to thank Ray Harris for critically reviewing the manuscript. The laboratory of Mark de Caestecker was supported by the National Institutes of Health Grants 1R01 HL093057 and 1RC4DK090770. The laboratory of Neil Hukriede was supported by the National Institutes of Health Grants 2R01 DK069403, 1RC4 DK090770, and 1P30DK079307, Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 2R01 HD053287.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Development.
Rights and permissions
About this article
Cite this article
Chiba, T., Hukriede, N. & de Caestecker, M.P. Kidney Regeneration: Lessons from Development. Curr Pathobiol Rep 3, 67–79 (2015). https://doi.org/10.1007/s40139-015-0069-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-015-0069-z