Abstract
Hybrid, polyploid, and clonal fishes are found naturally in wild populations, but they can also be induced artificially by cross-breeding and chromosome manipulation. The dojo loach Misgurnus anguillicaudatus includes various naturally occurring as well as artificially induced hybrid, polyploid, and clonal biotypes. This review aims to organize the results from previous works that used the dojo loach as the model animal for a better understanding of the interrelationship among the constitution of chromosome sets, the meiotic configuration, and the resultant gametogenesis. Autopolyploids with an even number of extra sets of homologous chromosomes were observed to be fertile. However, autopolyploids with an odd number of extra sets of homologous chromosomes and allopolyploids (polyploid hybrids) with exotic non-homologous chromosomes were found to exhibit a broad range of sterility ranging from retarded gonadal development to the production of aneuploid gametes with various abnormal characteristics. Sterile biotypes often showed meiotic configurations, including univalents. Past hybridization events likely triggered the atypical reproduction phenomena, such as the formation of unreduced isogenic gametes by doubling each chromosome for sister chromosome pairing, the elimination of a non-homologous chromosome set by meiotic hybridogenesis, and clonal development by spontaneous gynogenesis of unreduced eggs. The results obtained by studying a series of works using the dojo loach as the model organism highlight the mechanisms of sterility in hybrids and polyploids as well as of unisexuality in isogenic clones. These results contribute to the understanding of basic and aquaculture-oriented reproductive biology and genetics in fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromosome manipulation, including hybridization, is a set of techniques used to control the number and combination of sets of chromosomes derived from phylogenetically identical, similar, or distant species. The major techniques of chromosome manipulation are heterospecific fertilization for hybridization, inhibition of meiotic or mitotic divisions for ploidy level elevation, and genetic inactivation of gametes for induced gyno- and androgenesis (Fig. 1a–h). These techniques have been used to understand the basic biology of fish and their commercial applications, as already discussed in previous publications (Chevassus 1983; Arai 2000, 2001, 2002; Bartley et al. 2001; Scribner et al. 2001; Komen and Thorgaard 2007; Piferrer et al. 2009; Arai and Fujimoto 2013, 2019; Benfey 2016; Rahman et al. 2019). In aquaculture-oriented research, reproduction in hybrid and triploid fishes has been examined in relation to gonadal, gametic, and zygotic sterilities. Sterility often reallocates energy from maturation to somatic growth, and therefore an improvement in growth traits is predictable. Hybridization and triploidization also serve as reproductive control tools to diminish the risk of the accidental escape of exotic species and genetically modified organisms, including transgenics and genome-edited organisms, into indigenous ecosystems (Cotter et al. 2000; Devlin et al. 2006, 2010; Piferrer et al. 2009; Benfey 2016; Arai and Fujimoto 2019; Rahman et al. 2019); such organisms could seriously disturb biodiversity in indigenous ecosystems through genetic contamination and introgression. Both artificially produced hybrid and/or induced triploid strains are widely used in commercial fish culture (Arai 2000, 2001, 2002; Bartley et al. 2001; Scribner et al. 2001; Komen and Thorgaard 2007; Piferrer et al. 2009; Arai and Fujimoto 2013, 2019; Rahman et al. 2019; Havelka and Arai 2019). Occasionally, products of hybridization and triploidization, i.e., triploid hybrids or allotriploids, are developed as sterile all-female strains, especially in salmonids, to vitalize the local economy (Arai 2001, 2002; Kohara and Denda 2008; Arai and Fujimoto 2019). Moreover, sterile hybrid and triploid fishes can be readily applied as ideal hosts (recipients) in germ-line chimeras, because they do not undergo normal gametogenesis, and only transplanted exogenous germ cells can develop into fertile gametes in sterilized gonads (Piva et al. 2018; Goto and Saito 2019).
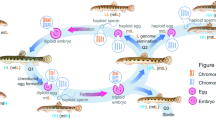
Basic techniques of chromosome (set) manipulation in teleosts as exemplified in the dojo loach Misgurnus anguillicaudatus. a Diploid induction by normal fertilization (2n female × 2n male). Mature eggs at the metaphase of second meiosis can accept sperm. After sperm intrusion, the second polar body (2 PB) is released and a 1n female (egg) pronucleus and a 1n male (sperm) pronucleus fuse to form a 2n zygote, which begins cleavage and development. b Triploid induction by the inhibition of second polar body (2 PB) release (PBI) after fertilization (2n × 2n/PBI). c Tetraploid induction by the inhibition of cleavage (CVI) after fertilization (2n × 2n/CVI). d Gynogenetic haploid induction by the fertilization of normal eggs with UV-irradiated genetically inert sperm (2n × UV). e Gynogenetic (heterozygous) diploid induction by PBI after the fertilization of normal eggs with UV-irradiated genetically inert sperm (2n × UV/PBI). f Gynogenetic (homozygous) diploid (doubled haploid) induction by CVI after the fertilization of normal eggs with UV-irradiated sperm (2n × UV/CVI). Doubled haploids produce isogenic eggs from which a clonal strain can be formed by second-round gynogenesis followed by artificial diploidization (PBI or CVI). g Androgenetic haploid induction by the fertilization of UV-irradiated genetically inert eggs with normal sperm of a diploid male (UV × 2n). h Androgenetic diploid (doubled haploid) induction by CVI after the fertilization of UV-irradiated genetically inert eggs with normal sperm of a diploid male (UV × 2n/CVI). Doubled haploids produce isogenic eggs or sperm from which a clonal strain can be formed by second round-gynogenesis or androgenesis followed by diploidization (PBI or CVI for gynogenesis, CVI for androgenesis). 2 PB second polar body, UV genetic inactivation of gametic nucleus by UV irradiation, open arrow timing of inhibition of 2 PB release (PBI), solid arrow timing of inhibition of the cleavage (CVI)
Certain kinds of artificial hybrid females exhibit unreduced egg formation, as previously reported in the hybrids of medaka (Shimizu et al. 2000), salmonid (Johonson and Wright 1986), sunfish (Dawley et al. 1985), cyprinid (Liu et al. 2001; Liu 2010), and cobitid (Choleva et al. 2012; Marta et al. 2023) fishes. These fertile hybrid females produced triploid progeny when backcrossed with parental species. Hybrids laying gynogenetically developed unreduced eggs were rare (Johonson and Wright 1986; Lampert et al. 2007; Choleva et al. 2012). The hybrid males had undeveloped abnormal testes, at best, with aneuploid sperm which could fertilize normal eggs but produced inviable progeny (Hamaguchi and Sakaizumi 1992; Shimizu et al. 1997; Arai and Fujimoto 2013, 2019; Marta et al. 2023). However, exceptional cases of fertile unreduced sperm were reported in Iberian minnow (Alves et al. 1999) and a crucian carp × common carp hybrid (Liu et al. 2001).
Hybridization between remotely distant species often results in inviable progeny (Chevassus 1983; Devlin et al. 2022). Although hybridization between closely related species produces viable progeny, in most cases, the resultant progeny is sterile (Chevassus 1983). On the contrary, hybrids between moderately distant species sometimes bypass the expression of sterility and acquire the ability to produce fertile gametes by atypical modes of reproduction, as mentioned later in the text. This phenomenon is generally known as the “balanced hypothesis” advocated by Moritz et al. (1989). In natural hybrids of Poeciliopsis (Schultz 1969) and Hexagrammus (Kimura-Kawaguchi et al. 2014), hybridogenesis produces hemiclonal haploid eggs by eliminating the paternally derived haploid set of chromosomes in the course of oogenesis and then exclusively transmitting the maternally derived haploid set of chromosomes into the eggs without any recombination (Fig. 2a). When backcrossed with paternal species, this progeny would comprise a haploid set of isogenic chromosomes from the mother and a haploid set of chromosomes from the father, generating only hybrid progeny in the next generation. Thus, through hybridogenesis, natural hemiclonal hybrids can be perpetuated by backcrossing with the paternal parent. Interestingly, hybrid-origin allodiploid and allopolyploid fish species reproduce asexually through the gynogenesis of unreduced isogenic eggs (Fig. 2b), as previously reported in Poecilia (Monaco et al. 1984; Dedukh et al. 2022), Squalius (Rutilus) (Collares-Pereira et al. 2013), Carassius (Yamashita et al. 1993; Mishina et al. 2021), Cobitis (Janko et al. 2018; Dedukh et al. 2020a), and others (Beukeboom and Vrijenhoek 1998; Goddard et al. 1998). Thus, hybridization and subsequent elevation of the ploidy level in the resultant progeny sometimes confer a capacity for clonal reproduction of unreduced eggs by gynogenesis. Such kinds of natural clonal lines are essentially different from the artificially established clonal strains that are induced by chromosome manipulation techniques (Arai 2000, 2001, 2002; Komen and Thorgaard 2007; Arai and Fujimoto 2013, 2019), wherein the second cycle of gyno- or androgenesis of isogenic gametes laid by completely homozygous doubled haploids (DH) can be induced by inhibiting the first cleavage after gyno- or androgenetic haploid development initiated by fertilization with genetically inactivated UV-irradiated sperm or eggs (Fig. 1f, h). As mentioned above, hybrid, polyploid, and asexual clones are closely interrelated. However, the gametogenic and meiotic mechanisms responsible for atypical reproduction, comprising unreduced gametogenesis, unisexual gynogenesis, or hybridogenesis, are not well understood. Moreover, the meiosis of artificial hybrid and induced polyploid fishes is also not understood conclusively. Thus, our knowledge of the cytogenetic mechanisms behind the expression of various kinds of sterilities in both natural and induced hybrids and triploids is still fragmentary and insufficient. The behavior and configuration of meiotic chromosomes, i.e., the capacity for synapsis formation between homologous, homoeologous, and/or orthologous chromosomes to form regular bivalents, have not yet been well examined in most cases of hybrid and polyploid fishes due to technical difficulties brought on by the relatively large numbers and small sizes of fish chromosomes.
Atypical reproductive modes found in teleosts. a Hybridogenesis (hemiclonal reproduction). This system presumably originates from a past hybridization event between genetically differentiated ancestors (differences are shown by green and yellow chromosomes). Paternally derived (yellow) chromosomes are eliminated in the course of oogenesis, while maternally derived (green) chromosomes are transmitted to haploid eggs without any recombination, and thus all resultant eggs are isogenic hemiclonal. The precise mechanism for isogenic haploid egg formation has not been identified. The fertilization of hemiclonal eggs with sperm of the paternal species generates the perpetual occurrence of hybrid progeny. b Gynogenesis (clonal reproduction). This system also originates from a past hybridization and subsequent triploidization event between genetically differentiated ancestors (shown by green and yellow chromosomes). The resultant hybrids and (allo)triploids generate unreduced 2n and 3n eggs, respectively, both of which develop naturally without any genetic contribution from sperm donors (shown by orange chromosomes), i.e., sperm-dependent parthenogenesis, gynogenesis. c Meiotic hybridogenesis. Triploid hybrids (two sets of green chromosomes and one set of yellow chromosomes) produce fertile recombinant haploid eggs after regular meiosis between homologous (green) chromosomes after elimination of non-homologous (yellow) chromosomes. Haploid eggs produce diploid progeny by fertilization in the next generation
Dojo loach Misgurnus anguillicaudatus is an excellent model animal to study the biological significance of both naturally occurring and artificially induced hybrid, polyploid, and clonal biotypes (Arai and Fujimoto 2013). This is because it is considered to be a species complex comprising genetically independent groups, which are presumably equivalent to species and their hybrids as well as to various levels of natural polyploid and clonal biotypes (Arai and Fujimoto 2013). In addition, as an experimental animal, dojo loach is generally easy to mature, ovulate, spermiate, and fertilize in relatively small-scale laboratory conditions, and procedures to breed, raise, and mature resultant progeny have already been established (Suzuki 1982; Arai and Fujimoto 2013). Therefore, dojo loach and related species have been used as model organisms in basic biological and biotechnological studies on fertilization (Yanagimachi et al. 2017) (Fig. 1a), developmental staging (Fujimoto et al. 2004, 2006), sex differentiation (Fujimoto et al. 2010a), sex reversal (Nomura et al. 1998; Yoshikawa et al. 2007, 2009), induced polyploidy (Suzuki et al. 1985a; Fujimoto et al. 2010b, 2013) (Fig. 1b, c), UV-ray-induced gynogenesis (Suzuki et al. 1985b; Suwa et al. 1994) (Fig. 1d), UV ray-induced androgenesis (Arai et al. 1992; Masaoka et al. 1995; Fujimoto et al. 2007; Yasui et al. 2010) (Fig. 1g), cold-shock-induced androgenesis (Morishima et al. 2011; Hou et al. 2013, 2014), nucleo-cytoplasmic hybrids (Fujimoto et al. 2010c), nuclear transplantation (Tanaka et al. 2009), intracytoplasmic sperm injection (Yasui et al. 2018), germ cell transplantation (Yasui et al. 2011), cryobanking (Kusuda et al. 2004; Yasui et al. 2008, 2009, 2010, 2011, 2012; Inoue et al. 2012), gene knockdown (Fujimoto et al. 2010a; Yasui et al. 2011), gene transfer (Nam et al. 2002), mitochondrial DNA markers (Morishima et al. 2008a; Shibata et al. 2020), nuclear gene markers (Yamada et al. 2015; Kuroda et al. 2021a), repetitive DNA markers (Fujimoto et al. 2017), allozyme markers (Khan and Arai 2000), microsatellite markers (Morishima et al. 2001, 2008b; Arias-Rodriguez et al. 2007), and molecular cytogenetics (Li et al. 2010, 2011, 2013, 2015, 2016; Kuroda et al. 2018, 2019, 2021b; Shibata et al. 2023).
In the first section of this review, the genetic structure and genomic constitution of the dojo loach species complex are briefly overviewed. The dojo loach was previously considered a single-species entity (Saitoh 1989), but a series of genetic and cytogenetic studies revealed that it included genetically independent groups (Khan and Arai 2000; Arias-Rodriguez et al. 2007; Morishima et al. 2008a; Yamada et al. 2015; Fujimoto et al. 2017; Kuroda et al. 2018, 2021a; b; Shibata et al. 2020, 2023), and the recent invasion of Japan by exotic loaches through the transportation of, e.g., food and fishing baits (Shimizu 2014) has made the situation with the natural populations much more complicated. Natural tetraploids, triploids, and other polyploids with higher DNA contents were often found in market samples but rarely in wild populations (Ojima and Takai 1979; Arai et al. 1991a; Arai 2003; Zhang and Arai 2003; Zhao et al. 2012a). Progeny from their hybridization and chromosome manipulation exhibited atypical reproductive modes that had never been observed in wild-type diploid dojo loach (Arai and Fujimoto 2013). After that, meiosis and gametogenesis in several kinds of artificial allodiploid hybrids, natural autopolyploids, allopolyploids, and clonal biotypes are focused upon to reveal certain relationships between reproductive capacity (fertility/sterility) and genome (chromosome set) constitution. The expression of atypical reproductive modes, such as sterility at the gonadal, gametic, and zygotic levels and fertility by the formation of unreduced gametes, is discussed in relation to mechanisms for altering meiosis and gametogenesis.
Brief overview on loach taxonomy and phylogeny
An overview on the phylogenetic and taxonomical relationship among Misgurnus loaches is schematically shown in Fig. 3. The dojo loach M. anguillicaudatus has been treated as a single-species entity for a long time (Saitoh 1989), but its taxonomical status is inconclusive, unstable, and confusing at present (Shimizu 2014). Taxonomic and nomenclature problems with Misgurnus loaches were also recognized in Russian Asia (Vasil’eva 2001). This is because genetic studies using classic allozyme electrophoresis (Khan and Arai 2000), microsatellite genotypes (Arias-Rodriguez et al. 2007), certain nuclear gene sequences (Yamada et al. 2015), characteristics of repetitive DNA sequences (Fujimoto et al. 2017; Kuroda et al. 2021a), and mitochondrial DNA haplotypes (Morishima et al. 2008a) revealed the presence of the genetically diverse groups A and B and the further differentiation of group B into B1 and B2 (Fig. 3). Other research groups also obtained the same results: the presence of three genetically independent groups in Japan (Koizumi et al. 2009). The genetic distance between these groups is almost equivalent to the inter-specific level (Morishima et al. 2008a; Koizumi et al. 2009). Recent molecular cytogenetic analyses using repetitive sequences as fluorescence in situ hybridization (FISH) probes clearly distinguished the chromosomes between groups A and B (Kuroda et al. 2018, 2021b). Thus, the Japanese population of dojo loach is most likely a species complex that includes some independent groups with diversification equivalent to inter-specific distances. In Japanese natural populations, group A is specific to the east part of Hokkaido and the Notojima District, Ishikawa Prefecture, Honshu, while most wild-type diploid individuals belonging to the indigenous group B1 are distributed in the central and south parts of Hokkaido, the northeast and west parts of Honshu, and Shikoku (Morishima et al. 2008a) (Fig. 3). Group B2 loaches have been shown to be distributed mainly in the central parts of Honshu and Kyushu (Morishima et al. 2008a) (Fig. 3).
An overview of the taxonomical and phylogenetic relationships among Misgurnus loaches, including M. fossilis, M. mizolepis, M. dabryanus, M. nikolskyi, M. chipisaniensis, M. bipartitus, M. mohoity, and three groups (A, B1, and B2) of M. anguillicaudatus, with brief descriptions of their cytogenetics and distributions in Japan and adjacent countries. Lengths in the phylogenetic tree do not reflect real genetic distances between clades. Relationships shown by solid lines (concluded) and dotted lines (estimated) are based on Morishima et al. (2008a), Perdices et al. (2012), and Shedko and Vasil’eva (2022). Red arrows indicate the transportation of exotic loaches from China to Japan. See main text for more details
Molecular studies on the complete mitochondrial genome showed that group A is phylogenetically close to M. mohoity and M. nikolskyi, while being genetically distant from group B1, which is close to M. bipartitus (Shibata et al. 2020). However, M. bipartitus is considered as a synonym of M. mohoity (Perdices et al. 2012), and thus the species identification and nomenclature of samples used for sequence data are unreliable. Thus, the relationship between group B1 and M. mohoity and/or M. bipartitus needs to be re-examined in the near future by integrating precise taxonomical identification, nomenclature, and molecular phylogeny data. On the other hand, mtDNA haplotypes of group B2 were the same or close to those of loaches in China, and thus B2 is inferred to be exotic in origin (Morishima et al. 2008a; Li et al. 2017). The group A loach is most likely an independent entity because Shedko and Vasil’eva (2022) described a new species of Misgurnus loaches from the south of Sakhalin Island, M. chipisaniensis, comprising mitochondrial cyt b, Rag I gene, and IRBP gene sequences—the gene sequences described in the Japanese group A (Yamada et al. 2015; Shibata et al. 2020). Shedko and Vasil’eva (2022) also revealed a close relation between M. chipisaniensis and M. nikolskyi (Fig. 3), and the presence of two clades in group B of M. anguillicaudatus. Thus, taxonomical studies are required to conclusively establish a relationship between the new species M. chipinsaniensis and the Japanese group A loach. A recently published illustrated book on Japanese freshwater fishes uses a new Japanese name, “Kitadojo,” and the English name “northern weather loach” for the group A dojo loach (Fujita 2019).
