Abstract
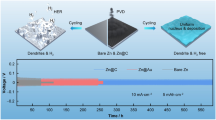
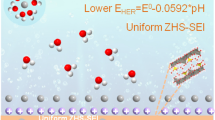
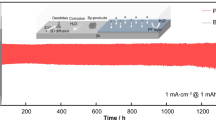
Aqueous zinc-based battery is usually plagued by serious dendrites and side reactions including Zn corrosion and water decomposition on the anode. To address the drawbacks, constructing coating layers with high conductivity and anti-catalytic effects on hydrogen evolution reaction has been considered as an efficient strategy. Herein, cheap and abundant two-dimensional (2D) conductive graphite (KS-6) coating layer with high electronic conductivity (∼ 106 S·m−1) could directly form strong bonding with Zn foil due to high zincophilicity, which correspondingly protects Zn metal from liquid electrolyte to inhibit parasitic hydrogen evolution and guide uniform Zn electrodeposition during cycling. The KS-6 layer owns a profitable charge redistribution effect to endow Zn anode with a lower nucleation energy barrier and a more uniformly distributed electric field compared with bare Zn. Therefore, such integrated Zn anode exhibits low voltage hysteresis (∼ 38 mV) and excellent cycling stability with dendrite-free behaviors (1 mA·cm−2 and 2 mA·cm−2) over 2,000 h, far outperforming many reported Zn metal anodes in aqueous systems. Encouragingly, in light of the superior Zn@KS-6 anode, VNOx powders and Prussian blue analogs Mn2Fe(CN)6 are applied as the cathode materials to assemble full batteries, which show remarkable cycling stabilities and high Coulombic efficiencies (CEs) over 200 cycles with capacity retention of 81.5% for VNOx//Zn@KS-6 battery and over 400 cycles with capacity retention of 94.6% for Mn2Fe(CN)6//Zn@KS-6 battery, respectively.

Similar content being viewed by others
References
Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456.
Ma, L.; Schroeder, M. A.; Borodin, O.; Pollard, T. P.; Ding, M. S.; Wang, C. S.; Xu, K. Realizing high zinc reversibility in rechargeable batteries. Nat. Energy 2020, 5, 743–749.
Song, M.; Tan, H.; Chao, D. L.; Fan, H. J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564.
Zheng, J. X.; Huang, Z. H.; Ming, F. W.; Zeng, Y.; Wei, B. B.; Jiang, Q.; Qi, Z. B.; Wang, Z. C.; Liang, H. F. Surface and interface engineering of Zn anodes in aqueous rechargeable Zn-ion batteries. Small, in press, https://doi.org/10.1002/smll.202200006.
Shin, J.; Lee, J.; Park, Y.; Choi, J. W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044.
Shi, Y. C.; Chen, Y.; Shi, L.; Wang, K.; Wang, B.; Li, L.; Ma, Y. M.; Li, Y. H.; Sun, Z. H.; Ali, W. et al. An overview and future perspectives of rechargeable zinc batteries. Small 2020, 16, 2000730.
Hsu, P. C.; Seol, S. K.; Lo, T. N.; Liu, C. J.; Wang, C. L.; Lin, C. S.; Hwu, Y.; Chen, C. H.; Chang, L. W.; Je, H. et al. Hydrogen bubbles and the growth morphology of ramified zinc by electrodeposition. J. Electrochem. Soc. 2008, 155, D400–D407.
Wang, R. Y.; Kirk, D. W.; Zhang, G. X. Effects of deposition conditions on the morphology of zinc deposits from alkaline zincate solutions. J. Electrochem. Soc. 2006, 153, C357–C364.
Ma, L.; Schroeder, M. A.; Pollard, T. P.; Borodin, O.; Ding, M. S.; Sun, R. M.; Cao, L. S.; Ho, J.; Baker, D. R.; Wang, C. S. et al. Critical factors dictating reversibility of the zinc metal anode. Energy Environ. Mater. 2020, 3, 516–521.
Yufit, V.; Tariq, F.; Eastwood, D. S.; Biton, M.; Wu, B.; Lee, P. D.; Brandon, N. P. Operando visualization and multi-scale tomography studies of dendrite formation and dissolution in zinc batteries. Joule 2019, 3, 485–502.
Cogswell, D. A. Quantitative phase-field modeling of dendritic electrodeposition. Phys. Rev. E 2015, 92, 011301.
Matsushita, M.; Sano, M.; Hayakawa, Y.; Honjo, H.; Sawada, Y. Fractal structures of zinc metal leaves grown by electrodeposition. Phys. Rev. Lett. 1984, 53, 286–289.
Wheeler, A. A.; Murray, B. T.; Schaefer, R. J. Computation of dendrites using a phase field model. Phys. D 1993, 66, 243–262.
Ji, X. L.; Jiang, H. A perspective: The technical barriers of Zn metal batteries. Chem. Res. Chin. Univ. 2020, 36, 55–60.
