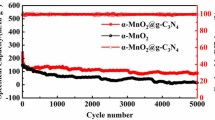
Abstract
Rechargeable aqueous zinc ion batteries (AZIBs) based on manganese dioxide (MnO2) have received much attention for large-scale energy storage applications, however, their energy density is mainly limited by the one-electron reaction of Mn4+/Mn3+ redox. Herein, Mo doped δ-MnO2 (Mo-MnO2) is prepared and used as a high-performance cathode for AZIBs, which delivers an ultrahigh specific capacity of 652 mAh·g−1 at 0.2 A·g−1 based on the two-step two-electron redox reaction of Mn4+ ⇌ Mn3+ ⇌ Mn2+. Ex-situ structural analysis and density functional theory calculation reveal that the Mo5+ dopant plays an important role in enhancing the electronic conductivity of Mo-MnO2 and promoting Jahn—Teller distortion of octahedral [MnO6] in ZnMn2O4, which facilitates the second step redox reaction of Mn3+/Mn2+. This work provides a novel cathode materials design with multi-electron redox chemistry to achieve high energy density in AZIBs.

Similar content being viewed by others
References
Kang, K.; Meng, Y. S.; Breger, J.; Grey, C. P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980.
Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134.
Zhong, C.; Liu, B.; Ding, J.; Liu, X. R.; Zhong, Y. W.; Li, Y.; Sun, C. B.; Han, X. P.; Deng, Y. D.; Zhao, N. Q. et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 2020, 5, 440–449.
Wang, C. L.; Gao, Y. X.; Sun, L. S.; Zhao, Y.; Yin, D. M.; Wang, H. R.; Cao, J. C.; Cheng, Y.; Wang, L. M. Anti-catalytic and zincophilic layers integrated zinc anode towards efficient aqueous batteries for ultra-long cycling stability. Nano Res. 2022, 15, 8076–8082.
Wang, R.; Wu, Q. F.; Wu, M. J.; Zheng, J. X.; Cui, J.; Kang, Q.; Qi, Z. B.; Ma, J. D.; Wang, Z. C.; Liang, H. F. Interface engineering of Zn meal anodes using electrochemically inert Al2O3 protective nanocoatings. Nano Res. 2022, 15, 7227–7233.
Zhuang, Z. C.; Li, Y. H.; Yu, R. H.; Xia, L. X.; Yang, J. R.; Lang, Z. Q.; Zhu, J. X.; Huang, J. Z.; Wang, J. O.; Wang, Y. et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat. Catal. 2022, 5, 300–310.
Faqing Cao, Baohu Wu, Tianyu Li, Shengtong Sun, Yucong Jiao, Peiyi Wu. Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance. Nano Research 2022, 15, 2030–2039.
Blanc, L. E.; Kundu, D.; Nazar, L. F. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020, 4, 771–799.
Yufang Cao, Xiaohui Tang, Linge Li, Haifeng Tu, Yuzhen Hu, Yingying Yu, Shuang Cheng, Hongzhen Lin, Liwen Zhang, Jiangtao Di. et al. Fast Zn2+ mobility enabled by sucrose modified Zn2+ solvation structure for dendrite-free aqueous zinc battery. Nano Research 2022, https://doi.org/10.1007/s12274-022-4726-3.
Liu, N. N.; Wu, X.; Fan, L. S.; Gong, S.; Guo, Z. K.; Chen, A. S.; Zhao, C. Y.; Mao, Y. C.; Zhang, N. Q.; Sun, K. N. Intercalation pseudocapacitive Zn2+ storage with hydrated vanadium dioxide toward ultrahigh rate performance. Adv. Mater. 2020, 32, 1908420.
Wang, S.; Yuan, Z. S.; Zhang, X.; Bi, S. S.; Zhou, Z.; Tian, J. L.; Zhang, Q. C.; Niu, Z. Q. Non-metal ion co-insertion chemistry in aqueous Zn/MnO2 batteries. Angew. Chem., Int. Ed. 2021, 60, 7056–7060.