In Japanese wild populations, most dojo loach specimens in groups A, B1, and B2 show diploidy, with 2n = 50 chromosomes categorized into 10 metacentric (m), 4 submetacentric (sm), and 36 telocentric (t) chromosomes and no morphologically distinct sex chromosomes (Ojima and Takai 1979; Arai et al. 1991a; Itono et al. 2006). However, in the market samples, tetraploid (4n = 100, 20m + 8sm + 72t) and triploid (3n = 75, 15m + 6sm + 54t) specimens have often been recognized (Arai et al. 1991a); other unusual polyploids, such as hyper-tetraploid, hyper-triploid, pentaploid-range, hexaploid-range, heptaploid-range, and mosaic individuals, were also infrequently detected by flow cytometry (Zhao et al. 2012a). Unusual aneuploid or hyper-diploid specimens with supernumerary B chromosomes were also reported (Zhang and Arai 2003). Although polyploid dojo specimens, mainly triploid and tetraploid, have often been found in market samples (Fig. 3), natural polyploids are scarce, and most wild-type individuals are diploids (2n = 50) in Japanese wild populations (Ojima and Takai 1979; Zhang and Arai 1999a; Arai 2003; Arai and Fujimoto 2013). Later, based on genetic results, these polyploids were strongly suggested to be exotic fishes (Fig. 3) because the diploid-tetraploid complex is present in the Chang Jiang River basin, China (Li et al. 1983, 2008, 2010, 2012, 2017). As described later in the section “Natural tetraploids—cytogenetic and experimental evidence of genetic autotetraploidy,” M. anguillicaudatus with 100 chromosomes was concluded to be a true tetraploid.
The fish population in Hirokami, Niigata Prefecture, Honshu Island, has relatively high frequencies (2.0–15.8%) of natural triploid specimens (Zhang and Arai 1999a). A special diploid individual was reported to lay both fertile haploid and diploid eggs simultaneously, and therefore triploidy may have arisen by cross-breeding between such a special diploid female and a wild-type diploid male in Hirokami (Zhang and Arai 1999a). In the east part of Hokkaido Island, high frequencies of natural triploids (4.3–21.3%) were recorded, along with spontaneous diploid clonal lineages which reproduce gynogenetically, as described in detail in a later section (Morishima et al. 2002). The clonal diploid loach lays unreduced diploid eggs, and these isogenic eggs develop by gynogenesis without any genetic contribution of sperm from sympatric wild-type gonochoristic males (Morishima et al. 2002). Sympatric natural triploids are concluded to arise from the diploid clonal eggs by accidental incorporation of sperm nuclei (Morishima et al. 2002). A similar occurrence of natural triploids was also detected in the population of Notojima Island, Nanao City, Ishikawa Prefecture, Japan (Morishima et al. 2008a). The clonal loach is considered to originate in past hybrid events between the ancestors of group A females and those of group B1 males (Fig. 3), as discussed later (in the section “Clonal lineage and its hybrid origin”).
The dojo loach M. anguillicaudatus and the related species M. nikolskyi and M. mohoity have a diploid chromosome number of 2n = 50 (Arai et al. 1991a; Itono et al. 2006; Vasil’ev and Vasil’eva 2008), but the bisexually reproducing tetraploid M. anguillicaudatus with 4n = 100 and putative inter-ploidy triploids with 3n = 75 exist in China (Li et al. 1983, 2008, 2010, 2012, 2013) (Fig. 3). European weatherfish M. fossilis shows 2n = 100 chromosomes (Neyfakh 1964; Timofeeva and Kaviani 1964; Ene and Radu 2000), but this loach is not tetraploid but diploid, as mentioned later in the section “Natural tetraploids—cytogenetic and experimental evidence of genetic autotetraploidy.” An exotic species, mud loach M. mizolepis, has been detected in a wide range of wild populations in Japan (Shimizu 2014). Mud loach M. mizolepis was considered synonymous with the large-scale loach Paramisgurnus dabryanus or M. dabryanus (Vasil’ev and Vasil’eva 2008; Perdices et al. 2012; Shedko and Vasil’eva 2022), but recent genetic studies disclosed the apparent presence of two genetically diverse types of exotic loaches, and thus it is difficult to label all the exotic loach specimens as P. dabryanus (Shimizu 2014). Both M. mizolepis and P. dabryanus showed 2n = 48 chromosomes (Li et al. 1983; Ueno et al. 1985), which were karyologically distinguishable from the 2n = 50 chromosomes of M. anguillicaudatus (Fig. 3). Such non-native exotic species issues make the taxonomical status of the dojo loach and its related species much more complicated. The complexity of the situation also makes the conservation of indigenous biodiversity difficult.
Hybridization
Inter-familial hybrids
Distant inter-familial hybridization was carried out between female dojo loach (the family Cobitidae) and male goldfish Carassius auratus, minnow Gnathopogon elongatus elongatus, or common carp Cyprinus carpio (family Cyprinidae) (Kijima et al. 1996a, b). These inter-familial hybrids were produced by successful hetero-specific fertilization, but no progeny survived beyond the larval stage due to the expression of various abnormalities during embryogenesis. In salmonids, the inviable embryogenesis of some hybrids could be explained by aneuploidies due to the elimination of chromosomes during early development (Arai 1984; Fujiwara et al. 1997). Similar elimination of chromosomes was also observed in the medaka hybrids in which all paternally derived chromosomes were eliminated until the haploid status was obtained (Sakai et al. 2007). However, inter-familial hybrids between the female dojo loach and the male goldfish, minnow, or carp showed intermediate chromosome numbers and karyotypes between parental species (Kijima et al. 1996a, b). In these inviable hybrids, chromosome losses did not occur during embryogenesis, and therefore they are considered true allodiploid hybrids with chromosomes from two parental species. Aberrant gene expression patterns may be reasoned to be the cause of abnormal embryogenesis in inviable salmonid hybrids without chromosome losses (Arai 1984). However, the molecular and cellular mechanisms responsible for abnormal development in inviable hybrids have not been examined thoroughly so far, and further studies are required in the future.
In certain salmonid hybrids, an increase in maternally derived chromosome set(s) obtained by allotriploidization resulted in a drastic recovery in abnormal embryonic development, and the resultant viable allotriploids showed a normal appearance, hatched successfully, and grew to adult stages (Arai 1984, 1986, 1988, 2002; Fujiwara et al. 1997; Piferrer et al. 2009; Arai and Fujimoto 2019). A similar morphogenic recovery was also observed in the allotriploid hybrids of female dojo loach × male goldfish, minnow, or carp. The external appearance of these allotriploid hybrids was much better than that of the allodiploid hybrids (Kijima et al. 1996a, b). Similar phenomena were also observed in allopentaploid inter-familial hybrids between natural tetraploid female dojo loach or spinous loach Cobitis biwae and male carp (Kijima et al. 1996b). However, all inter-familial loach hybrids died before the adult stage.
Inter-generic hybrids
Classic hybridization studies reported that inter-generic cross-breeding between Misgurnus dojo loach and Cobitis spinous loach resulted in inviable progeny (Minamori 1953). In contrast, when we conducted reciprocal inter-generic hybridizations between dojo loach and spinous loach, all the resultant progeny hatched larvae at more than 90% of the normal rates and grew to adult size (Kusunoki et al. 1994a). In spinous loach C. biwae, there were two local races, large tetraploid (4n = 96, 32m + 44sm, subtelocentric (st) + 20 acrocentric (a)) and small diploid (2n = 48, 16m + 22sm, st + 10a), but the inter-generic hybrids produced were sufficiently viable (Kusunoki et al. 1994a). All the resultant inter-generic hybrids between the dojo loach and spinous loach were true hybrids because they showed karyotypes intermediate between the two parental species (Kusunoki et al. 1994a). This conclusion was also supported by allozyme genotypes (Arai et al. 1994).
Inter-specific hybrids
Artificial hybrids between the dojo loach and mud loach were viable and males fertile, as suggested by Korean scientists on histological grounds (Park et al. 2006). Fujimoto et al. (2008) also produced viable diploid and triploid hybrids between female dojo loach and male mud loach. Triploid hybrids, i.e., allotriploids, were produced by inhibiting the second polar body release (Fig. 1b), and their sterility has been reported previously (Nam et al. 2004).
Among the diploid hybrids between female dojo loach and male mud loach, different ploidy statuses were seen in the sperm. Some hybrids showed a predominance of haploid and tetraploid sperm cells, while others comprised exclusively of tetraploid sperm cells (Table 1). Tetraploid sperm cells seemed to be arrested at the replication stage without entering meiotic divisions, as previously reported in interspecific medaka hybrids (Hamaguchi and Sakaizumi 1992; Shimizu et al. 1997). As summarized in Table 1, tetraploid spermatozoa which exhibited low motility had heads larger (length 2.83 μm, width 2.80 μm) than those of the control diploids (length 1.80 μm, width 1.80 μm) (Fujimoto et al. 2008; Zhao et al. 2016). Some of them had no flagellum (36%), while others were bi-flagellate (17%) (Zhao et al. 2016). The length of the flagellum of tetraploid spermatozoa (12.5 μm) was much shorter than that of control diploids (23.9 μm). ATP contents of hybrids were significantly higher than those of control diploids. Numbers of mitochondria per spermatozoon in the hybrids showed a wider range (4–19) than those in the control diploids (7–14). As described by Zhao et al. (2016), flow cytometry revealed a larger total volume of mitochondrial mass in the hybrids than in the control. The shorter flagellum and larger sperm head may have caused the decrease in motility and subsequent survival rates of the progeny due to the failure of fertilization. High energy-related factors such as ATP content and the larger numbers and volume of mitochondria could not compensate for the low motility of the tetraploid spermatozoa of hybrids.
Sperms of male hybrids backcrossed with dojo loach did not result in larvae in most cases, but some crosses gave viable larvae at very low rates (1.0–2.2%) (Fujimoto et al. 2008). Microsatellite genotyping in these progeny indicated that haploid spermatozoa were produced in the diploid hybrid by regular meiotic divisions, as in the wild-type diploids, and both dojo-loach-derived and mud-loach-derived alleles were segregated (Fujimoto et al. 2008). The difference in karyotype between the dojo loach (2n = 50, 10m + 4sm + 36t) and mud loach (2n = 48, 12m + 4sm + 32t) could be explained by Robertsonian translocation, and thus balanced chromosomes might successfully proceed to meiotic divisions and subsequent regular spermatogenesis. At present, the mechanisms for the production of a small quantity of fertile haploid and diploid spermatozoa have not been elucidated.
Inter-populational hybrids
As described in the previous section, dojo loach show the presence of the genetically divergent groups A, B1, and B2. Before successful genetic identification, hybridization between female dojo loach from group A from a dominant locality (formerly Memanbetsu town, presently Ozora town, Abashiri district, Hokkaido) and male dojo loach from group B1 from another dominant locality (formerly Kita village, presently Iwamizawa city, Hokkaido) had been carried out in 2004, and about 50% of the fertilized eggs had hatched (Arias-Rodriguez et al. 2009).
The eggs of these inter-populational hybrids were backcrossed with putative group A males, hybridized with goldfish males, and gynogenetically induced with UV-irradiated sperm, and the ploidy status and microsatellite genotypes were examined in the resultant progeny (Arias-Rodriguez et al. 2009). In the inter-populational hybrid females, oogenesis could be categorized into two cases: (1) the production of unreduced isogenic diploid eggs which incorporate sperm to produce triploid progeny and (2) the simultaneous production of a large number of meiotic haploid (63–96%) and a small number of diploid eggs which also incorporate sperm. Both cases had a rate of approximately 50% in the female hybrid individuals. In the second case, some females produced isogenic unreduced eggs, but others did not. Isogenic eggs are presumably produced by premeiotic endomitosis, as discussed later in the section “Cytogenetic mechanisms for triploid and haploid egg formation,” but mechanisms for the production of non-isogenic diploid eggs have not been identified. Together with the haploid and/or diploid eggs, some females laid a few tetraploid and other higher-polyploid eggs with hexaploid and heptaploid ranges (Arias-Rodriguez et al. 2009). Thus, inter-populational hybrid females showed atypical oogenesis, such as unreduced egg formation and simultaneous generation of both haploid and diploid eggs.
In contrast, inter-populational hybrid males produced fewer sperms of low motility (0–20%) and with morphological abnormalities such as no flagellum or multi-flagella (Arias-Rodriguez et al. 2010). Thus, such a hybrid male was considered gametically sterile. As summarized in Table 1, flow cytometry revealed the presence of haploid, diploid, and tetraploid cell populations and larger sperm head sizes in the hybrids (2.90 μm) as compared to the wild-type diploids (1.80 μm) (Arias-Rodriguez et al. 2010).
Fertilization experiments with hybrid sperms resulted in zero or extremely low (4.5% at best) survival rates of the resultant progeny, and few diploid and triploid progeny occurred in the backcross, suggesting the simultaneous production of haploid and diploid spermatozoa. Microsatellite genotyping of a few progeny revealed that haploid spermatozoa are generated by regular meiosis, and the diploid spermatozoa, since they had two alleles of hybrids, were presumably unreduced genotypes (Arias-Rodriguez et al. 2010).
Meiotic configurations were carefully analyzed in similar inter-populational hybrids between female group B1 and male group A dojo loach (Kuroda et al. 2019). In the wild-type dojo loach spermatocytes (2n = 50), 25 bivalents are normally observed. In these hybrids, synapses were seen between group A and group B1 chromosomes, and an average number of bivalents ± standard deviation (SD) of 21.8 ± 1.6 were observed by FISH using the group-B-specific repetitive sequences Man-Dra B. An average of 3.2 ± 1.6 univalents exclusively with group A chromosomes and 3.2 ± 1.6 univalents with group B1 chromosomes were also observed together with the bivalents (Kuroda et al. 2019). Thus, approximately 22 chromosomes in each group could pair to form inter-group bivalents, while approximately three chromosomes in each group could not find any counterpart to form a bivalent and, therefore, formed univalents (Table 2). In these meiotic configurations, the formation of aneuploid spermatozoa or the arrest of spermatogenesis is predicted, but a very small quantity of haploid and diploid spermatozoa was recorded by Arias-Rodriguez et al. (2010).
Inter-ploidy diploid hybrids
Chinese-origin natural tetraploid loach is genetically different from Japanese diploid dojo loach (Morishima et al. 2008a; Arai and Fujimoto 2013; Li et al. 2017). The Chinese tetraploid loach has 4n = 100 chromosomes categorized into four groups, as explained later in the “Autotetraploidy” section.
Although hybridization between tetraploid and diploid dojo loach produces triploid hybrids (Fig. 4c, e), the reproduction of any real diploid hybrid (allodiploid) with one set of chromosomes from a tetraploid and another set from a diploid has been difficult to investigate. Zhang et al. (2002) artificially induced a viable diploid individual from diploid eggs of a bisexually reproducing natural tetraploid female by artificial gynogenesis with UV-irradiated sperm (Fig. 4g). Then, cross-breeding between this tetraploid-origin gynogenetic diploid female and a wild-type diploid male was conducted to produce allodiploid hybrids comprising one set of 25 chromosomes from the natural tetraploid and another set of 25 chromosomes from the wild-type diploid (Fig. 4i). Such hybrids are considered inter-populational or inter-ploidy allodiploid hybrids between tetraploids and diploids.
Hybridization and chromosome manipulation using mature eggs (at the metaphase of second meiosis) and sperm of autotetraploid and diploid wild-type dojo loach. Chromosomes with different colors originate from different biotypes/genetic groups (blue: exotic autotetraploids, red: wild-type diploids). a Autotetraploid induction by cross-breeding between a tetraploid female and a tetraploid male (4n × 4n). b Autohexaploid induction by the inhibition of second polar body release (PBI) after 4n × 4n cross-breeding (4n × 4n/PBI). c Allotriploid induction by hybridization between a tetraploid female and a diploid male (4n × 2n). d Allopentaploid induction by PBI after 4n × 2n cross-breeding (4n × 2n/PBI). e Allotriploid induction by hybridization between a diploid female and a tetraploid male (2n × 4n). f Allotetraploid (neo-tetraploid) induction by PBI after 2n × 4n cross-breeding (2n × 4n/PBI). g Gynogenetic diploid induction by the fertilization of eggs of a tetraploid with UV-irradiated sperm (4n × UV). Note the viability of the 4n × UV progeny as compared to the 2n × UV progeny in Fig. 1d. h Androgenetic diploid induction by the fertilization of UV-irradiated eggs with sperm of tetraploids (UV × 4n). Note the viability of the UV × 4n progeny as compared to the UV × 2n progeny in Fig. 1g. i Inter-ploidy diploid hybrid (allodiploid) induction by the fertilization of haploid eggs laid by the gynogenetic diploid induced from eggs of tetraploids (4n × UV) with sperm of wild-type diploids ((4n × UV) × 2n). j Autotriploid (comprising three sets of chromosomes of autotetraploid) induction by PBI after the fertilization of haploid eggs laid by the gynogenetic diploids induced from eggs of tetraploids (4n × UV) with sperm of autotetraploids ((4n × UV) × 4n). 2 PB second polar body, UV genetic inactivation of gametic nucleus by UV irradiation, open arrow timing of inhibition of 2 PB release (PBI)
A majority (76%) of the oocytes from such inter-ploidy allodiploid hybrids showed the regular 25 bivalents, as in the wild-type diploid, but 16% comprised a few univalents, and the remaining 8% exhibited about 50 bivalents, indicating the presence of a chromosome duplication event before entering meiosis (Zhang et al. 2002) (Table 2). These meiotic configurations suggest the formation of both regular haploid and unreduced diploid eggs, along with a few aneuploid eggs, but the actual reproduction results have not been examined so far. In the testes, however, only a few spermatocytes (6%) showed the regular 25 bivalents, whereas 86% contained various numbers of univalents and the remaining 8% showed 50 bivalents (Zhang et al. 2002) (Table 2). No peaks of spermatozoa were identified flow cytometrically in the testes (Zhang et al. 2002). Thus, there was little possibility of the successful formation of haploid or diploid sperms, but the high rates of meiotic cells, including univalents, should result in the formation of aneuploid spermatozoa. A rate of spermatocytes with the regular 25 bivalents was much lower than that of oocytes with 25 bivalents (Zhang et al. 2002). The difference in the rates of meiotic germ cells with regular bivalents between the sexes cannot be explained at present, but females showed a high possibility of producing normal or near-normal haploid eggs. Although both female and male allodiploid hybrids showed the possibility of forming a small number of unreduced gametes, the reproductive modes of these hybrids have unfortunately not been examined so far.