Diggle, J. W.; Despic, A. R.; Bockris, J. O. The mechanism of the dendritic electrocrystallization of zinc. J. Eectrochem. Soc. 1969, 116, 1503–1514.
Zhang, Y. M.; Wu, Y. T.; You, W. Q.; Tian, M. K.; Huang, P. W.; Zhang, Y. F.; Sun, Z. J.; Ma, Y.; Hao, T. Q.; Liu, N. Deeply rechargeable and hydrogen-evolution-suppressing zinc anode in alkaline aqueous electrolyte. Nano Lett. 2020, 20, 4700–4707.
Blanc, L. E.; Kundu, D.; Nazar, L. F. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020, 4, 771–799.
Uekawa, N.; Yamashita, R.; Jun Wu, Y.; Kakegawa, K. Effect of alkali metal hydroxide on formation processes of zinc oxide crystallites from aqueous solutions containing Zn(OH)42− ions. Phys. Chem. Chem. Phys. 2004, 6, 442–446.
Cui, B. F.; Han, X. P.; Hu, W. B. Micronanostructured design of dendrite-free zinc anodes and their applications in aqueous zinc-based rechargeable batteries. Small Struct. 2021, 2, 2000128.
Zhang, Q.; Luan, J. Y.; Tang, Y. G.; Ji, X. B.; Wang, H. Y. Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2020, 59, 13180–13191.
Zhang, N. N.; Huang, S.; Yuan, Z. S.; Zhu, J. C.; Zhao, Z. F.; Niu, Z. Q. Direct self-assembly of MXene on Zn anodes for dendrite-free aqueous zinc-ion batteries. Angew. Chem., Int. Ed. 2021, 60, 2861–2865.
Yang, Q.; Guo, Y.; Yan, B. X.; Wang, C. D.; Liu, Z. X.; Huang, Z. D.; Wang, Y. K.; Li, Y. R.; Li, H. F.; Song, L. et al. Hydrogen-substituted graphdiyne ion tunnels directing concentration redistribution for commercial-grade dendrite-free zinc anodes. Adv. Mater. 2020, 32, 2001755.
Xie, X. S.; Liang, S. Q.; Gao, J. W.; Guo, S.; Guo, J. B.; Wang, C.; Xu, G. Y.; Wu, X. W.; Chen, G.; Zhou, J. Manipulating the iontransfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ. Sci. 2020, 13, 503–510.
Guo, W.; Zhang, Y.; Tong, X.; Wang, X.; Zhang, L.; Xia, X.; Tu, J. Multifunctional tin layer enabled long-life and stable anode for aqueous zinc-ion batteries. Mater. Today Energy 2021, 20, 100675.
Yin, Y. B.; Wang, S. N.; Zhang, Q.; Song, Y.; Chang, N. N.; Pan, Y. W.; Zhang, H. M.; Li, X. F. Dendrite-free zinc deposition induced by tin-modified multifunctional 3D host for stable zinc-based flow battery. Adv. Mater. 2020, 32, 1906803.
Zheng, J. X.; Huang, Z. H.; Zeng, Y.; Liu, W. Q.; Wei, B. B.; Qi, Z. B.; Wang, Z. C.; Xia, C.; Liang, H. F. Electrostatic shielding regulation of magnetron sputtered Al-based alloy protective coatings enables highly reversible zinc anodes. Nano Lett. 2022, 22, 1017–1023.
Li, C. P.; Shi, X. D.; Liang, S. Q.; Ma, X. M.; Han, M. M.; Wu, X. W.; Zhou, J. Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem. Eng. J. 2020, 379, 122248.
Zhang, Q.; Luan, J. Y.; Fu, L.; Wu, S. A.; Tang, Y. G.; Ji, X. B.; Wang, H. Y. The three-dimensional dendrite-free zinc anode on a copper mesh with a zinc-oriented polyacrylamide electrolyte additive. Angew. Chem., Int. Ed. 2019, 58, 15841–15847.
Zeng, Y. X.; Zhang, X. Y.; Qin, R. F.; Liu, X. Q.; Fang, P. P.; Zheng, D. Z.; Tong, Y. X.; Lu, X. H. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv. Mater. 2019, 31, 1903675.
Qin, R. Z.; Wang, Y. T.; Zhang, M. Z.; Wang, Y.; Ding, S. X.; Song, A. Y.; Yi, H. C.; Yang, L. Y.; Song, Y. L.; Cui, Y. H. et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021, 80, 105478.
Ma, L. T.; Li, Q.; Ying, Y. R.; Ma, F. X.; Chen, S. M.; Li, Y. Y.; Huang, H. T.; Zhi, C. Y. Toward practical high-areal-capacity aqueous zinc-metal batteries: Quantifying hydrogen evolution and a solid-ion conductor for stable zinc anodes. Adv. Mater. 2021, 33, 2007406.