Hao Ren, Jin Zhao, Lan Yang, Qinghua Liang, Srinivasan Madhavi, Qingyu Yan. Inverse opal manganese dioxide constructed by few-layered ultrathin nanosheets as high-performance cathodes for aqueous zinc-ion batteries. Nano Research 2019, 12, 1347–1353.
Xiong, T.; Yu, Z. G.; Wu, H. J.; Du, Y. H.; Xie, Q. D.; Chen, J. S.; Zhang, Y. W.; Pennycook, S. J.; Lee, W. S. V.; Xue, J. M. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery. Adv. Energy Mater. 2019, 9, 1803815.
Can Huang, Qiufan Wang, Daohong Zhang, Guozhen Shen. Coupling N-doping and rich oxygen vacancies in mesoporous ZnMn2O4 nanocages toward advanced. Nano Research 2022, 15, 8118–8127.
Zhao, Q. H.; Song, A. Y.; Zhao, W. G.; Qin, R. Z.; Ding, S. X.; Chen, X.; Song, Y. L.; Yang, L. Y.; Lin, H.; Li, S. N. et al. Boosting the energy density of aqueous batteries via facile Grotthuss proton transport. Angew. Chem., Int. Ed. 2021, 60, 4169–4174.
Zhuang, Z. C.; Li, Y.; Li, Y. H.; Huang, J. Z.; Wei, B.; Sun, R.; Ren, Y. J.; Ding, J.; Zhu, J. X.; Lang, Z. Q. et al. Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides. Energy Environ. Sci. 2021, 14, 1016–1028.
Fang, G. Z.; Zhu, C. Y.; Chen, M. H.; Zhou, J.; Tang, B. Y.; Cao, X. X.; Zheng, X. S.; Pan, A. Q.; Liang, S. Q. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv. Funct. Mater. 2019, 29, 1808375.
Wang, D. H.; Wang, L. F.; Liang, G. J.; Li, H. F.; Liu, Z. X.; Tang, Z. J.; Liang, J. B.; Zhi, C. Y. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019, 13, 10643–10652.
Zhang, Y.; Deng, S. J.; Pan, G. X.; Zhang, H. Z.; Liu, B.; Wang, X. L.; Zheng, X. S.; Liu, Q.; Wang, X. L.; Xia, X. H. et al. Introducing oxygen defects into phosphate ions intercalated manganese dioxide/vertical multilayer graphene arrays to boost flexible zinc ion storage. Small Methods 2020, 4, 1900828.
Huang, J.; Tang, X.; Liu, K.; Fang, G.; He, Z.; Li, Z. Interfacial chemical binding and improved kinetics assisting stable aqueous Zn-MnO2 batteries. Mater. Today Energy 2020, 17, 100475.
Guo, S.; Liang, S. Q.; Zhang, B. S.; Fang, G. Z.; Ma, D.; Zhou, J. Cathode interfacial layer formation via in situ electrochemically charging in aqueous zinc-ion battery. ACS Nano 2019, 13, 13456–13464.
Huang, J. D.; Zeng, J.; Zhu, K. J.; Zhang, R. Z.; Liu, J. High-performance aqueous zinc-manganese battery with reversible Mn2+/Mn4+ double redox achieved by carbon coated MnOx nanoparticles. Nano-Micro Lett. 2020, 12, 110.
Zhao, S.; Han, B.; Zhang, D. T.; Huang, Q.; Xiao, L.; Chen, L. B.; Ivey, D. G.; Deng, Y. D.; Wei, W. F. Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J. Mater. Chem. A 2018, 6, 5733–5739.
Zeng, X. H.; Liu, J. T.; Mao, J. F.; Hao, J. N.; Wang, Z. J.; Zhou, S.; Ling, C. D.; Guo, Z. P. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 2020, 10, 1904163.
Xie, C. X.; Li, T. Y.; Deng, C. Z.; Song, Y.; Zhang, H. M.; Li, X. F. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energy Environ. Sci. 2020, 13, 135–143.
Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871.
Kohn, W.; Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138.
Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Chen, C.; Xu, K.; Ji, X.; Zhang, B.; Miao, L.; Jiang, J. J. Enhanced electrochemical performance by facile oxygen vacancies from lower valence-state doping for ramsdellite-MnO2. J. Mater. Chem. A 2015, 3, 12461–12467.
Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465.
Kang, Q.; Zhao, J.; Li, X.; Zhu, G. Y.; Feng, X. M.; Ma, Y. W.; Huang, W.; Liu, J. A single wire as all-inclusive fully functional supercapacitor. Nano Energy 2017, 32, 201–208.
Rong, S. P.; Zhang, P. Y.; Yang, Y. J.; Zhu, L.; Wang, J. L.; Liu, F. MnO2 framework for instantaneous mineralization of carcinogenic airborne formaldehyde at room temperature. ACS Catal. 2017, 7, 1057–1067.
Chen, Y. T.; Gu, S.; Wu, S. L.; Ma, X. X.; Hussain, I.; Sun, Z. P.; Lu, Z. G.; Zhang, K. L. Copper activated near-full two-electron Mn4+/Mn2+ redox for mild aqueous Zn/MnO2 battery. Chem. Eng. J. 2022, 450, 137923.
Zhu, C. Y.; Fang, G. Z.; Zhou, J.; Guo, J. H.; Wang, Z. Q.; Wang, C.; Li, J. Y.; Tang, Y.; Liang, S. Q. Binder-free stainless steel@Mn3O4 nanoflower composite: A high-activity aqueous zinc-ion battery cathode with high-capacity and long-cycle-life. J. Mater. Chem. A 2018, 6, 9677–9683.
Zhang, N.; Cheng, F. Y.; Liu, J. X.; Wang, L. B.; Long, X. H.; Liu, X. S.; Li, F. J.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405.
Wei, C. G.; Xu, C. J.; Li, B. H.; Du, H. D.; Kang, F. Y. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage. J. Phys. Chem. Solids 2012, 73, 1487–1491.
Li, H. F.; Liu, Z. X.; Liang, G. J.; Huang, Y.; Huang, Y.; Zhu, M. S.; Pei, Z. X.; Xue, Q.; Tang, Z. J.; Wang, Y. K. et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018, 12, 3140–3148.
Zhang, N.; Dong, Y.; Jia, M.; Bian, X.; Wang, Y. Y.; Qiu, M. D.; Xu, J. Z.; Liu, Y. C.; Jiao, L. F.; Cheng, F. Y. Rechargeable aqueous Zn-V2O5 battery with high energy density and long cycle life. ACS Energy Lett. 2018, 3, 1366–1372.
Wan, F.; Zhang, L. L.; Dai, X.; Wang, X. Y.; Niu, Z. Q.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656.
Kundu, D.; Vajargah, S. H.; Wan, L. W.; Adams, B.; Prendergast, D.; Nazar, L. F. Aqueous vs. nonaqueous Zn-ion batteries:Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892.
Hu, P.; Zhu, T.; Wang, X. P.; Wei, X. J.; Yan, M. Y.; Li, J. T.; Luo, W.; Yang, W.; Zhang, W. C.; Zhou, L. et al. Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery. Nano Lett. 2018, 18, 1758–1763.
Zhang, L. Y.; Chen, L.; Zhou, X. F.; Liu, Z. P. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy Mater. 2015, 5, 1400930.
Liu, Z.; Pulletikurthi, G.; Endres, F. A prussian blue/zinc secondary battery with a bio-ionic liquid-water mixture as electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164.
Hou, Z. G.; Zhang, X. Q.; Li, X. N.; Zhu, Y. C.; Liang, J. W.; Qian, Y. T. Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery. J. Mater. Chem. A 2017, 5, 730–738.
Zhang, N.; Cheng, F. Y.; Liu, Y. C.; Zhao, Q.; Lei, K. X.; Chen, C. C.; Liu, X. S.; Chen, J. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J. Am. Chem. Soc. 2016, 138, 12894–12901.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21935001 and 22101015), the National Key Research and Development Program of China (No. 2018YFA0702002), and the Program for Changjiang Scholars and Innovation Research Team in the University (No. IRT1205).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xia, X., Zhao, Y., Zhao, Y. et al. Mo doping provokes two electron reaction in MnO2 with ultrahigh capacity for aqueous zinc ion batteries. Nano Res. 16, 2511–2518 (2023). https://doi.org/10.1007/s12274-022-5057-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5057-0