Autotetraploidy
Induced tetraploids
Tetraploids can theoretically be induced by inhibiting early cleavage after normal fertilization (Fig. 1c), but induction is very difficult in reality due to an extremely low survival capacity (Arai 2000, 2001, 2002; Piferrer et al. 2009; Arai and Fujimoto 2013, 2019). Survival rates are poor due to the side effects of manipulation, which may cause unusual cell division without nuclear division (anuclear division), mosaicism comprising anuclear cells, and aneuploid macro- and micro-meres in the course of cleavage (Sakao et al. 2003). An abnormal vascular system causing edema was observed frequently, even in the larval stage after hatching (Sakao et al. 2006). Therefore, it is generally very difficult to obtain healthy adult tetraploids. However, once a fertile tetraploid family has been established, mature induced tetraploids are expected to generate diploid gametes, and then it is easy to produce a triploid family by cross-breeding between tetraploids and diploids. It is also easy to extend ploidy manipulation towards higher polyploid production by inhibiting polar body release after inter-ploidy hybridization (Arai 2000, 2001; Arai and Fujimoto 2013, 2019). In dojo loach, a few tetraploid progeny were successfully induced, but survivors (one tetraploid male and one diploid-tetraploid mosaic male) produced unexpected haploid sperm (Fujimoto et al. 2013), as previously reported in other fish species, including the closely related mud loach (Nam and Kim 2004).
Natural tetraploids—cytogenetic and experimental evidence of genetic autotetraploidy
Natural tetraploid dojo loach with 100 chromosomes have often been found among market samples, together with diploids and other polyploids (Arai et al. 1991a; Zhao et al. 2012a). They are likely to be exotic fish, Chinese in origin, because the diploid-tetraploid complex appears in the Chang Jiang River basin, China (Li et al. 2008, 2010, 2017), and also because such tetraploids have not been identified so far in wild populations in Japan, even after intensive screening trials (Zhang and Arai 1999a; Arai 2003; Arai and Fujimoto 2013).
Cytogenetic observation using conventional Giemsa staining revealed that natural tetraploids had four sets of homologous chromosomes (4n = 100, 20m + 8sm + 72t) (Arai et al. 1991a; Li et al. 2010). Diploid loach (2n = 50, 10m + 4sm + 36t) has two homologous chromosomes with rDNA sites detected by FISH signals, positive AG (silver stained)-NOR (nucleolus organizer regions), and CMA3 (chromomycin A3)/DA (distamycin A) differential staining, whereas tetraploids exhibited four chromosomes with rDNA sites (Li et al. 2010). These results showed that natural tetraploids have four sets of homologous chromosomes, i.e., they are autotetraploid.
In the case of diploid biotypes (2n = 50), UV-induced gynogenetic and androgenetic embryos developing from gametes of diploids could not survive due to the expression of abnormalities, which is referred to as haploid syndrome (Arai et al. 1991b, 1993, 1995) (Fig. 1d, g). This result strongly suggested that the diploid biotypes have two sets of homologous chromosomes, i.e., they are diploid. The European weather fish M. fossilis has 100 chromosomes, but gynogenetically induced progeny were inviable (Neyfakh 1964; Romashov and Belyaeva 1964). Thus, this species is no longer a genetic tetraploid fish (4n = 100), but a diploid (2n = 100) with two sets of homologous chromosomes. Similarly, common carp are generally considered tetraploid in origin with 100 chromosomes or chromosome arms, which is twice the number of other cyprinid species with 2n = 50, but their induced gynogenetic and androgenetic progeny were inviable because of haploid syndrome (Komen and Thorgaard 2007). Thus, these fish species are considered evolutionary tetraploids but genetically true diploids (2n = 100). Salmonid fishes of evolutionary tetraploid origin are also genetic diploids and thus their induced gynogenetic progeny are no longer viable due to the abnormal development caused by haploidy (Onozato 1982; Onozato and Yamaha 1983). On the contrary, when gynogenetic and androgenetic progeny were artificially induced from gametes of natural tetraploid loach with 100 chromosomes, the resultant progeny with 50 chromosomes were viable and showed a normal external appearance (Arai et al. 1991b, 1992, 1993, 1995) (Fig. 4g, h). These results indicate that the dojo loach with 100 chromosomes are not evolutionary diploids (2n = 100), but genetically true tetraploids with four sets of homologous chromosomes (4n = 100). A similar approach using induced gynogenesis revealed that a large race of spinous loach with 98 chromosomes was a genetic tetraploid with four sets of homologous chromosomes (Kusunoki et al. 1994b).
Characteristics of fertile diploid gametes of autotetraploids
Natural tetraploid dojo loach are gonochoristic and reproduce bisexually (Arai et al. 1991b, 1993, 1995; Matsubara et al. 1995; Zhang and Arai 1996; Arai 2003; Arai and Fujimoto 2013) with a female:male sex ratio of about 1: 1 (Arai et al. 1999; Arai 2003; Li et al. 2012; Arai and Fujimoto 2013; Zhao et al. 2014). They produce fertile gametes and can thus be used for intra- or inter-ploidy hybridization with diploid biotypes as well as for ploidy manipulation after hybridization (Arai et al. 1991b, 1993, 1995, 1999; Matsubara et al. 1995; Zhang and Arai 1996; Li et al. 2012; Arai and Fujimoto 2013; Zhao et al. 2014) (Fig. 4a–f). The diameters of fertile diploid eggs of natural tetraploids were larger (1.2–1.4 mm) than those of the control diploids (1.0–1.2 mm) (Arai et al. 1999). In the Chinese diploid–tetraploid complex, tetraploids laid eggs with diameters larger (1.0 mm) than those of control diploids (0.8 mm) (Li et al. 2012). In sperms and testes, the ploidy status of the major cell population was diploid spermatozoa, and the concentration and motility of such diploid spermatozoa were similar to or higher than those of the controls (Table 1), indicating fertility equivalent to haploid spermatozoa of the control diploids. The morphological structure of the diploid spermatozoa was essentially similar to that of the haploid spermatozoa of the control diploids. However, as seen in Table 1, head sizes of diploid spermatozoa of natural tetraploids (length 2.25 μm, width 2.24 μm) were larger than those of haploid spermatozoa of the control diploids (length 1.81 μm, width 1.80 μm). The flagellar length of the sperm in tetraploids (30.4 μm) was longer than that of the control (24.2 μm). The average number of mitochondria per spermatozoon (18.2) in natural tetraploids was higher than that in the control diploids (10.4). The volume of mitochondrial mass was also larger than that in the control (Zhao et al. 2014). The ATP contents of diploid spermatozoa were much higher than those of the haploid spermatozoa of the control. Higher energy, inferred from the higher numbers and larger volume of mitochondria, and the high ATP content presumably ensured that the diploid spermatozoa of tetraploids with larger head sizes had normal motility. The longer flagellum also supported stable motility to propel the diploid spermatozoa.
Meiosis with several quadrivalents and many bivalents
Autotetraploid fish are predicted to show a meiotic configuration that exclusively comprises quadrivalents, because the four homologous chromosomes are expected to form a quartet due to their high affinity. Such meiotic figures comprised exclusively of quadrivalents were reported in tetraploid frog species (Beçak et al. 1966). Thus, a meiotic configuration including only quadrivalents was expected in natural tetraploid dojo loach. In the spermatocyte metaphase and the germinal vesicles of the mature oocytes of the wild-type diploid loach, 25 bivalents were detected (Li et al. 2011) (Table 2). However, in the natural tetraploid loach, an unexpected configuration comprising a few quadrivalents and a relatively large number of bivalents was observed (Li et al. 2011) (Table 2). The most frequent meiotic configuration was 4 quadrivalents + 42 bivalents in spermatocytes and 3 quadrivalents and 44 bivalents in oocytes, together with various other kinds of configurations (Li et al. 2011) (Table 2). Although the duplication of chromosomes from diploidy (2n = 50) to tetraploidy (4n = 100) should give rise to a quadrivalent meiotic configuration only due to the high affinity among the four homologous chromosomes, many bivalents also appeared in the actual meiosis of tetraploid loach (Table 2). Such bivalent-rich configurations in the meiosis of the tetraploid loach can be explained by rapid pairwise diversification soon after chromosome duplication. Quadrivalents are likely unstable for regular meiotic divisions and thus the tetraploid biotype may have taken a stable configuration genetically as well as cytogenetically by decreasing quadrivalents and increasing bivalents in the process of meiosis. As diploid and tetraploid loach were reported to share very similar or identical mtDNA haplotypes (Morishima et al. 2008a; Li et al. 2017), tetraploids were considered to have arisen recently by genome duplication, but the meiotic configuration may have changed rapidly from unstable to stable.
Neo-tetraploidy as an allotetraploidy
As mentioned in the “Autotetraploid” section, it is generally difficult to induce a sufficient number of healthy tetraploid fishes and to maintain tetraploid strains. Similarly, allotetraploid fish are also difficult to produce (Arai 2000, 2001; Piferrer et al. 2009; Arai and Fujimoto 2013, 2019). As natural tetraploid biotypes are another source of diploid gametes in dojo loach, a “neo-tetraploid” strain can be produced by fertilizing haploid eggs of wild-type diploids with diploid sperm of natural tetraploids, followed by chromosome manipulation to inhibit the second polar body release (Fujimoto et al. 2010b) (Fig. 4f). Wild-type diploids from Japan are genetically different from the natural tetraploids present in the Chang Jiang River system, China (Morishima et al. 2008a; Li et al. 2017). Therefore, the neo-tetraploid strain is considered a kind of allotetraploidy comprising two sets of homologous chromosomes from Japanese wild-type diploids and the other two sets from Chinese tetraploid loach (Fig. 4f).
Out of 15 3-year-old neo-tetraploids, eight were males and the sexes of the other seven were not known (Fujimoto et al. 2010b). As summarized in Table 1, the spermatozoa of neo-tetraploids were exclusively diploid. Their concentration was lower than that of the haploid spermatozoa of the control diploids, but motility was similar to that of the control diploids. The size of the head of the diploid spermatozoa of neo-tetraploids (length 2.24 μm, width 2.24 μm) was larger than those of the haploid spermatozoa (length 1.82 μm, width 1.81 μm). The flagellum was also longer in the spermatozoa of the neo-tetraploids (30.83 μm) than in the control diploids (23.85 μm). The head size and flagellum length of spermatozoa of the neo-tetraploids were similar to those of natural autotetraploids (Table 1). Higher ATP content (360.5 nmol/109 cells) and a larger number of mitochondria per cell (14–22) were recorded (Table 1). The total volume of the mitochondrial mass was reported to be larger than that in the control (Zhao et al. 2012b). These energy-related factors and the longer flagellum length may explain the active motility of diploid spermatozoa with larger head sizes. These parameters were similar to those recorded in the diploid spermatozoa of natural autotetraploids (Table 1).
The diploid spermatozoa of neo-tetraploids and the eggs of wild-type diploids were crossbred to produce triploids as second-generation progeny. Second-generation androgenetic diploids were also produced by fertilizing UV-irradiated eggs of wild-type diploids with diploid spermatozoa of neo-tetraploids. The second generation of neo-tetraploids were induced by inhibiting the second polar body release after cross-breeding between wild-type diploid females and neo-tetraploid males.
The second-generation progeny of neo-tetraploids deviated from the balanced 1:1 sex ratio and were sterile, except for a triploid female (Fujimoto et al. 2010b). The triploid progeny displayed a predominance of males (22 males:1 female in one cross and 11 males:1 female in the other cross). All these triploid males produced small volumes of non-motile triploid or hexaploid sperms and were thus considered sterile. On the contrary, a triploid female laid fertile haploid eggs which developed into diploid progeny after fertilization with haploid spermatozoa of wild-type diploid males. Second-generation androgenetic diploids were all males and produced diploid progeny after fertilization with wild-type haploid eggs. In the second-generation neo-tetraploids, there were no females and males produced a small quantity of non-motile sperm. In the testes, tetraploid and octaploid cell populations were considered the result of meiotic arrest at the replication stage.
The predominance of males in the second-generation progeny of neo-tetraploids showed that the first-generation neo-tetraploid males must have produced diploid spermatozoa exclusively with the male-determination gene or chromosome. However, there is no good explanation for the extremely biased sex ratio (Fujimoto et al. 2010b). The other possible explanation was sex reversal caused by environmental factors (Nomura et al. 1998; Fujimoto et al. 2010b; Morishima et al. 2012). Further studies are required to determine the sex system in tetraploids.
Autohexaploidy
Cross-breeding using tetraploid pairs resulted in tetraploid families, and the inhibition of second polar body release after fertilization resulted in the production of hexaploid progeny (6n = 150) (Zhang and Arai 1996; Arai et al. 1999) (Fig. 4a, b). Hexaploid loach were viable and generated fertile triploid eggs and sperm (Arai et al. 1999). Fertile triploid gametes of hexaploids were further evidence that dojo loach with 100 chromosomes were genetically tetraploid. Had dojo loach with 100 chromosomes been diploid (2n = 100), polar-body inhibition would have produced sterile triploids (3n = 150). However, all the loach with 150 chromosomes produced fertile triploid gametes. The results indicated that loach with 100 chromosomes were not diploid but tetraploid. Egg sizes of hexaploids (approximately 1.4 mm diameter) were larger than those of tetraploids (1.2–1.4 mm), which in turn were larger than those of diploids (1.0–1.2 mm). Both tetraploid and hexaploid biotypes presumably underwent the regular process of meiosis in gametogenesis (Table 2). The sex ratio of the hexaploid family was 11 females:10 males (Arai et al. 1999). Unfortunately, the meiotic configuration has not been observed and the characteristics of the triploid spermatozoa have not been examined.
Autotriploidy
Sterile oogenesis and spermatogenesis
Autotriploidy comprises three sets of conspecific homologous chromosomes. Such a genomic constitution can be easily induced by inhibiting the second polar body release just after fertilization with physical treatments such as hydrostatic pressure or temperature (cold, heat) shocks (Fig. 1b). In dojo loach, artificial triploids had an egg nucleus (1n), a sperm nucleus (1n), and an additional second polar body nucleus (1n). Thus, these autotriploids had three sets of homologous chromosomes derived from the wild-type diploid dojo loach.
Histologically sterile gonads have been reported in both female and male 1-year-old induced autotriploid dojo loach (Suzuki et al. 1985a). In ovaries of 1-year-old wild-type diploids that were full of vitellogenic oocytes, only a few yolk-laden oocytes were detected in the undeveloped ovaries, even in 6- or 7-year-old autotriploids (Zhang and Arai 1999b). In contrast, same-age autotriploid males had almost the same-sized testes as diploids (Zhang and Arai 1999b). Those testes contained a small number of fertile but aneuploid spermatozoa with extremely low motility and various abnormalities (Fujimoto et al. 2008) (Table 1). Sperms of autotriploid males were aneuploidies with a mode of 1.3n, which generated 2.2–2.5n progeny and 3.0–3.7n progeny when backcrossed with diploid and tetraploid females, respectively (Zhang and Arai 1999b; Arai and Inamori 1999) (Table 2). Most progeny were morphologically abnormal and inviable, but a small number of hyper-diploid and hyper-triploid progeny survived in diploid × triploid (female × male) and tetraploid × triploid crosses, respectively (Zhang and Arai 1999b; Arai and Inamori 1999). Thus, almost complete sterility in females and aneuploid spermatogenesis in males were confirmed in autotriploids, including three sets of chromosomes derived from wild-type diploids.
Progeny of induced autotriploids often show putative B chromosomes as supernumerary chromosomes (Zhang and Arai 1999b; Arai and Inamori 1999). They are much smaller than the smallest chromosomes of the regular karyotype members, do not pair in meiosis, and show inter- and intra-individual variations. Such B chromosomes were reported to occur not only in the progeny of autotriploids, but also in the natural population (Zhang and Arai 2003) and in market hyper-tetraploid specimens (Zhao et al. 2012a).
The other type of autotriploids, with three out of four sets of homologous chromosomes derived from natural tetraploids, were produced by cross-breeding between haploid eggs of gynogenetic diploid progeny induced from eggs of tetraploid females and diploid sperm of tetraploid males (Zhang et al. 2002), as seen in Fig. 4g, j. In 6- to 8-month-old autotriploids comprising three sets of natural tetraploids, the ovary was small and the oocytes did not differentiate beyond the pachytene stage, indicating gonadal sterility of females (Table 2). Their testes exhibited triploid, hexaploid, and dodecaploid cell populations without the production of fertile spermatozoa. The presence of a relatively high peak of hexaploid-range sperm cells indicated the arrest of meiotic division at the replication phase before meiosis commenced (Table 2). This feature suggests gametic sterility in males. There was no essential difference in reproductive performance between autotriploids with three sets of chromosomes from wild-type diploids and those with three sets of chromosomes from natural tetraploids.
Aberrant meiosis
Meiotic configurations with 25 bivalents and 25 univalents were detected in the prophase and metaphase of 90% of the spermatocytes of both induced autotriploid males with three sets of chromosomes from wild-type diploids (Zhang and Arai 1999b) and those with three sets of chromosomes from natural tetraploids (Zhang et al. 2002) (Table 2). Although trivalents were previously predicted as the major meiotic configuration in induced autotriploid fishes (Piferrer et al. 2009), no such meiosis was observed in autotriploid dojo loach. However, 75 bivalents probably from duplicated germ cells were occasionally detected in both types of autotriploids (Zhang and Arai 1999b; Zhang et al. 2002), suggesting a very low possibility of producing unreduced triploid gametes.
In the spermatocytes of autotriploids, equal segregation of bivalents and random segregation of univalents are predicted to provide a theoretical formation of aneuploid spermatozoa with a mode of 1.5n. However, a shift towards the lower chromosome number was observed and the actual mode was detected at 1.3n among spermatozoa of autotriploids (Zhang and Arai 1999b). Selective production of more hyper-haploid (1.1–1.5n) spermatozoa than hypo-diploid (1.6–1.9n) ones was observed (Zhang and Arai 1999b; Arai and Inamori 1999; Zhang et al. 2002).
Classic cytogenetic microscopy of pachytene-stage oocytes in autotriploids artificially induced from wild-type diploids revealed the presence of 25 densely stained thick and 25 lightly stained thin elements, which were likely to be bivalents and univalents, respectively (Zhang and Arai 1999b) (Table 2). In autotriploid females, 25 bivalents + 25 univalents were also observed in the oocytes of autotriploids with three sets of chromosomes from natural tetraploids (Zhang et al. 2002) (Table 2). Meiotic chromosomes were reported to become vague and then uncountable in the late pachytene stage (Zhang and Arai 1999b). The meiotic chromosomes became invisible and autotriploid oocytes underwent a kind of degeneration; such an aberrant event after synapsis would lead to the arrest of oogenesis and ovarian development. Although the sterility of autotriploids was essentially caused by impaired synapsis among the three homologous chromosomes, the reason why the same meiotic configurations in both oocytes and spermatocytes resulted in different gonadal development between females (degenerated oocytes and ovary) and males (aneuploid spermatozoa) has not been elucidated so far.
Allotriploidy
Inter-specific allotriploids
Allotriploid (triploid hybrid) males from female dojo loach and male mud loach showed three different types of spermatozoa (Fujimoto et al. 2008): type 1, exclusively non-motile and infertile hexaploid spermatozoa; type 2, the simultaneous presence of a small quantity of haploid and a large quantity of hexaploid spermatozoa; and type 3, only fertile haploid spermatozoa (Table 1). Only allotriploid males with type 3 spermatozoa produced viable progeny on backcrossing with female dojo loach because the concentration and motility of those spermatozoa were sufficient. Although sterility was suggested for such allotriploids by histological observation in previous studies (Park et al. 2006; Zhao et al. 2016), some allotriploid males apparently showed fertility (Fujimoto et al. 2008).
Surprisingly, microsatellite genotyping of backcrossed progeny revealed that such type 3 spermatozoa had only maternally derived dojo loach alleles (Fujimoto et al. 2008). These genetic results strongly suggest that some allotriploids differentiated into fertile haploid spermatozoa by meiotic hybridogenesis (Fig. 2c), i.e., meiosis might occur between two homologous chromosomes derived maternally from dojo loach, while unmatched chromosomes derived paternally from mud loach are presumably eliminated before maturation. However, the germ cell stage in which the elimination of paternal chromosomes takes place has not been identified so far, and the mechanisms underlying chromosome elimination in inter-specific allotriploid loach are unknown.
Inter-ploidy allotriploids
Reciprocal inter-ploidy hybridizations were carried out between wild-type diploid and natural tetraploid dojo loach to produce allotriploids comprising one set of chromosomes from diploids and two sets of chromosomes from tetraploids (Matsubara et al. 1995; Zhang and Arai 1996) (Fig. 4c, e). When a series of these hybridizations was conducted experimentally, the genetic relationship between diploid and tetraploid as well as that between Japanese and Chinese loach had not yet been clarified. Inter-ploidy allotriploid males were sterile without any functional spermatozoa, while females laid two types of fertile eggs: large (diameter 1.4 mm) and small (diameter 1.1 mm) (Matsubara et al. 1995) (Table 2, Fig. 5). The proportions of large and small eggs differed among the individuals (Matsubara et al. 1995). Some exhibited a predominance of large eggs (100–70%), while others showed a predominance of small eggs (100–76%). When large eggs were fertilized with haploid spermatozoa of wild-type diploids and UV-irradiated spermatozoa, the progeny obtained were tetraploid and gynogenetic triploid, respectively (Matsubara et al. 1995; Zhang and Arai 1996) (Fig. 5a, c). When these progeny were subjected to inhibition of the second polar body release, ploidy status of the resultant progeny was heptaploid and hexaploid (Fig. 5b, d), respectively, except for a very small number of unpredicted polyploids (Matsubara et al. 1995; Zhang and Arai 1996). When small eggs were fertilized with haploid spermatozoa and UV-irradiated spermatozoa, the progeny were diploid and gynogenetic haploid, respectively (Matsubara et al. 1995; Zhang and Arai 1996) (Fig. 5e, g). When these progeny were subjected to inhibition of the second polar body release, the ploidy status of the resultant progeny was triploid and diploid, respectively (Matsubara et al. 1995; Zhang and Arai 1996) (Fig. 5f, h). In light of these results, most large eggs had a triploid egg nucleus and a triploid second polar body nucleus, while the small eggs had a haploid egg nucleus and a haploid polar body nucleus. Besides the triploid polar body nucleus of large triploid eggs, the possible involvement of haploid and diploid polar bodies in the production of progeny was also suggested because tetraploid and pentaploid progeny arose occasionally after the inhibition of the second polar body release in the induced gynogenesis of large triploid eggs (Matsubara et al. 1995). In gynogenetic allotriploid progeny induced from eggs of inter-ploidy allotriploid females (Fig. 5c), the infrequent occurrence of aneuploid eggs (diameter 1.3–1.4 mm) was observed along with large and small eggs (Momotani et al. 2002) (Table 2).
Progeny of unreduced triploid (3n) large (L) and reduced haploid (1n) small (S) eggs laid by allotriploids produced by reciprocal cross-breeding between wild-type diploid and autotetraploid dojo loach. Mature eggs at the metaphase of second meiosis can accept sperm. Blue chromosomes are from autotetraploids, while red chromosomes are from wild-type diploids. a Allotetraploid induction by the fertilization of unreduced 3n L-eggs with 1n sperm of wild-type diploids (3n L-egg × 2n). b Alloheptaploid induction by the inhibition of second polar body (2 PB) release (PBI) after fertilization of unreduced 3n L-eggs with 1n sperm of wild-type diploids (3n L-egg × 2n/PBI). c Gynogenetic allotriploid induction by the fertilization of unreduced 3n L-eggs with UV-irradiated sperm (3n L-egg × UV). d Gynogenetic allohexaploid induction by PBI after the fertilization of unreduced 3n L-eggs with UV-irradiated sperm (3n L-egg × UV/PBI). e Diploid (allodiploid) induction by the fertilization of reduced 1n S-eggs with 1n sperm of wild-type diploids (1n S-egg × 2n). f Triploid (allotriploid) induction by PBI after the fertilization of reduced 1n S-eggs with 1n sperm of wild-type diploids (1n S-egg × 2n/PBI). g Gynogenetic haploid induction by the fertilization of reduced 1n S-eggs with UV-irradiated sperm (1n S-egg × UV). h Gynogenetic diploid induction by PBI after the fertilization of reduced 1n S-eggs with UV-irradiated sperm (1n S-egg × UV/PBI). 2 PB second polar body, UV genetic inactivation of gametic nucleus by UV irradiation, L-egg large-size egg, S-egg small-size egg, open arrow timing of inhibition of the second polar body release (PBI)
Genetics of inter-ploidy allotriploids
Genetic characteristics were analyzed in gynogenetic allotriploid progeny induced from large eggs of triploid females by fertilization with UV-irradiated genetically inert sperm and in gynogenetic allohexaploid progeny induced by inhibiting the second polar body release after the induction of gynogenetic allotriploids (Arai and Mukaino 1997) (Fig. 5c, d). Multi-locus DNA fingerprinting with minisatellite 33.15 and 33.6 probes revealed that all the gynogenetic allohexaploids examined showed uniform genotypes identical to those of the allotriploid mother (Arai and Mukaino 1997). On the contrary, 16 out of the 22 gynogenetic allotriploids examined showed genotypes isogenic to their allotriploid mother, proving their clonal nature (Arai and Mukaino 1997). The other gynogenetic allotriploids showed very similar (96.2–99.2% similarity in band sharing indices) but aclonal genotypes without a few fragments (Arai and Mukaino 1997). These results strongly suggested that allotriploid females from inter-ploidy diploid × tetraploid hybridization produced fertile unreduced large-size eggs which were genetically identical or highly similar to the somatic cells of the mother.
Diploid progeny were produced by fertilizing haploid small eggs of allotriploid females (diploid × tetraploid) with haploid sperm of wild-type diploid dojo loach (Arai and Mukaino 1998) (Fig. 5e). Gynogenetic haploids appeared when small eggs were fertilized with UV-irradiated sperm (Fig. 5g). When the second polar body release was inhibited in these crosses, triploids and gynogenetic diploids appeared (Fig. 5f, h). Different electrophoretic genotypes were detected in two allozyme loci between diploid and tetraploid dojo loach (Arai and Mukaino 1998). Allozyme genotypes analyzed in the diploid progeny of allotriploids suggested preferential pairing between the two homologous chromosomes derived from the tetraploid parent because of their higher affinity during meiosis (Arai and Mukaino 1998). Thus, the genetic results strongly suggested an elimination of non- or low-homologous chromosomes derived from the diploid parent and regular meiosis between two homologous chromosomes derived from the tetraploid parent, i.e., meiotic hybridogenesis (Fig. 2c).
Cytogenetic mechanisms for triploid and haploid egg formation
Cytogenetic observations of immature ovaries of inter-ploidy allotriploids from crosses between wild-type diploids and natural tetraploids revealed a high percentage of hexaploid metaphases with 150 chromosomes (Zhang et al. 1998). The results were strongly suggestive of the occurrence of chromosome doubling in the oogonial stages and that an elevation of ploidy from triploid to hexaploid should cause the development of large oocytes (Zhang et al. 1998). An increase in cell size due to the elevation of ploidy is a well-known phenomenon in polyploid organisms (Arai 2000, 2001; Piferrer et al. 2009; Arai and Fujimoto 2019).
The oocytes at the pachytene stage in diploid wild-type controls (2n = 50) showed a meiotic configuration with 25 bivalents (Zhang et al. 1998, 2002; Zhang and Arai 1999b) (Table 2). The small oocytes of allotriploids (3n = 75) showed 25 thick, densely stained elements and 25 thin, faintly stained elements, which seemed to be 25 bivalents and 25 univalents, respectively (Zhang et al. 1998) (Table 2). Thus, the haploid eggs are likely formed by the segregation of the 25 bivalents, with no involvement of the 25 univalents. The majority of the large oocytes were observed to have no fewer than 60 thick elements and an estimated 75 bivalents that were theoretically paired from the 150 chromosomes transmitted from the hexaploid oogonia (Zhang et al. 1998) (Table 2). Although there was some uncertainty about the meiotic chromosome count due to the technical limitations of classic cytogenetic observations, the outline of the meiotic configurations in both small and large oocytes was observed. In the near future, the results may be reconfirmed by using new techniques such as immunochemical staining with an antibody to synaptonemal complexes (Araya-Jaime et al. 2015; Dedukh et al. 2020a, b, 2022; Tichopád et al. 2022).
Cytological observations revealed that both small and large oocytes showed normal bipolar spindle formation in the metaphase of first meiosis (MI) and then entered into the anaphase of MI to exclude the first polar body normally (Zhang et al. 1998). After this event, a normal bipolar spindle was formed at the metaphase of second meiosis (MII) (Zhang et al. 1998). These cytogenetic and cytological observations indicate that both small and large oocytes of allotriploid females undergo the same meiotic processes as seen in the normal diploids (Fig. 6a).
Mechanisms for atypical reproductive modes in the dojo loach. Genetic differences between parental fish are shown by different colors of chromosomes (dark blue, light blue, white). Dark blue and light blue chromosomes are closely related homologous chromosomes, while white chromosomes are non-homologous chromosomes. a Reduced haploid egg formation in wild-type diploids by normal meiosis: homologous chromosomes replicate, synapse, and recombine, and reduced haploid eggs are formed after two regular meiotic divisions. b Clonal triploid egg formation by premeiotic endomitosis in allotriploids: a triploid oogonium becomes a hexaploid oogonium by duplicating each chromosome at a certain stage before entering meiosis. Two consecutive meiotic divisions produce triploid clonal eggs after replication, synapsis between sister chromosomes, and recombination. Recombination does not generate genetic variation because of the exchange of identical elements. c Aclonal triploid egg formation by premeiotic endomitosis in allotriploids: a triploid oogonium becomes a hexaploid oogonium by duplicating each chromosome at a certain stage before entering meiosis. After replication, synapsis occasionally happens between a few homologous (dark blue and light blue) chromosome pairs to generate genetic variation. Two consecutive meiotic divisions produce aclonal (but very similar) triploid eggs after this partial recombination. d Reduced haploid egg formation by meiotic hybridogenesis in allotriploids: a non-homologous chromosome set is eliminated and two sets of homologous chromosomes enter regular meiosis to form reduced (genetically variant) haploid eggs. e Clonal diploid egg formation by premeiotic endomitosis in clonal diploids: a diploid oogonium with heterozygosity between non-homologous chromosomes becomes a tetraploid oogonium by duplicating each chromosome. The resultant sister chromosomes behave as homologous chromosomes, undergoing replication, synapsis between sister chromosomes, and recombination. Two consecutive meiotic divisions produce diploid clonal eggs. Recombination does not generate genetic variation because of the exchange between identical elements. This system also occurs in sex-reversed clonal males to produce clonal diploid sperm. f Clonal diploid egg formation by meiosis in clonal tetraploids: no duplication of chromosomes occurs; the tetraploid oocyte enters the regular meiotic process and homologous (identical) chromosomes undergo replication, synapsis, and recombination. Recombination does not generate genetic variation because of the exchange between identical elements. This system also occurs in sex-reversed clonal males to produce clonal diploid sperm. MI first meiosis, MII second meiosis, 1 PB first polar body, 2 PB second polar body
The doubled bivalents and the presence of two successive divisions in the meiotic process, as observed in the large-sized oocytes, indicate that large-sized triploid eggs are formed by premeiotic endomitosis wherein chromosome numbers of germ cells get duplicated before entering meiosis (Zhang et al. 1998). Thus, unreduced triploid eggs of a large size are likely produced by normal meiosis of hexaploid oocytes. All the chromosomes of triploid germ cells are doubled before entering meiosis in the course of oogonial proliferation, and then preferential pairing between sister chromosomes duplicated from each chromosome occurs to give rise to 75 bivalents in the hexaploid oocytes (Zhang et al. 1998) (Fig. 6b). Pairing between sister replicates is essential in this system (Fig. 6b), but the possibility of synapsis between non-sister homologous chromosomes from tetraploid parents cannot be excluded (Fig. 6c) because of the presence of a small number of aclonal triploid eggs with a slight variation in DNA fingerprints (Arai and Mukaino 1997).
Among the other cytogenetic variations, two poles were occasionally detected at one side or both sides of the bipolar spindle of MII (Zhang et al. 1998). Such a formation likely induces unusual segregation of chromosomes, as seen in the following example: when the second polar body release was inhibited after gynogenetic induction of unreduced triploid eggs, a majority of the progeny were gynogenetic hexaploids, but a small number of gynogenetic pentaploid (triploid egg nucleus + diploid polar body nucleus) and tetraploid (triploid egg nucleus + haploid polar body nucleus) progeny also appeared, presumably due to the partial success of MII inhibition (Matsubara et al. 1995).
Small haploid eggs may differentiate from triploid oogonia without premeiotic chromosome doubling (Fig. 6d). In small oocytes, 25 bivalents and 25 univalents were observed (Zhang et al. 1998) (Table 2). Such a bivalent-univalent complex often gives rise to the occurrence of aneuploid gametes because bivalents segregate equally while univalents segregate randomly. In autotriploid dojo loach (Zhang and Arai 1999b; Zhang et al. 2002) and other autotriploid fish (Thorgaard and Gall 1979), such a configuration apparently caused the occurrence of aneuploid spermatozoa, as described in the previous section. Cytological examination of small oocytes showed that they underwent two successive meiotic divisions, just as normal control diploids do (Zhang et al. 1998) (Fig. 6d). Thus, only bivalents should be involved in meiotic events, and univalents might be eliminated before final maturation (Zhang et al. 1998) (Fig. 6d). As discussed in a later section on clonal biotypes, germinal vesicles of clone-derived allotriploids, which also lay haploid eggs, exhibited 25 bivalents, and no residual univalents were identified in the oocyte nucleus (Morishima et al. 2008c). In the small oocytes of allotriploid females, chromosome-like bodies, presumably detached from the equatorial plate, were found in the cytoplasm around the bipolar spindle of MI (Zhang et al. 1998). These elements were no longer visible in the metaphase of the second meiotic division (Zhang et al. 1998). However, conclusive evidence of the timing and the germ cell stage of the preferential elimination of chromosomes derived exclusively from one parent before final maturation has not been obtained yet. In relation to examples of other hybridogenetic animals, two different theories were provided: (1) chromosomal elimination by the formation of a unipolar spindle just before the beginning of meiosis (Cimino 1972), and (2) gradual elimination during the gonial proliferation period (Turner and Heppich 1981; Heppich et al. 1982; Turner and Heppich-Turner 1991; Chmielewska et al. 2018; Dedukh et al. 2020b). Considering genetic results based on allozyme genotypes (Arai and Mukaino 1998) and the cytological results summarized above (Zhang et al. 1998), meiotic hybridogenesis (Fig. 2c) is involved in the formation of a small haploid egg of allotriploid dojo loach. Homologous chromosomes from a tetraploid parent likely pair to form bivalents due to their high affinity, and then these bivalents produce haploid eggs by regular meiosis (Fig. 6d).
Aneuploid gametogenesis in inter-ploidy allotriploids in China
In the Chang Jiang River system, tetraploid dojo loach with 100 chromosomes co-exist with gonochoristic diploid wild types (2n = 50) and low frequencies of natural triploids (3n = 75) (Li et al. 2008, 2010, 2012, 2017). The loach with 100 chromosomes are not examples of diploids devolved from tetraploid ancestors but genetically true tetraploids with four sets of homologous chromosomes (4n = 100) because viable gynogenetic progeny can be produced from their eggs on fertilization with UV-irradiated sperm (Li et al. 2013) (Fig. 4g). When reproductive capacity was examined in these Chinese polyploids (Li et al. 2012), tetraploid males exhibited fertile performance in terms of concentration and motility of sperm, while tetraploid females laid fertile diploid eggs with sizes larger than the haploid eggs of the diploid wild type (Table 1). Examples of natural triploid females laid fertile haploid eggs (Li et al. 2012), and this reproduction is similar to the case of small eggs in other inter-ploidy allotriploids between Japanese wild-type diploids and unknown-origin (putative Chinese) natural tetraploids, as described in the previous section (Fig. 5e–h). The allotriploid males generated aneuploid spermatozoa (1.2–2.2n) (Li et al. 2012), like in autotriploid males and another type of inter-ploidy allotriploid described in the next paragraph.
Inter-ploidy hybridization was performed in China between diploids from Liaoning Province and tetraploids from Hubei Province to produce viable triploid progeny. Population genetic studies on Chinese dojo loach and related species have not been well conducted. However, natural tetraploids in Hubei belong to the group B2 (Morishima et al. 2008a; Li et al. 2017), while diploids in Liaoning belong to the clade close to group A (Li et al. 2017). The major meiotic configuration of these inter-ploidy allotriploids is composed of 25 bivalents and 25 univalents in both germinal vesicles and spermatocytes, but other configurations comprising several trivalents and more univalents are also seen (Li et al. 2015) (Table 2). The identification of three homologous chromosomes bearing NOR with FISH using an rDNA probe and differential staining with silver nitrates and chromomycin A3 revealed that two-thirds of the triploid meiotic cells had one bivalent with two NORs and one univalent with one NOR (Li et al. 2015). The other one-third of the meiotic cells exhibited three univalents, each of which had one NOR, showing a failure of homologous chromosomes to pair (Li et al. 2015).
Inter-ploidy allotriploids produced fertile gametes in both females and males (Li et al. 2016). The majority of the males exhibited spermatozoa with around 1.5–1.6n, but the others showed triploid and hexaploid cell populations in sperm, suggesting the arrest of spermatogenesis at the replication phase before meiotic division was commenced (Li et al. 2016). When allotriploids from diploids and tetraploids were backcrossed with wild-type diploids (triploid female × diploid male, diploid female × triploid male) and inter-crossed (triploid female × triploid male), progeny from these crosses were inviable and died in embryonic and/or larval stages (Li et al. 2016). In diploid female × triploid male crosses, embryos had a mean chromosome number of 61.6 (2.4n), while triploid female × diploid male progeny showed a mean chromosome number of 61.1 (2.4n) (Li et al. 2016). In triploid female × triploid male crosses, the mean chromosome number was 73.0 (2.9n) (Li et al. 2016). In other sets of crosses, diploid female × triploid male and triploid female × diploid male crosses gave mean chromosome numbers of 58.8 (2.4n) and 67.2 (2.7n), respectively, while triploid female × triploid male crosses showed 73.5 (2.9n) chromosomes (Li et al. 2016). These results indicated the production of aneuploid gametes with a mode of around 1.5n in both male and female inter-ploidy allotriploids in China (Table 2). These aneuploid eggs and spermatozoa are considered to be generated by the equal segregation of 25 bivalents and the random segregation of 25 univalents, as predicted by the most frequent meiotic configuration in both oocytes and spermatocytes.
Allotriploids between Chinese diploids (Liaoning Province) and Chinese tetraploids (Hubei Province) did not produce any unreduced or hybridogenetic eggs. They produced aneuploid eggs and sperm because they formed bivalent-univalent meiotic configurations in both oocytes and spermatocytes (Li et al. 2016) (Table 2). This meiotic event was similar in both autotriploid sexes, although autotriploid females often exhibited complete sterility. Distant inter-ploidy hybridization between Japanese diploids and Chinese tetraploids resulted in atypical reproduction such as unreduced and/or hybridogenetic oogenesis and sterile spermatogenesis (Matsubara et al. 1995; Zhang et al. 1998) (Tables 1, 2) (Fig. 5). These results suggested that hybridization between more remotely related parents might have resulted in atypical reproduction such as unreduced egg formation and meiotic hybridogenesis (Fig. 2bc).
Allopentaploidy
When eggs of natural autotetraploid females were fertilized with sperm of wild-type diploid males and the second polar body release was inhibited, allopentaploid progeny (5n = 125) comprising four sets of chromosomes derived from autotetraploids (of putative Chinese origin) and one set of chromosomes derived from wild-type diploids (of Japanese origin) were successfully produced (Fig. 4d). These allopentaploids were viable, and mature females produced diploid eggs with diameters larger (1.2 mm) than those of the wild-type diploids (1.1 mm) but smaller than those of the large triploid eggs (1.4 mm) laid by inter-ploidy allotriploid females (Matsubara et al. 1995). Although the meiotic process has not been cytogenetically examined in these allopentaploids so far, pairing may have arisen between the four homologous chromosomes derived from the autotetraploids after the probable elimination of unmatched non-homologous chromosomes derived from wild-type diploids. This reproductive mode seems to be similar to meiotic hybridogenesis (Fig. 2c), which has been observed in the course of small haploid egg formation in inter-ploidy allotriploid females (Zhang et al. 1998) (Fig. 6d). Actually, cross-breeding between a female allopentaploid and a male diploid produced viable triploid progeny (Matsubara et al. 1995; Zhang and Arai 1996). In contrast, allopentaploid males were reported to produce 2.3n spermatozoa (Zhang and Arai 1996) (Table 2). Although the meiotic process is not known, the simultaneous formation of 50 bivalents, i.e., 100 chromosomes derived from autotetraploids, and 25 univalents derived from wild-type diploids in pentaploid 5n = 125 chromosomes show the presumable involvement of aneuploid spermatogenesis by the equal segregation of bivalents and the random segregation of univalents. An experimental cross using sperms of allopentaploid males has not been performed, and thus the developmental capacity of its progeny is unknown at present. As mentioned above, allopentaploid females laid diploid eggs, whereas the males showed aneuploid sperm. Therefore, the same allopentaploid combination of chromosome sets did not give the same results for both sexes.
Unusual hyper-triploidy and hyper-tetraploidy
Flow cytometry analyses of about 450 live loach samples taken from the central wholesale market in Tokyo revealed the remarkable occurrence of polyploid specimens with the following DNA content range: triploid (11.5%), tetraploid (6.7%), pentaploid (0.2%), hexaploid (0.2%), and heptaploid (0.2%) (Zhao et al. 2012a). Among them, hyper-triploids and hyper-tetraploids were also evident flow cytometrically (Zhao et al. 2012a).
In the sperm of hyper-triploid males, haploid-, triploid-, and hexaploid-range cell populations were evident, but the hexaploid-range cell population was dominant (72%), suggesting arrest at the replication phase before meiotic division was commenced (Zhao et al. 2014). Hexaploid-range sperm cells had larger-sized heads (length 3.45 μm, width 3.42 μm) than those of the haploid spermatozoa of the control (length 1.82 μm, width 1.81 μm), but their flagellar length (17.4 μm) was shorter than that of the control (24.2 μm), as shown in Table 1. Aflagellate and biflagellate sperm cells were also observed. All these sperm cells showed extremely low motility despite their higher ATP content and total mitochondrial mass (Zhao et al. 2014). The number of mitochondria was similar to that seen in the control (Table 1). These high-energy-related factors could not compensate for the low motility of the unusual sperm cells with bigger-sized heads and a shorter flagellum.
Hyper-tetraploids produced hyper-diploid spermatozoa with vigorous motility (Zhao et al. 2014) (Table 1). Hyper-diploid spermatozoa had larger-sized heads (length 2.24 μm, width 2.23 μm) as well as longer flagella (30.6 μm). ATP content and number of mitochondria were higher than those of the control and similar to those observed in diploid spermatozoa of natural tetraploids and neo-tetraploids (Table 1). When eggs of wild-type diploids were fertilized with hyper-diploid spermatozoa, triploid-range larvae with a normal appearance were produced (Zhao et al. 2012a). When genetically inert eggs irradiated with UV were fertilized with hyper-diploid spermatozoa, no viable androgenetic larvae were produced (Zhao et al. 2012a). The resultant hyper-diploid androgenetic progeny had 2n = 54, which clearly indicated the presence of supernumerary micro-chromosomes (Zhao et al. 2012a).
Natural clones
Clonal lineage and its hybrid origin
Tetraploid dojo loach have not been discovered in Japanese wild populations, despite thorough screening with flow cytometry (Zhang and Arai 1999a; Arai 2003; Arai and Fujimoto 2013). However, the east part of Hokkaido is one of the hot spots for high frequencies of triploids (Morishima et al. 2002). In this case, the involvement of certain diploid individuals which may produce unreduced eggs is predicted, as in the case reported in Hirokami, Niigata Prefecture (Zhang and Arai 1999a). The eggs of mature dojo loach collected from the east part of Hokkaido were used for three different experimental setups: they were fertilized with the sperm of wild-type diploids, hybridized with hetero-specific goldfish sperm, and gynogenetically activated with UV-irradiated sperm. As a result, some of the females in all three experiments produced viable diploid dojo larvae with a normal appearance (Morishima et al. 2002). They were verified to be genetically identical to the somatic cells of the mother by microsatellite genotyping and multi-locus DNA fingerprinting (Morishima et al. 2002). Cytological observations revealed that the sperm intruding the clonal eggs failed to decondense and the male pronucleus was not formed for subsequent syngamy (Itono et al. 2007). The condensed sperm nucleus could not contribute to the zygotic nucleus and disappeared in the course of cleavage (Itono et al. 2007). The egg nucleus became the female pronucleus by decondensation, and it alone acted as the zygotic nucleus (Itono et al. 2007). These results indicated the presence of clonal dojo loach which laid isogenic diploid eggs that developed by sperm-dependent parthenogenesis, i.e., gynogenesis. Such clonal lineages were also found in Notojima Island, Ishikawa Prefecture (Morishima et al. 2008a). In the cross-fertilization experiment with wild-type diploids, certain percentages of triploids were also found (Morishima et al. 2002; Itono et al. 2007). Microsatellite genotyping and DNA fingerprinting revealed that the resultant triploids had both diploid clonal alleles and haploid wild-type alleles (Morishima et al. 2002; Itono et al. 2007). Cytological observations also showed delayed decondensation of the sperm nucleus and subsequent syngamy with the blastomere of a clonal diploid embryo (Itono et al. 2007). These results explain the occurrence of the relatively high frequencies of triploids: accidental incorporation of the sperm nucleus into the blastomere of a clonal diploid embryo.
A total of four clonal lineages (families) with high genetic similarities have been detected in the Hokkaido and Ishikawa prefectures (Morishima et al. 2002, 2008a). Previous genetic studies using allozymes (Khan and Arai 2000), microsatellites (Arias-Rodriguez et al. 2007), and mitochondrial DNA (Morishima et al. 2008a; Shibata et al. 2020) disclosed the presence of genetically divergent groups, A and B, in dojo loach. More precise recent studies further revealed the heterozygosity of the clonal loach because the clone had both group-A-specific and group-B-specific sequences of recombination activating gene 1 (RAG1) and interphotoreceptor retinoid binding protein 2 (IRBP2) (Yamada et al. 2015). The hybrid status of the clonal loach was also verified by the presence of both group-A-specific and group-B-specific repetitive sequences (Fujimoto et al. 2017). Molecular cytogenetic analyses using FISH with group-A-specific ManDra-A and group-B-specific ManDra-B probes indicated that in the clonal dojo loach, one set of chromosomes was derived from group A and the other from group B (Kuroda et al. 2018, 2021b). These results led to the conclusion that clonal dojo loach should have arisen from a past hybridization event between ancestors of group A and those of group B. Based on the mitochondrial DNA haplotype, the group A ancestor was taken to be a female (Morishima et al. 2008a). Recent FISH studies showed further structural differentiation in chromosomes between ancestral and contemporary group A dojo loach (Kuroda et al. 2021b). A newly described Misgurnus species, M. chipisaniensis, was reported to have mtDNAcytb, Rag1, and IRBP2 sequences belonging to the same phylogenic clade as the group A dojo loach (Shedko and Vasil’eva 2022). Although the precise taxonomical relationship between the M. chipisaniensis in Sakhalin and the group A in Hokkaido and Honshu has not yet been determined, both loaches are similar. This also suggested the presence of a clonal loach in the southern part of Sakhalin Island because the mtDNA haplotype specific to the clone was detected (Shedko and Vasil’eva 2022).
Sex-reversed clonal males
For most unisexual fishes which reproduce gynogenetically, males are either absent or sterile (Arai and Fujimoto 2013). In the east area of Hokkaido, where clonal lineages appear, clone-origin triploids were formed. The triploid females were fertile but reproduced by meiotic hybridogenesis (Fig. 2c), while the triploid males were sterile (Oshima et al. 2005; Morishima et al. 2008c) (Table 2). Sympatrically, the diploid-triploid mosaic dojo loach was found at a very low rate (Morishima et al. 2004). In this mosaic, the diploid cell populations had a clonal genotype while the triploid cell populations had clonal diploid sets of chromosomes and a sperm-derived haploid set of chromosomes (Morishima et al. 2004). After the triggering of gynogenetic development in a clonal egg, delayed syngamy might have occurred between the re-decondensed sperm nucleus and a somatic cell nucleus in the blastomere of the clonal embryo to produce the triploid cell lineage. Remarkably, diploid-triploid mosaic males produced functional diploid spermatozoa with a clonal genotype (Morishima et al. 2004). However, the concentration and motility of the diploid spermatozoa were very low, and their head sizes (length 2.48 μm, width 2.38 μm) were larger than those of haploid spermatozoa of the control diploids (length 1.87 μm, width 1.70 μm), as shown in Table 1. Thus, the low hatching rates of eggs fertilized with such diploid spermatozoa can be explained by the failure to achieve fertilization due to the low spermatozoa concentration and motility. Large heads of diploid spermatozoa generally make it difficult for them to enter the eggs through the micropyle (Morishima et al. 2004).
Since male heterogametic sex determination (XX female, XY male) is predicted from the exclusive occurrence of females in induced gynogenetic diploids in dojo loach (Suzuki et al. 1985b), clonal diploids are essentially all female because the Y chromosome is absent. In the mosaics, triploid germ cells with the Y chromosome produce sterile testes, which induce the spermatogenesis of germ cells with the clonal diploid genotype because the undifferentiated germ cells have bipotency in sex differentiation according to the gonadal environment, as reported in germ-line chimerism. In several teleosts, germ cells taken from genetically female donors could differentiate into male gametes under chimeric germ-line conditions (Shinomiya et al. 2002; Yamaha et al. 2003; Yoshizaki et al. 2012), which are equivalent to the mosaic conditions.
All-female clonal loach were sex reversed to physiological males by 17-α-methyltestosterone treatment (Yoshikawa et al. 2007). These sex-reversed clonal males produced fertile diploid spermatozoa isogenic to their father’s clonal lineage (Yoshikawa et al. 2007), as in the diploid-triploid clone-origin mosaic (Morishima et al. 2004). The characteristics of the diploid spermatozoa of the sex-reversed clonal loach were significantly different from those of the haploid spermatozoa of wild-type diploids (Zhao et al. 2012b) but were similar to those of the diploid spermatozoa of diploid-triploid mosaics (Morishima et al. 2004). As seen in Table 1, diploid spermatozoa of sex-reversed clonal males showed a very low concentration and motility (less than 5%) and had larger-sized sperm heads (length 2.25 μm, width 2.25 μm) and longer flagella (30.75 μm) than those of the haploid spermatozoa of the control loach (length 1.82 μm, width 1.81 μm, flagellum 23.85 μm). In spite of the large-sized sperm head, the number of mitochondria per spermatozoon was not different from that in the control, while the ATP content was lower than that of fertile diploid spermatozoa of autotetraploids and neo-tetraploids. These differences in energy-related parameters may explain the low motility of the diploid spermatozoa of the clonal males. Sex-reversed clonal males were not sterile like males of other unisexual fishes (Janko et al. 2018; Dedukh et al. 2020a), but their spermatozoa exhibited a low potential for fertility due to their low concentration, low motility, and large head size.
Mechanisms for clonal reproduction
When the mature oocytes of the clonal biotypes were isolated from the ovary and incubated in vitro with 17-α-20-β-dihydroxy-4-pregnene-3-one to induce final maturation, cytological microscopy showed bipolar spindle formation at the metaphase of the first meiosis, the subsequent release of the first polar body, and the formation of the bipolar spindle at the metaphase of the second meiosis before ovulation (Itono et al. 2006). These observations confirmed the presence of two consecutive meiotic divisions in the clonal dojo loach, as seen in the final maturation of the wild-type diploid dojo loach. The result indicated that apomixis was not involved in unreduced egg formation in clonal dojo loach because of the presence of the first polar body release. Apomixis is a mechanism responsible for the abortion of the first meiotic division, resulting in the production of clonal eggs only by the second meiotic division; a mechanism identified as a reproductive system in crucian carp (Yamashita et al. 1993) and Amazon molly (Monaco et al. 1984; Dedukh et al. 2022). Germinal vesicles isolated from fully grown oocytes of wild-type diploids exhibited 25 bivalents formed by pairing among 2n = 50 chromosomes, while the germinal vesicles of the clones showed 50 bivalents, clearly indicating the presence of twice the number of bivalents formed by the pairing of 100 chromosomes before the first meiotic division (Itono et al. 2006) (Table 2). These observations indicated that all the chromosomes of the germ cells were duplicated from 2n = 50 to 4n = 100 by endomitosis without cytokinesis before entering meiosis. The presence of twice the number of bivalents in the germinal vesicles and the evidence for two consecutive meiotic divisions conclusively proved the involvement of premeiotic endomitosis in unreduced oogenesis (Fig. 6e).
The spermatocytes of sex-reversed clonal males also revealed twice the number of bivalents, indicating the involvement of the same premeiotic endomitosis in unreduced spermatogenesis (Yoshikawa et al. 2009). Further FISH analyses using the group-specific sequences ManDra-A and -B and chromosome-specific rDNA as probes in spermatocytes showed that pairing between sister chromosomes duplicated from every single chromosome derived from each ancestral group assured the existence of isogenic clonal gametes, as recombination or crossing over did not generate genetic variations due to the exchange of identical elements between sister chromosomes (Kuroda et al. 2018, 2021b). Pairing between sister chromosomes in diplotene chromosomes from germinal vesicles of the clonal females was also confirmed (Kuroda et al. 2018). Spontaneous cell fusion of adjacent germ cells was proposed as an alternative mechanism in unisexual crucian carp (Wang et al. 2016). However, no histological image showing cell fusion has been obtained in the case of clonal dojo loach.
Classic microscopic studies carried out to ascertain the endomitotic stage in the formation of unreduced eggs in artificial hybrid fish suggest that the chromosome doubling time is just before the germ cells enter meiosis or one or few divisions before meiosis (Hamaguchi and Sakaizumi 1992; Shimizu et al. 2000). Dedukh et al. (2021) observed unreduced oogenesis, using immunolabeling with antibodies against the lateral and central component of the synaptonemal complexes as well as FISH analyses with chromosome-specific probes, in clonally reproduced Cobitis diploid and triploid hybrids, and supported the above-mentioned results. They concluded that duplication occurred in only a minor fraction of clusters (“nests”) of oogonial cells and that the vast majority of oogonial cells were unable to duplicate their chromosomes. Consequently, only oogonial cells with duplicated chromosomes could enter meiotic divisions by forming bivalents between sister chromosomes, while the other oogonial cells could not proceed beyond the pachytene stage due to the “pachytene checkpoint” barrier (Dedukh et al. 2021).
However, a different timing of chromosome doubling was shown in sex-reversed clonal dojo loach (Yoshikawa et al. 2009). In the testes of clonal males, germ cells with larger nuclei were observed histologically in type B spermatogonia (Yoshikawa et al. 2009). In contrast, among early germ cells with sizes similar to those of wild-type diploid males, larger type A spermatogonia were also histologically detected (Yoshikawa et al. 2009). By performing flow cytometrical analyses for DNA content in isolated and then separated germ cells of clonal males, tetraploidy with 4C DNA content, i.e., chromosome doubling, was exclusively verified in type A and early type B spermatogonia, which were immunohistochemically identified by antispermatogonia-specific antigen 1 (Yoshikawa et al. 2009). These results indicate conclusively that chromosome doubling arises at the type A spermatogonia before the beginning of active proliferation by mitosis, which is a characteristic feature of type B spermatogonia in clonal male dojo loach. Chromosome doubling in the early stage of spermatogonia for diploid spermatogenesis indicated that most clonal germ cells underwent premeiotic endomitosis to differentiate into fertile diploid spermatozoa.
In clonal diploid females of dojo loach, twice the number of bivalents could be clearly observed in the germinal vesicles, and thus endomitosis had already occurred in the oocytes (Itono et al. 2006), but the exact timing of endomitosis in unreduced oogenesis has not yet been identified. The differences in the timing and the germ-cell type of premeiotic chromosome doubling between Cobitis clonal females (Dedukh et al. 2021) and Misgurnus clonal males (Yamashita et al. 1993) is difficult to explain. These difference may indicate a species-specific or sex-specific timing of endomitosis. Further studies are required in the near future to identify the timing of premeiotic endomitosis in dojo loach clonal females, probably using new techniques such as immunolabeling of lateral and central components of the synaptonemal complexes (Araya-Jaime et al. 2015; Dedukh et al. 2020a, 2021, 2022; Tichopád et al. 2022). The complete sterility of clonal Cobitis males makes it difficult to analyze the timing of chromosome duplication in them, and hence the necessity (Dedukh et al. 2021).
Clonal tetraploids
When clonal diploid eggs were fertilized with clonal diploid sperm of sex-reversed clonal males, clonal diploid sperm triggered gynogenetic development in a large number of clonal eggs (Morishima et al. 2012). However, a certain number of diploid eggs incorporated the clonal diploid sperm nucleus to generate clonal tetraploid progeny (Morishima et al. 2012). On the contrary, when clonal diploid eggs were fertilized with UV-irradiated sperm to initiate gynogenesis, tetraploid progeny were produced as a result of inhibition of the second polar body release (Morishima et al. 2012). There was no genetic difference between diploid and tetraploid clonal loach except for the number of chromosome sets or ploidy status. Although clonal diploid loach produced unreduced diploid eggs, clonal tetraploid loach did not produce unreduced tetraploid eggs; it laid reduced diploid eggs by regular meiosis (Morishima et al. 2012). The diploid eggs laid by clonal tetraploids were isogenic and gynogenetically reproduced, similar to the unreduced eggs laid by clonal diploids (Morishima et al. 2012). Clonal tetraploid males, which appeared by sex reversal due to environmental factors (Nomura et al. 1998), apparently produced fertile isogenic diploid spermatozoa (Morishima et al. 2012). Clonal tetraploids are considered amphidiploids with two sets of chromosomes: chromosomes from group A and group B ancestors. Thus, two homologous chromosomes from the two different groups were able to form bivalents due to their high affinity, and regular meiosis starting from tetraploid germ cells proceeded in both oocytes and spermatocytes with an amphidiploid or allotetraploid constitution without the necessity to double each chromosome derived from the different groups by premeiotic endomitosis (Fig. 6f). These results suggest that unreduced gametogenesis may be controlled not by any kind of major gene(s) for the expression of premeiotic endomitosis but by the constitution of the chromosomes to ensure the occurrence of a normal meiotic process by the formation of bivalents and the two subsequent consecutive divisions. Recent germ cell transplantation experiments in a related species, Cobitis, indicated that spermatogonial stem cells from unisexual triploid hybrid males (C. elongatoides-taenia-taenia) that were transplanted into sexual diploid females (C. elongatoides) apparently differentiated into unreduced triploid eggs which underwent clonal reproduction, whereas those transplanted into sexual diploid males resulted in sterility (Tichopád et al. 2022). These results suggested that the capacity for unreduced gametogenesis should be intrinsic to the heterozygous genomic constitution of the hybrid germ cells rather than dependent on special gene(s) to initiate chromosome doubling.
Clone-origin allotriploids and meiotic hybridogenesis
In the localities where diploid clonal loach arose, natural triploids were found at relatively high rates (Morishima et al. 2002, 2008a). They were clone-origin triploids comprising two sets of chromosomes (A and B) from the clone and one set of chromosomes (A) from the wild type (Itono et al. 2007; Morishima et al. 2008c). In germinal vesicles of full-grown oocytes of clone-origin triploids, 25 bivalents—that is, 2n = 50 chromosomes—were detected (Morishima et al. 2008c) (Table 2). The majority of the progeny of clone-origin triploids were diploids after fertilization with haploid sperm of diploid wild-type males (Itono et al. 2007; Morishima et al. 2008c). This showed that clone-origin triploid females produced haploid eggs. Unreduced triploid eggs were expected at very low rates because of the presence of oocytes with 75 bivalents (Morishima et al. 2008c). Microsatellite genotyping in the diploid progeny of clone-origin triploids demonstrated not only the presence of preferential pairing between homologous chromosomes of the same genetic group, A or B, but also the elimination of unmatched chromosomes during the process of haploid oogenesis (Morishima et al. 2008c). Therefore, one chromosome set is eliminated before the resumption of meiosis in the clone-origin triploid, and the other two chromosome sets proceed to regular meiotic divisions (Fig. 6d). This reproductive mode is defined as meiotic hybridogenesis (Fig. 2c). Such a mode was also observed in inter-ploidy allotriploids from hybridization between Japanese wild-type diploids and Chinese tetraploids (Arai and Mukaino 1998; Zhang et al. 1998). Meiotic hybridogenetic reproduction is not female specific because fertile spermatozoa were occasionally produced by this mechanism in inter-specific allotriploid hybrids between dojo loach and mud loach (Fujimoto et al. 2008).
The timing and mechanism of elimination of the haploid set of chromosomes of low affinity have not yet been determined in fish. However, recent studies on the natural hybridogenetic water frog proposed that the elimination proceeded with the emergence of micronuclei as detached nuclear buds during the interphase of primary oogonia and prespermatogonia, based on electron microscopy, immunofluorescence, and cytochemistry analyses (Chmielewska et al. 2018; Dedukh et al. 2020b).
Clone-origin triploid females were fertile, as mentioned above, but males were sterile (Oshima et al. 2005; Morishima et al. 2008c). In clone-origin triploid males comprising two sets of chromosomes derived from group A and one set of chromosomes derived from group B, i.e., a genomic composition of AAB, both bivalents and univalents were present, but trivalents and other multivalents were scarce (Kuroda et al. 2019). Preferential pairing was seen between chromosomes derived from the same group, group A (an average of 17.4 bivalents), and therefore univalents of group A occurred less frequently (an average of 11.3 univalents) as compared to those of group B (an average of 21.2 univalents) (Kuroda et al. 2019). This indicates that pairing between chromosomes from the same group arose with higher frequency but that between chromosomes from the different groups was also possible (an average of 3.8 bivalents) (Kuroda et al. 2019) (Table 2). Such a configuration, comprising both bivalents and univalents, causes the occurrence of aneuploid spermatozoa because bivalents segregate regularly to each pole, whereas univalents segregate randomly, as already reported in artificially induced autotriploid males (Zhang and Arai 1999b; Zhang et al. 2002). According to Oshima et al. (2005), replication would generate and accumulate hexaploid cell populations in the testes of clone-origin triploids, but these would not enter meiotic division. An accumulation of tetraploid and hexaploid cell populations has often been detected in the testes of sterile hybrid males and triploid males, respectively. Such an aberrant accumulation of the replicated cell population indicates a failure to complete meiotic division, and thus no or very few functional spermatozoa are produced. Most sperm cells are abnormal as well as non-motile. However, the characteristics and energy-related parameters of sperm cells have not yet been examined in clone-origin triploid males.
Discussion
Meiotic configurations and reproductive modes
In this review, the author aimed to organize the previous experimental results obtained using various biotypes of the dojo loach to better understand the mechanisms underlying the changes in numbers and combinations of homologous and/or non-homologous chromosome sets that affect gametogenesis and reproduction. Polyploids with even numbers of homologous chromosome sets are generally fertile, as seen in autotetraploids and autohexaploids (Arai et al. 1999; Li et al. 2011), because of the ability of homologous chromosomes to form bivalents or relatively stable multivalents, such as quadrivalents, which can segregate in meiotic divisions. As a matter of fact, several quadrivalents and many bivalents have always been observed in both oocytes and spermatocytes in meiosis in fertile natural autotetraploids. In contrast, polyploids with an odd number of homologous chromosome sets, for example autotriploids, showed aberrant meiotic configurations, such as a bivalents-univalents complex (Zhang and Arai 1999b; Zhang et al. 2002). In males, aneuploid sperms are formed. In females, bivalent-univalent configurations presumably give rise to the degeneration of oocytes. The reason why the same meiotic configurations in both oocytes and spermatocytes end in different results in females and males has not been discovered yet.
Similarly, aberrant meiosis brought on by the formation of a bivalent-univalent configuration was also recognized in allotriploids between less remotely related diploids and tetraploids, both from China (Li et al. 2015, 2016). In such inter-ploidy allotriploids, aneuploid eggs and sperm were produced by aberrant meiosis due to the presence of univalents. In contrast, allotriploids between remotely related Japanese diploids and exotic tetraploids exhibited atypical reproduction. Allotriploid females simultaneously generated triploid eggs with isogenic or near-isogenic genotypes as well as haploid eggs with recombinant genotypes (Matsubara et al. 1995; Arai and Mukaino 1997, 1998; Zhang et al. 1998). While the former unreduced eggs were found to be produced by the mechanism of premeiotic endomitosis—chromosome doubling before meiosis is entered (Arai and Mukaino 1997; Zhang et al. 1998), the latter haploid eggs were generated by the mechanism of meiotic hybridogenesis (Arai and Mukaino 1998; Zhang et al. 1998). A similar reproductive mechanism was also detected in allopentaploid females, and meiotic hybridogenesis was presumably involved in their oogenesis (Matsubara et al. 1995; Zhang and Arai 1996). However, allotriploid and allopentaploid males were sterile, and accumulations of hexaploid- and decaploid-range cells, respectively, were frequently observed in the testes (Matsubara et al. 1995; Zhang and Arai 1996; Zhang et al. 1998). This predominance of polyploid testicular cells suggested that replication of triploid and pentaploid germ cells followed by the accumulation of the resultant hexaploid and decaploid cells, respectively, took place without them entering meiotic division (Zhang and Arai 1996; Zhang et al. 1998, 2002). An accumulation of replicated germ cells was a common characteristic of sterile male autotriploids and inter-specific or inter-populational male hybrids (Fujimoto et al. 2008; Zhao et al. 2016), as previously reported in interspecific hybrids of other fish species (Shimizu et al. 1997).
Hybridization and clonal reproduction
As described above, meiotic incompatibility between hetero-specific genomes or non-homologous chromosome sets derived from two genetically different biotypes may trigger the mechanisms that shift the reproductive system from sterility to atypical fertility in allopolyploids. Atypical clonal reproduction in the dojo loach likely originated in a past hybridization event between ancestors of two diversified groups, A and B (Yamada et al. 2015; Fujimoto et al. 2017; Kuroda et al. 2018, 2021b). In diploid clonal females and sex-reversed clonal males, meiotic incompatibility, presumably detected by early germ cells in advance before meiosis, may have induced chromosome doubling by endomitosis to ensure the formation of isogenic diploid gametes by sister chromosome pairing, as shown by the 50 bivalents (Itono et al. 2006; Yoshikawa et al. 2009; Kuroda et al. 2018, 2021b).
It was reported that unreduced eggs laid by artificially synthesized hybrids between contemporary different bisexual species or biotypes could not automatically initiate gynogenesis in almost all cases (Johnson and Wright 1986; Shimizu et al. 2000; Lampert et al. 2007; Arias-Rodriguez et al. 2009; Choleva et al. 2012; Marta et al. 2023). Thus, synthetic hybrids can produce unreduced eggs, but they normally incorporate a sperm nucleus to become triploid zygotes in most cases. Among the huge numbers of such unreduced eggs of hybrid-origin females, gynogenetic ability might have been rarely obtained in a very limited number of unreduced eggs by mutation, leading to the establishment of clonal lineages. However, how such a gynogenetic mutation takes place in an ancestral hybrid loach and then maintains clonal lineages by stable reproduction using sperms of sympatric gonochoristic parental wild-type fish is still unknown.
Timing of chromosome duplication and elimination
There is another argument about the differences in the timing and germ cell stage in the occurrence of chromosome doubling or endomitosis in the all-female Cobitis (Dedukh et al. 2021) and the sex-reversed male Misgurnus clone (Yoshikawa et al. 2009). This ambiguity may be resolved in the near future by the identification of the duplication time and germ cells in female Misgurnus clones by molecular cytogenetic, immunohistochemical, ultrastructural (electron microscopic), transcriptomic, and other effective approaches. In the Cobitis clones, males are absent or sterile. Thus, analyses of both sexes of Misgurnus clones are especially important for drawing any conclusions. Although sex-reversed clonal males produced isogenic diploid spermatozoa (Yoshikawa et al. 2007), their concentration and motility were poorer than those of control haploid spermatozoa, and thus it is generally difficult to fertilize enough eggs (Zhao et al. 2012a, b). This lower fertility may be a state preceding the occurrence of male-specific sterility of the clone, a state often found in other unisexual fishes (Arai and Fujimoto 2013; Dedukh et al. 2021; Tichopád et al. 2022).
Natural allotriploids which occurred by the accidental incorporation of a haploid sperm nucleus into the clonal diploid genotype exhibited meiotic hybridogenesis (Morishima et al. 2008c). This mechanism had already been observed in allotriploid females between tetraploids and diploids. Clone-origin allotriploid males were sterile, and meiotic configurations comprising inter-group and intra-group bivalents and intra-group univalents were observed (Kuroda et al. 2019). As reported in other allotriploid biotypes, males with a bivalent-univalent complex turned sterile (Morishima et al. 2008c). The question that arises is: why do the clone-origin allotriploids reproduce not by unreduced egg formation but by meiotic hybridogenesis? A similar question may also be raised for allotriploids between Japanese diploids and exotic tetraploids, which simultaneously produced both unreduced triploid eggs and hybridogenetic haploid eggs. We do not know the mechanism responsible for triggering the duplication and elimination of unmatched chromosomes that are unable to find counterparts to form bivalents at the synapsis stage.
Presence or absence of major gene(s) for clonal reproduction
Very recently, molecular cytogenetic and transcriptomic approaches were employed in unisexual Amazon molly, which reproduces by apomixis (Dedukh et al. 2022). The researchers successfully found the involvement of achiasmatic meiosis in the failure to form bivalents, but did not identify any involvement of known meiotic genes in unisexual reproduction. In apomictic Amazon molly, meiotic genes are found to be expressed as in bisexual parental species (Dedukh et al. 2022). Further studies, especially on the dysregulation of proteins presumably synthesized by meiosis-related genes in the process of gametogenesis, are necessary to answer these questions. In different kinds of unisexual fishes, such as dojo loach, which produce gynogenetically developing unreduced eggs by the mechanism of premeiotic chromosome duplication, the transcriptomes in the course of meiosis and gametogenesis have not been analyzed so far. However, clonal diploid loaches undergo unreduced gametogenesis due to premeiotic genome doubling, while clonal tetraploid loaches, which are genetically identical to the clonal diploid except for the number of chromosome sets, proceed through regular meiosis to form diploid gametes (Morishima et al. 2012). Thus, in the dojo loach, clonal reproduction may be determined not by special major gene(s) but presumably by certain biological changes brought on by the different constitution and dose of chromosomes in the germ cells. Furthermore, unreduced gametogenesis is suggested to be intrinsic to the hybrid status, based on the results from germ cell transplantation experiments in Cobitis (Tichopád et al. 2022).
Genetic sex and aberrant or atypical reproduction
The sex determination system in polyploid fishes remains an important issue. A balanced sex ratio (females:males = 1:1) was seen in the progenies of both autotetraploid and autohexaploid dojo loach, just as in wild-type diploids, although genotypes including multiple numbers of sex-determining Y chromosomes (or genes), such as XXYY, XXXY in tetraploids and XXXXYY in hexaploids, are predicted (Arai et al. 1999; Arai and Fujimoto 2013). In hybrids and polyploids using various biotypes of the dojo loach and its related species, the relationship between reproductive capacity and genetic sex is still unknown because the sex-determining gene has not been identified in the loach species. Future studies are needed to comprehend the interfaces among genetic sex, meiotic configuration, aberrant (sterile or aneuploid) or atypical (unreduced or hybridogenetic) gametogenesis, and unisexual (clonal) development in hybrid, polyploid, and clonal (unisexual) biotypes of the dojo loach. These studies may give insights into the mechanisms and evolution of atypical reproduction in vertebrates and aid the development of new biotechnology to establish aquaculture strains with improved performance.
Change history
21 August 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12562-023-01716-3
References
Alves MJ, Coelho MM, Próspero MI, Collares-Pereira MJ (1999) Production of fertile unreduced sperm by hybrid males of the Rutilus alburnoides complex (Teleostei, Cyprinidae): an alternative route to genome tetraploidization in unisexuals. Genetics 151:277–283
Arai K (1984) Developmental genetic studies on salmonids: morphogenesis, isozyme phenotypes and chromosomes in hybrid embryos. Mem Fac Fish Sci Hokkaido Univ 31:1–94
Arai K (1986) Effect of allotriploidization on development of the hybrid between female chum salmon and male brook trout. Bull Jpn Soc Sci Fish 52:823–825
Arai K (1988) Viability of allotriploids in salmonids. Nippon Suisan Gakkaishi 54:1695–1701
Arai K (2000) Chromosome manipulation in aquaculture: recent progress and perspective. Aquacult Sci (Suisanzoshoku) 48:295–303
Arai K (2001) Genetic improvement of aquaculture finfish species by chromosome manipulation techniques in Japan. Aquaculture 197:205–228
Arai K (2002) Significance and prospect of chromosome manipulation in aquaculture of salmonids. Fish Sci 68(Suppl. I):34–737
Arai K (2003) Genetics of the loach, Misgurnus anguillicaudatus: recent progress and perspective. Folia Biol (Krakow) 51S:107–117
Arai K, Fujimoto T (2013) Genomic constitution and atypical reproduction in polyploid and unisexual lineages of the Misgurnus loach, a teleost fish. Cytogenet Genome Res 140:226–240
Arai K, Fujimoto T (2019) Chromosome manipulation techniques and applications to aquaculture. In: Wang H-P et al (eds) Sex control in aquaculture, vol 1. Wiley, Hoboken, pp 137–162
Arai K, Inamori Y (1999) Viable hyperdiploid progeny between diploid female and induced triploid male in the loach, Misgurnus anguillicaudatus. Aquacult Sci (Suisanzoshoku) 47:489–495
Arai K, Mukaino M (1997) Clonal nature of gynogenetically induced progeny of triploid (diploid × tetraploid) loach, Misgurnus anguillicaudatus (Pisces: Cobitididae). J Exp Zool 278:412–421
Arai K, Mukaino M (1998) Electrophoretic analysis of the diploid progenies from triploid × diploid crosses in the loach Misgurnus anguillicaudatus (Pisces: Cobitidae). J Exp Zool 280:368–374
Arai K, Matsubara K, Suzuki R (1991a) Karyotype and erythrocyte size of spontaneous tetraploidy and triploidy in the loach Misgurnus anguillicaudatus. Nippon Suisan Gakkaishi 57:2167–2172
Arai K, Matsubara K, Suzuki R (1991b) Chromosomes and developmental potential of progeny of spontaneous tetraploid loach Misgurnus anguillicaudatus. Nippon Suisan Gakkaishi 57:2173–2178
Arai K, Masaoka T, Suzuki R (1992) Optimum condition of UV ray irradiation for genetic inactivation of loach eggs. Nippon Suisan Gakkaishi 58:1197–1201
Arai K, Matsubara K, Suzuki R (1993) Production of polyploids and viable gynogens using spontaneously occurring tetraploid loach, Misgurnus anguillicaudatus. Aquaculture 117:227–235
Arai K, Tanaka A, Kusunoki T, Suzuki R (1994) Allozyme expression in hybrids between diploid and tetraploid races of spinous loach, Cobitis biwae and loach, Misgurnus anguillicaudatus. Aquacult Sci (Suisanzoshoku) 42:585–591 (In Japanese)
Arai K, Ikeno M, Suzuki R (1995) Production of androgenetic diploid loach Misgurnus anguillicaudatus using spermatozoa of natural tetraploids. Aquaculture 137:131–138
Arai K, Taniura K, Zhang Q (1999) Production of second generation progeny of hexaploid loach. Fish Sci 65:186–192
Arias-Rodriguez L, Morishima K, Arai K (2007) Genetically diversified populations in the loach Misgurnus anguillicaudatus inferred from newly developed microsatellite markers. Mol Ecol Notes 7:82–85
Arias-Rodriguez L, Yasui GS, Arai K (2009) Disruption of normal meiosis in artificial inter-populational hybrid females of Misgurnus loach. Genetica 136:49–56
Arias-Rodriguez L, Yasui GS, Kusuda S, Arai K (2010) Reproductive and genetic capacity of spermatozoa of inter-populational hybrid males in the loach, Misgurnus anguillicaudatus. J Appl Ichthyol 26:653–658
Araya-Jaime C, Serrano EA, de Andrade Silva DMZ, Yamashita M, Iwai T, Oliveira C, Foresti F (2015) Surface-spreading technique of meiotic cells and immunodetection of synaptonemal complex proteins in teleostean fishes. Mol Cytogenet 8:4
Bartley DM, Rana K, Immink AJ (2001) The use of inter-specific hybrids in aquaculture and fisheries. Rev Fish Biol Fisher 10:325–337
Beçak MI, Beçak W, Rabello MN (1966) Cytological evidence of constant tetraploidy in the bisexual South American frog Odontohyrnus americanus. Chromosoma 19:188–192
Benfey T (2016) Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Rev Aquacult 8:264–282
Beukeboom LW, Vrijenhoek RC (1998) Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J Evol Biol 11:755–782
Chevassus B (1983) Hybridization in fish. Aquaculture 17:113–128
Chmielewska M, Dedukh D, Haczkiewicz K, Rozenblunt-Kościsty B, Kaźmierczak M, Kolenda K, Serwa E, Pietras-Lebioda A, Krasikova A, Ogielska M (2018) The programmed DNA elimination and formation of micronuclei in germ line cells of the natural hybridogenetic water frog Pelophylax esculentus. Sci Rep 8:7870
Choleva L, Janko K, De Gelas K, Bohlen J, Šlechtova V, Rábová M, Ráb P (2012) Synthesis of clonality and polyploidy in vertebrate animals by hybridization between two sexual species. Evolution 66:2191–2203
Cimino MC (1972) Egg-production, polyploidization and evolution in a diploid all-female fish of the genus Poeciliopsis. Evolution 26:294–306
Collares-Pereira MJ, Matos I, Morgado-Santos M, Coelho MM (2013) Natural pathways towards polyploidy in animals: the Squalius alburnoides fish complex as a model system to study genome size and genome reorganization in polyploids. Cytogenet Genome Res 140:97–116
Cotter D, O’Donovan V, O’Maoiléidigh N, Rogan G, Roche N, Wilkins NP (2000) An evaluation of the triploid Atlantic salmon (Salmo salar L.) in minimizing the impact of escaped farmed salmon on wild population. Aquaculture 186:61–75
Dawley RM, Graham JH, Schultz RJ (1985) Triploid progeny of pumpkinseed x green sunfish hybrids. J Hered 76:251–257
Dedukh D, Majtánová Z, Marta A, Pšenička M, Kotusz J, Klíma J, Juchno D, Boron A, Janko K (2020a) Parthenogenesis as a solution to hybrid sterility: the mechanistic basis of meiotic distortions in clonal and sterile hybrids. Genetics 215:975–987
Dedukh D, Riumin S, Chmielewska M, Rozenblunt-Kościsty B, Kolenda K, Kaźmierczak M, Dudzik A, Ogielska M, Krasikova A (2020b) Micronuclei in germ cells of hybrid frogs from Pelophylax esculentus complex contain gradually eliminated chromosomes. Sci Rep 10:8720
Dedukh D, Marta A, Janko K (2021) Challenges and costs of asexuality: variation in premeiotic genome duplication in gynogenetic hybrids from Cobitis taenia complex. Int J Mol Sci 22:12117
Dedukh D, da Cruz I, Kneitz S, Marta A, Ormanns J, Tichopád T, Lu Y, Alsheimer M, Janko K, Schartl M (2022) Achiasmatic meiosis in the unisexual Amazon molly, Poecilia formosa. Chromo Res 30:443–457
Devlin RH, Sundröm LF, Muir WM (2006) Interface of biotechnology and ecology for environmental risk assessments of transgenic fish. Trends Biotechnol 24:89–97
Devlin RH, Sakhrani D, Biagi CA, Eom K-W (2010) Occurrence of incomplete paternal chromosome retention in GH-transgenic coho salmon being assessed for reproductive containment by pressure-shock-induced triploidy. Aquaculture 304:66–78
Devlin RH, Biagi CA, Sakhrani D, Fujimoto T, Leggatt RA, Smith JL, Yeasaki TY (2022) An assessment of hybridization potential between Atlantic and Pacific salmon. Can J Fish Aquat Sci 79:670–676
Ene C, Radu S (2000) Chromosome study of Misgurnus fossilis from Danube delta biosphere reserve, Romania. Folia Zool (Praha) 49:91–955
Fujimoto T, Kataoka T, Otani S, Saito T, Aita T, Yamaha E, Arai K (2004) Embryonic stages from cleavage to gastrula in the loach Misgurnus anguillicaudatus. Zool Sci 21:747–755
Fujimoto T, Kataoka T, Sakao S, Saito T, Yamaha E, Arai K (2006) Developmental stages and germ cell lineage of the loach (Misgurnus anguillicaudatus). Zool Sci 23:977–989
Fujimoto T, Sakao S, Yamaha E, Arai K (2007) Evaluation of different doses of UV irradiation to loach eggs for genetic inactivation of the maternal genome. J Exp Zool 307A:449–462
Fujimoto T, Yasui GS, Yoshikawa H, Yamaha E, Arai K (2008) Genetic and reproductive potential of spermatozoa of diploid and triploid males obtained from interspecific hybridization of Misgurnus anguillicaudatus female with M. mizolepis male. J Appl Ichthyol 24:430–437
Fujimoto T, Nishimura T, Goto-Kazeto R, Kawakami Y, Yamaha E, Arai K (2010a) Sexual dimorphism of gonadal structure and gene expression in germ cell deficient loach, a teleost fish. Proc Natl Acad Sci USA 107:17211–17216
Fujimoto T, Yasui GS, Hayakawa M, Sakao S, Yamaha E, Arai K (2010b) Reproductive capacity of neo-tetraploid loaches produced using diploid spermatozoa from a natural tetraploid male. Aquaculture 308:S133–S139
Fujimoto T, Saito T, Sakao S, Arai K, Yamaha E (2010c) Developmental potential of embryonic cells of the nucleocytoplasmic hybrid comprising haploid nucleus of goldfish and egg cytoplasm of loach. Intl J Dev Biol 54:827–835
Fujimoto T, Sakao S, Oshima K, Yamaha E, Arai K (2013) Heat-shock induced tetraploid and diploid/tetraploid mosaic in the loach, Misgurnus anguillicaudatus. Aquacult Int 21:769–781
Fujimoto T, Yamada A, Kodo Y, Nakaya K, Okubo-MurataM ST, Ninomiya K, Inaba M, Kuroda M, Arai K, Murakami M (2017) Development of nuclear DNA markers to characterize genetically diverse groups of Misgurnus anguillicaudatus and its closely related species. Fish Sci 83:743–756
Fujita T (2019) Northern weather loach. In: Hosoya K et al (eds) Freshwater fishes of Japan. Yamakei, Tokyo, p 178 (In Japanese)
Fujiwara A, Abe S, Yamaha E, Yamazaki F, Yoshida CM (1997) Uniparental chromosome elimination in the early embryogenesis of the inviable salmonid hybrids between masu salmon female and rainbow trout male. Chromosoma 106:44–52
Goddard KA, Megwinoff O, Wessner LL, Giamo F (1998) Confirmation of gynogenesis in Phoxinus eos-neogaeus (Pisces: Cyprinidae). J Hered 89:151–157
Goto R, Saito T (2019) A state-of-the-art review of surrogate propagation in fish. Theriogenology 133:216–227
Hamaguchi S, Sakaizumi M (1992) Sexually differentiated mechanisms of sterility in interspecific hybrids between Oryzias latipes and O. curvinotus. J Exp Zool 263:323–329
Havelka M, Arai K (2019) Hybridization and polyploidization in sturgeon. In: Wang H-P et al (eds) Sex control in aquaculture, vol 2. Wiley, Hoboken, pp 669–687
Heppich S, Turner HG, Greilhugen J (1982) Premeiotic chromosome doubling after genome elimination during spermatogenesis of the species hybrid Rana esculenta. Theor Appl Genet 61:101–104
Hou J, Fujimoto T, Yamaha E, Arai K (2013) Production of androgenetic diploid loach by cold-shock of eggs fertilized with diploid sperm. Theriogenology 80:125–130
Hou J, Saito T, Fujimoto T, Yamaha E, Arai K (2014) Induced androgenetic doubled haploids without irradiation of eggs in loach Misgurnus anguillicaudatus. Aquaculture 420–421:S57–S63
Itono M, Morishima K, Fujimoto T, Yamaha E, Arai K (2006) Premeiotic endomitosis produces diploid eggs in the natural clone loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J Exp Zool 305A:513–523
Itono M, Okabayashi N, Morishima K, Fujimoto T, Yoshikawa H, Yamaha E, Arai K (2007) Cytological mechanisms of gynogenesis and sperm incorporation in unreduced diploid eggs of the clonal loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J Exp Zool 307A:35–50
Inoue D, Fujimoto T, Yasui GS, Kawakami Y, Yamaha E, Arai K (2012) Vitrification of primordial germ cells using whole embryos for gene-banking in loach, Misgurnus anguillicaudatus. J Appl Ichthyol 28:919–924
Janko K, Pačes J, Wilkinson-Herbots H, Costa RJ, Roslein J, Drozd P, Iakovenko N, Rídl J, Hroudová M, Kocí J, Reifová R, Šlechtova V, Choleva L (2018) Hybrid asexuality as a primary postzygotic barrier between nascent species: on the interconnection between sexuality, hybridization and speciation. Mol Ecol 27:248–263
Johonson KR, Wright JM (1986) Female brown trout × male Atlantic salmon hybrids produce gynogens and triploids when backcrossed to male Atlantic salmon. Aquaculture 57:345–358
Khan MMR, Arai K (2000) Allozyme variation and genetic differentiation of the Misgurnus anguillicaudatus. Fish Sci 66:211–222
Kijima K, Arai K, Suzuki R (1996a) Induced allotriploidy in inviable interfamilial hybrids, female loach × male goldfish and female loach x male minnow. J Fac Appl Biol Sci Hiroshima Univ 35:1–12 (In Japanese)
Kijima K, Arai K, Suzuki R (1996b) Induction of allotriploids and allopentaploids in interfamilial hybrids, female spinous loach × male carp and female loach x male carp. J Fac Appl Biol Sci Hiroshima Univ 35:13–26 (In Japanese)
Kimura-Kawaguchi MR, Horita M, Abe S, Arai K, Kawata M, Munehara H (2014) Identification of hemiclonal reproduction in three species of Hexagrammos marine reef fishes. J Fish Biol 85:189–209
Kohara M, Denda I (2008) Production of allotriploid “Shinsyu Salmon” by chromosome manipulation. Fish Genet Breed Sci (Suisan-Ikusyu) 37:61–66 (in Japanese)
Koizumi N, Takemura T, Watabe K, Mori A (2009) Genetic variation and diversity of Japanese loach inferred from mitochondrial DNA—phylogenetic analysis using the cytochrome b gene sequence. Trans Jap Soc Irrig Drain Rural Engin 77:7–16 (in Japanese)
Komen H, Thorgaard GH (2007) Androgenesis, gynogenesis and the production of clones in fishes: a review. Aquaculture 269:150–173
Kuroda M, Fujimoto T, Murakami M, Yamaha E, Arai K (2018) Clonal reproduction assured by sister chromosome pairing in dojo loach, a teleost fish. Chromo Res 26:243–253
Kuroda M, Fujimoto T, Murakami M, Yamaha E, Arai K (2019) Aberrant meiotic configurations cause sterility in clone-origin triploid and inter-group hybrid males of the dojo loach, Misgurnus anguillicaudatus. Cytogenet Genome Res 158:46–54
Kuroda M, Fujimoto T, Yamaha E, Arai K (2021a) Improvement in group identification of dojo loach, Misgurnus anguillicaudatus, using PCR-restriction fragment length polymorphism. Conserv Genet Reour 13:457–463
Kuroda M, Shibata K, Fujimoto T, Murakami M, Yamaha E, Arai K (2021b) FISH identifies chromosome differentiation between contemporary genomes of wild types and the ancestral genome of unisexual clones of dojo loach, Misgurnus anguillicaudatus. Cytogenet Genome Res 161:178–186
Kusuda S, Teranishi T, Koide N, Nagai T, Arai K, Yamaha E (2004) Pluripotency of cryopreserved blastomeres of the goldfish. J Exp Zool 301A:131–138
Kusunoki T, Arai K, Suzuki R (1994a) Viability and karyotypes of interracial and intergeneric hybrids in loach species. Fish Sci 60:415–422
Kusunoki T, Arai K, Suzuki R (1994b) Production of viable gynogens without chromosome duplication in the spinous loach Cobitis biwae. Aquaculture 117:227–235
Lampart KP, Lamatsch DK, Fischer P, Epplen JT, Nanda I, Schmid M, Schartl M (2007) Automictic reproduction in interspecific hybrids of poeciliid fish. Curr Biol 17:1948–1953
Li K, Li Y, Zhou D (1983) A comparative study of the karyotypes in two species of mud loaches. Zool Res (china) 4:75–81
Li YJ, Jie Y, Wang JB, Xu Y, Jie W, Sun XW, Arai K (2008) A study on the distribution of polyploid loaches in China. Nippon Suisan Gakkaishi 74:177–182 (in Japanese)
Li YJ, Tian Y, Zhang MZ, Tian PP, Yu Z, Abe S, Arai K (2010) Chromosome banding and FISH with rDNA in the diploid and tetraploid loach Misgurnus anguillicaudatus. Ichthyol Res 57:358–366
Li YJ, Yu Z, Zhang MZ, Qian C, Abe S, Arai K (2011) The origin of natural tetraploid loach Misgurnus anguilllicaudatus (Teleostei: Cobitidae) inferred from meiotic chromosome configurations. Genetica 139:805–811
Li YJ, Zhang MZ, Qian C, Gao M, Arai K (2012) Fertility and ploidy of gametes of diploid, triploid and tetraploid loaches, Misgurnus anguillicaudatus, in China. J Appl Ichthyol 28:900–905
Li YJ, Yu Z, Zhang MZ, Qian C, Abe S, Arai K (2013) Induction of viable gynogenetic progeny using eggs and UV irradiated sperm from Chinese tetraploid loach, Misgurnus anguiiilicaudatus. Aquacult Int 21:759–768
Li YJ, Gao YC, Zhou H, Ma HY, Li JQ, Arai K (2015) Meiotic chromosome configurations in triploid progeny from reciprocal crosses between wild-type diploid and natural tetraploid loach Misgurnus anguillicaudatus in China. Genetica 143:555–562
Li YJ, Gao YC, Zhou H, Ma HY, Lin ZQ, Ma TY, Sui Y, Arai K (2016) Aneuploid progenies of triploid hybrids between diploid and tetraploid loach Misgurnus anguillicaudatus in China. Genetica 144:601–609
Li YJ, Li A, Zhou H (2017) Study on genetics of natural polyploidy loach Misgurnus anguillicaudatus in China. Chinese Agriculture Science, Beijing, p 186 (In Chinese)
Liu SJ (2010) Distant hybridization leads to different ploidy fishes. Sci China Life Sci 53:416–425
Liu SJ, Liu Y, Zhou G, Zhang X, Luo C, Feng H, He X, Zhu G, Yang H (2001) The formation of tetraploid stocks of red crucian carp × common carp hybrids as an effect of interspecific hybridization. Aquaculture 192:171–186
Marta A, Tichopád T, Bartoš O, Klima J, Shah MA, Bohlem VS, Bohlen J, Haračka K, Choleva L, Stöck M, Dedukh D, Janko K (2023) Genetic and karyotype divergence between parents affect clonality and sterility in hybrids. bioRxiv. https://doi.org/10.1101/2023.04.11.536494
Masaoka T, Arai K, Suzuki R (1995) Production of androgenetic diploid loach, Misgurnus anguillicaudatus from UV irradiated eggs by suppression of the first cleavage. Fish Sci 61:716–717
Matsubara K, Arai K, Suzuki R (1995) Survival potential and chromosomes of progeny of triploid and pentaploid females in the loach, Misgurnus anguillicaudatus. Aquaculture 131:37–48
Minamori S (1953) Physiological isolation in Cobitidae II. Inviability of hybrids between the mud loach and some local races of spinous loaches. J Sci Hiroshima Univ B1 14:125–149
Mishina T, Takeshima H, Takada M, Iguchi K, Zhang C, Zhao Y, Kawahara-Miki R, Hashiguchi Y, Tabata R, Sasaki T, Nishida M, Watanabe K (2021) Interploidy gene flow involving the sexual-asexual cycle facilitates the diversification of gynogenetic triploid Carassius fish. Sci Rep 11:22485
Momotani S, Morishima K, Zhang Q, Arai K (2002) Genetic analyses of the progeny of triploid gynogens induced from unreduced eggs of triploid (diploid female × tetraploid male) loach. Aquaculture 204:311–322
Monaco PJ, Rasch EM, Balsano JS (1984) Apomictic reproduction in the Amazon molly, Poecilia formosa, and its triploid hybrids. In: Turner BJ (ed) Evolutionary genetics of fishes. Plenum, New York, pp 311–328
Morishima K, Nakayama I, Arai K (2001) Microsatellite-centromere mapping in the loach Misgurnus anguillicaudatus. Genetica 111:59–69
Morishima K, Horie S, Yamaha E, Arai K (2002) A cryptic clonal line of the loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) evidenced by induced gynogenesis, interspecific hybridization, microsatellite genotyping and multilocus DNA fingerprinting. Zool Sci 19:565–575
Morishima K, Oshima K, Horie S, Fujimoto T, Yamaha E, Arai K (2004) Clonal diploid sperm of the diploid-triploid mosaic loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J Exp Zool 301A:491–501
Morishima K, Nakamura-Shiokawa Y, Bando E, Li YJ, Boron A, Khan MMR, Arai K (2008a) Cryptic clonal lineages and genetic diversity in the loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) inferred from nuclear and mitochondrial DNA analyses. Genetica 132:159–171
Morishima K, Nakayama I, Arai K (2008b) Genetic linkage map of the loach Misgurnus anguillicaudatus (Teleostei: Cobitidae). Genetica 132:227–241
Morishima K, Yoshikawa H, Arai K (2008c) Meiotic hybridogenesis in triploid Misgurnus loach derived from a clonal lineage. Heredity 100:581–586
Morishima K, Fujimoto T, Sato M, Kawae A, Zhao Y, Yamaha E, Arai K (2011) Cold-shock eliminates female nucleus in fertilized eggs to induce androgenesis in the loach (Misgurnus anguillicaudatus), a teleost fish. BMC Biotech 11:116
Morishima K, Yoshikawa H, Arai K (2012) Diploid clone produces unreduced diloid gametes but tetraploid clone generates reduced diploid gametes in the Misgurnus loach. Biol Reprod 86(33):1–8
Moritz C, Brown WM, Densmore L, Wright JW, Vyas D, Donnellan S, Adams M, Baverstock P (1989) Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus (Teiidae) and Heteronotia (Gekkonidae). In: Dawley RM, Bogart JP (eds) Evolution and ecology of unisexual vertebrate. New York State Museum, Albany, pp 87–112
Nam YK, Kim DS (2004) Ploidy status of progeny from the crosses between tetraploid males and diploid females in mud loach (Misgurnus mizolepis). Aquaculture 236:575–582
Nam YK, Cho YS, Cho HJ, Kim DS (2002) Accelerated growth performance and stable germ-line transmission in androgenetically derived homozygous transgenic mud loach, Misgurnus mizolepis. Aquaculture 209:257–270
Nam YK, Park IS, Kim DS (2004) Triploid hybridization of fast-growing transgenic mud loach Misgurnus mizolepis male to cyprinid loach Misgurnus anguillicaudatus female: the first performance study on growth and reproduction of transgenic polyploid fish. Aquaculture 231:559–572
Neyfakh A (1964) Radiation investigation of nucleo-cytoplasmic interactions in morphogenesis and biochemical differentiation. Nature 201:880–884
Nomura T, Arai K, Hayashi T, Suzuki R (1998) Effect of temperature on sex ratios of normal and gynogenetic diploid loach. Fish Sci 64:753–758
Ojima T, Takai A (1979) The occurrence of spontaneous polyploidy in the Japanese common loach, Misgurnus anguillicaudatus. Proc Jpn Acad 55B:487–491
Onozato H (1982) The “Hertwig effect” and gynogenesis in chum salmon Oncorhunchus keta eggs fertilized with 60Co γ-ray irradiated milt. Bull Jpn Soc Sci Fish 48:1237–1244
Onozato H, Yamaha E (1983) Induction of gynogenesis with ultraviolet rays in four species of salmoniformes. Bull Jpn Soc Sci Fish 49:693–699
Oshima K, Morishima K, Yamaha E, Arai K (2005) Reproductive capacity of triploid loaches obtained from Hokkaido Island, Japan. Ichthyol Res 52:1–8
Park IS, Nam YK, Kim DS (2006) Growth performance, morphometric traits and gonad development of induced reciprocal diploid and triploid hybrids between mud loach (Misgurnus mizolepis Günther) and cyprinid loach (Misgurnus anguillicaudatus Cantor). Aquacult Res 37:1246–1253
Perdices A, Vasil’ev V, Vasil’eva E (2012) Molecular phylogeny and intraspecific structure of loaches (genera Cobitis and Misgurnus) from the far east region of Russia and some conclusions on their systematics. Ichthyol Res 59:113–123
Piferrer F, Beaumont A, Falguiére J-C, Flajšhans M, Haffray P, Colombo L (2009) Polyploid fish and shellfish: production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293:125–156
Piva LH, de Siqueira-Silva DH, Goes CAG, Fujimoto T, Saito T, Dragone LV, Senhorini JA, Porto-Foresti F, Ferraz JBS, Yasui GS (2018) Triploid or hybrid tetra: which is the ideal sterile host for surrogate technology. Theriogenology 108:239–244
Rahman MA, Lee S-G, Yusoff FM, Rafiquzzaman SM (2019) Hybridization and its application in aquaculture. In: Wang H-P et al. (eds) Sex control in aquaculture, vol. 1. Wiley, Hoboken, pp 163–78
Romashov DD, Belyaeva VV (1964) The cytology of the radiation gynogenesis and androgenesis in the loach (Misgurnus fossilis). Dokl Acad Nauk SSSR 157:964–967
Saitoh K (1989) Asian pond loach. In: Kawanabe et al (eds) Freshwater fishes of Japan. Yamakei, Tokyo, pp 382–385 (In Japanese)
Sakai C, Konno F, Nakano O, Iwai T, Yokota J, Lee J, Nishida-Umehara C, Kuroiwa A, Matsuda Y, Yamashita M (2007) Chromosome elimination in the interspecific hybrid medaka between Oryzias latipes and O. hubbsi. Chromo Res 15:697–709
Sakao S, Fujimoto T, Tanaka M, Yamaha E, Arai K (2003) Aberrant and arrested embryos from masu salmon eggs treated for tetraploidization by inhibition of the first cleavage. Nippon Suisan Gakkaishi 69:738–748 (In Japanese)
Sakao S, Fujimoto T, Kimura S, Yamaha E, Arai K (2006) Drastic mortality in tetraploid induction results from elevation of ploidy in masu salmon Oncorhynchus masou. Aquaculture 252:147–160
Schultz RJ (1969) Hybridization, unisexuality and polyploidy in the teleost Poeciliopsis (Poecilidae) and other vertebrates. Am Natl 103:605–619
Scribner KT, Page KS, Barton ML (2001) Hybridization in freshwater fishes: a review of case studies and cytonuclear methods of biological inference. Rev Fish Biol Fisher 10:293–323
Shedko SV, Vasil’eva ED (2022) A new species of the pond loaches Misgurnus (Cobitidae) from the south of Sakhalin Island. J Ichthyol 62:356–372
Shibata K, Yen DT, Fujimoto T, Arai K (2020) Comparative analysis of mitochondrial genomes in genetically distinct groups of the dojo loach Misgurnus anguillicaudatus. Mitochondrial DNA Part B 5:3810–3812
Shibata K, Kuroda M, Yamaha E, Arai K, Fujimoto T (2023) Nucleotide sequence and chromosome mapping of 5S ribosomal DNA from the dojo loach, Misgurnus anguillicaudatus. Cytogenet Genome Res. https://doi.org/10.1159/000529150
Shimizu T (2014) Genetic status and consequences of commercial utilization of the oriental weather loach Misgurnus anguillicaudatus in Japan. Jpn J Ichthyol 61:36–40 (In Japanese)
Shimizu Y, Shibata N, Yamashita M (1997) Spermiogenesis without preceding meiosis in the hybrid medaka between Oryzias latipes and O. curvinotus. J Exp Zool 279:102–112
Shimizu Y, Shibata N, Sakaizumi M, Yamashita M (2000) Production of diploid eggs through premeiotic endomitosis in the hybrid medaka between Oryzias latipes and O. curvinotus. Zool Sci 17:951–958
Shinomiya A, Shibata N, Sakaizumi M, Hamaguchi S (2002) Sex reversals of genetic females (XX) induced by transplantation of XY somatic cells in the medaka, Oryzias latipes. Int J Dev Biol 46:711–717
Suwa M, Arai K, Suzuki R (1994) Suppression of the first cleavage and cytogenetic studies on the gynogenetic loach. Fish Sci 60:673–681
Suzuki R (1982) Aquaculture of loach with diagraphic illustrations (Zukai/Dojo no Youshoku). Midori Shobou, Tokyo, p 101 (In Japanese)
Suzuki R, Nakanishi T, Oshiro T (1985a) Survival, growth and sterility of induced triploids in the cyprinid loach Misgurnus anguillicaudatus. Bull Jpn Soc Sci Fish 51:889–894
Suzuki R, Oshiro T, Nakanishi T (1985b) Survival, growth and fertility of gynogenetic diploids induced in the cyprinid loach, Misgurnus anguillicaudatus. Aquaculture 48:45–55
Tanaka D, Takahashi A, Ueno K (2009) Morphometric characteristics and reproductive capacity of nuclear transplants derived from embryonic cells of loach, Misgurnus anguillicaudatus. J Exp Zool 311A:11–19
Thorgaard GH, Gall GAE (1979) Adult triploids in a rainbow trout family. Genetics 93:961–973
Tichopád T, Franĕk R, Doležálková-Kaštánková M, Dedukh D, Marta A, Halačka K, Steinbach C, Janko K, Pšenička M (2022) Clonal gametogenesis is triggered by intrinsic stimuli in the hybrid’s germ cells but is dependent on sex differentiation. Biol Reprod 2022:1–12. https://doi.org/10.1093/biolre/ioac074
Timofeeva MY, Kaviani KA (1964) Nucleis acids and unfertilized eggs and developing grounding embryos. Biokimiia 29:96–100
Turner HG, Heppich S (1981) Premeiotic genome exclusion during oogenesis in the common edible frog, Rana esculenta. Naturwissenschaften 68:207–208
Turner HG, Heppich-Turner S (1991) Genome exclusion and two strategies of chromosome duplication in oogenesis of a hybrid frog. Naturwissenschaften 78:32–34
Ueno K, Senou H, Kim IS (1985) A comparative study on five species of Korean cobitid fish. Jpn J Genet 60:539–544
Vasil’eva ED (2001) Loaches (genus Misgurnus, Cobitidae) of Russian Asia. I. The species composition in waters of Russia (with a description of a new species) and some nomenclature and taxonomic problems of related forms from adjacent countries. J Ichthyology 41:553–563
Vasil’ev VP, Vasile’eva ED (2008) Comparative karyology of species of the genera Misgurnus and Cobitis (Cobitidae) from the Amur River Basin in connection with their taxonomic relations and the evolution of karyotypes. J Ichthyology 48:1–13
Wang J, Liu Q, Luo K, Chen X, Xiao J, Zhang C, Tao M, Zhao R, Liu S (2016) Cell fusion as the formation mechanism of unreduced gametes in the gynogenetic diploid fish. Sci Rep 6:31658
Yamada A, Kodo Y, Murakami M, Kuroda M, Aoki T, Fujimoto T, Arai K (2015) Hybrid origin of gynogenetic clones and the introgression of their mitochondrial genome into sexual diploids through meiotic hybridogenesis in the loach Misgurnus anguillicaudatus. J Exp Zool 323A:593–606
Yamaha E, Murakami M, Hada K, Otani S, Fujimoto T, Tanaka M, Sakao S, Kimura S, Sato S, Arai K (2003) Recovery of fertility in male hybrids of a cross between goldfish and common carp by transplantation of PGC (primordial germ cell)-containing graft. Genetica 119:121–131
Yamashita M, Jiang J, Onozato H, Nakanishi T, Nagahama Y (1993) A tripolar spindle formed at meiosis I assures the retention of the original ploidy in the gynogenetic triploid crucian carp, Ginbuna Carassius auratus langsdorfii. Dev Growth Differ 35:631–636
Yanagimachi R, Harumi T, Matsubara H, Yan W, Yuan S, Hirohashi N, Iida T, Yamaha E, Arai K, Matsubara T, Andoh T, Vine C, Cherr GN (2017) Chemical and physical guidance of fish spermatozoa into the egg through the micropyle. Biol Reprod 96:780–799
Yasui GS, Arias-Rodriguez L, Fujimoto T, Arai K (2008) Simple and inexpensive method for cryopreservation of fish sperm combining straw and powdered dry ice. Cryoletter 29:383–390
Yasui GS, Arias-Rodriguez L, Fujimoto T, Arai K (2009) A sperm cryopreservation protocol for the loach Misgurnus anguillicaudatus and its applicability for other species. Anim Reprod Sci 116:335–345
Yasui GS, Fujimoto T, Arai K (2010) Restoration of the loach Misgurnus anguillicaudatus from cryopreserved diploid sperm and induced androgenesis. Aquaculture 308:S140–S144
Yasui GS, Fujimoto T, Sakao S, Yamaha E, Arai K (2011) Production of loach (Misgurnus anguillicaudatus) germ line chimera using transplantation of primordial germ cells isolated from cryopreserved blastomeres. J Anim Sci 89:2380–2388
Yasui GS, Fujimoto T, Arias-Rodriguez L, Takagi Y, Arai K (2012) The effect of ions and cryoprotectants upon sperm motility and fertilization success in the loach Misgurnus anguillicaudatus. Aquaculture 344–349:147–152
Yasui GS, Saito T, Zhao Y, Fujimoto T, Yamaha E, Arai K (2018) Intra-ooplasmic injection of a multiple number of sperm to induce androgenesis and polyploidy in the dojo loach Misgurnus anguillicaudatus (Teleostei: Cobitidae). Zygote 26:408–416
Yoshikawa H, Morishima K, Kusuda S, Yamaha E, Arai K (2007) Diploid sperm produced by artificially sex-reversed clone loach. J Exp Zool 307A:75–83
Yoshikawa H, Morishima K, Fujimoto T, Saito T, Kobayashi T, Yamaha E, Arai K (2009) Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol Reprod 80:973–979
Yoshizaki G, Ichikawa M, Hayashi M, Iwasaki Y, Miwa M et al (2012) Sexual plasticity of ovarian germ cells in rainbow trout. Development 137:1227–1230
Zhang Q, Arai K (1996) Flow cytometry for DNA contents of somatic cells and spermatozoa in the progeny of natural tetraploid loach. Fish Sci 62:870–877
Zhang Q, Arai K (1999a) Distribution and reproductive capacity of natural triploid individuals and occurrence of unreduced eggs as a cause of polyploidization in the loach, Misgurnus anguillicaudatus. Ichthyol Res 46:153–161
Zhang Q, Arai K (1999b) Aberrant meioses and viable aneuploid progeny of induced triploid loach (Misgurnus anguillicaudatus) when crossed to natural tetraploids. Aquaculture 175:63–76
Zhang Q, Arai K (2003) Extensive karyotype variation in somatic and meiotic cells of the loach Misgurnus anguillicaudatus (Pisces: Cobitidae). Folia Zool 52:423–429
Zhang Q, Arai K, Yamashita M (1998) Cytogenetic mechanisms for triploid and haploid egg formation in the triploid loach Misgurnus anguillicaudatus. J Exp Zool 281:608–619
Zhang Q, Hanada K, Arai K (2002) Aberrant meiosis in diploid and triploid progeny of gynogenetic diploids produced from eggs of natural tetraploid loach. Folia Zool 51:165–176
Zhao Y, Toda M, Hou J, Aso M, Arai K (2012a) The occurrence of hyper-tetraploid and other unusual polyploid loaches Misgurnus anguillicaudatus among market specimens in Japan. Fish Sci 78:1219–1227
Zhao Y, Pšenička M, Fujimoto T, Saito T, Yasui GS, Yamaha E, Arai K (2012b) Motility, morphology, mitochondria and ATP content of diploid spermatozoa from sex-reversed clonal diploid and neo-tetraploid loach, Misgurnus anguillicaudatus. J Appl Ichthyol 28:1006–1012
Zhao Y, Saito T, Pšenička M, Fujimoto T, Arai K (2014) Comparison of spermatozoa parameters, fine structures, and energy-related factors among tetraploid, hyper-tetraploid, and hyper-triploid loaches (Misgurnus anguillicaudatus). J Exp Zool 321A:198–206
Zhao Y, Fujimoto T, Pšenička M, Saito T, Arai K (2016) Non-motile tetraploid spermatozoa of Misgurnus loach hybrids. Fish Sci 82:127–135
Acknowledgements
I would like to thank the Japanese Society of Fisheries Science, which invited me to be an author of a review paper for the journal Fisheries Science. I wish to express my sincere gratitude to all the co-authors of scientific papers related to the biology and biotechnology of the dojo loach. I also thank Dr. T. Fujimoto for his critical reading of the manuscript. I would also like to thank Editage (www.editage.com) for English language editing. This review was based on original works supported by grants-in-aid for KAKENHI (grant nos. 15H02457, 24380100, 21380114, 18380108, 14656073, 13460079, 10660181, 09556044, 06660239 and 03806029) from the Japan Society for the Promotion of Science (JSPS), Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published with support by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant no. JP19HP2002.
The original online version of this article was revised to update figure 3.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arai, K. Meiosis and gametogenesis in hybrid, polyploid, and clonal fishes: case studies in the dojo loach Misgurnus anguillicaudatus. Fish Sci 89, 537–570 (2023). https://doi.org/10.1007/s12562-023-01703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-023-01703-8