Zhuang, Z. C.; Li, Y.; Huang, J. Z.; Li, Z. L.; Zhao, K. N.; Zhao, Y. L.; Xu, L.; Zhou, L.; Moskaleva, L. V.; Mai, L. Q. Sisyphus effects in hydrogen electrochemistry on metal silicides enabled by silicene subunit edge. Sci. Bull. 2019, 64, 617–624.
Zhang, S. L.; Ao, X.; Huang, J.; Wei, B.; Zhai, Y. L.; Zhai, D.; Deng, W. Q.; Su, C. L.; Wang D. S.; Li, Y. D. Isolated single-atom Ni-N5 catalytic site in hollow porous carbon capsules for efficient lithium-sulfur batteries. Nano Lett. 2021, 21, 9691–9698.
Zhuang, Z. C.; Kang, Q.; Wang, D. S.; Li, Y. D. Single-atom catalysis enables long-life, high-energy lithium-sulfur batteries. Nano Res. 2020, 13, 1856–1866.
Xie, F. X.; Li, H.; Wang, X. S.; Zhi, X.; Chao, D. L.; Davey, K.; Qiao, S. Z. Mechanism for zincophilic sites on zinc-metal anode hosts in aqueous batteries. Adv. Energy Mater. 2021, 11, 2003419.
Yuan, D.; Zhao, J.; Ren, H.; Chen, Y. Q.; Chua, R.; Jie, E. T. J.; Cai, Y.; Edison, E.; Manalastas, W. Jr.; Wong, M. W. et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew. Chem., Int. Ed. 2021, 60, 7213–7219.
Liang, P. C.; Yi, J.; Liu, X. Y.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y. Y.; Wang, Y. G.; Xia, Y. Y.; Zhang, J. J. Highly reversible Zn anode enabled by controllable formation of nucleation sites for Zn-based batteries. Adv. Funct. Mater. 2020, 30, 1908528.
Hao, J. N.; Li, X. L.; Zhang, S. L.; Yang, F. H.; Zeng, X. H.; Zhang, S.; Bo, G. Y.; Wang, C. S.; Guo, Z. P. Designing dendrite-free zinc anodes for advanced aqueous zinc batteries. Adv. Funct. Mater. 2020, 30, 2001263.
Han, C.; Li, W. J.; Liu, H. K.; Dou, S. X.; Wang, J. Z. Principals and strategies for constructing a highly reversible zinc metal anode in aqueous batteries. Nano Energy 2020, 74, 104880.
Yang, Q.; Huang, Z. D.; Li, X. L.; Liu, Z. X.; Li, H. F.; Liang, G. J.; Wang, D. H.; Huang, Q.; Zhang, S. J.; Chen, S. et al. A wholly degradable, rechargeable Zn-Ti3C2 MXene capacitor with superior anti-self-discharge function. ACS Nano 2019, 13, 8275–8283.
Yuksel, R.; Buyukcakir, O.; Seong, W. K.; Ruoff, R. S. Metal-organic framework integrated anodes for aqueous zinc-ion batteries. Adv. Energy Mater. 2020, 10, 1904215.
Wang, Z.; Huang, J. H.; Guo, Z. W.; Dong, X. L.; Liu, Y.; Wang, Y. G.; Xia, Y. Y. A metal-organic framework host for highly reversible dendrite-free zinc metal anodes. Joule 2019, 3, 1289–1300.
Wang, C. L.; Sun, L. S.; Li, M. X.; Zhou, L.; Cheng, Y.; Ao, X.; Zhang, X. Y.; Wang, L. M.; Tian, B. B.; Fan, H. J. Aqueous Zn2+/Na+ dual-salt batteries with stable discharge voltage and high columbic efficiency by systematic electrolyte regulation. Sci. China Chem. 2022, 65, 399–407.
Cao, F. Q.; Wu, B. H.; Li, T. Y.; Sun, S. T.; Jiao, Y. C.; Wu, P. Y. Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance. Nano Res. 2022, 15, 2030–2039.
Yang, X. Z.; Li, W. P.; Lv, J. Z.; Sun, G. J.; Shi, Z. X.; Su, Y. W.; Lian, X. Y.; Shao, Y. Y.; Zhi, A. M.; Tian, X. Z. et al. In situ separator modification via CVD-derived N-doped carbon for highly reversible Zn metal anodes. Nano Res., in press, https://doi.org/10.1007/s12274-021-3957-z.
Gunawardhana, N.; Park, G. J.; Thapa, A. K.; Dimov, N.; Sasidharan, M.; Nakamura, H.; Yoshio, M. Performance of a graphite (KS-6)/MoO3 energy storing system. J. Power Sources 2012, 203, 257–261.
Wang, R. Y.; Wang, J.; Qiu, T.; Chen, L. P.; Liu, H. M.; Yang, W. S. Effects of different carbon sources on the electrochemical properties of Li4Ti5O12/C composites. Electrochim. Acta 2012, 70, 84–90.
Lin, H. B.; Huang, W. Z.; Rong, H. B.; Hu, J. N.; Mai, S. W.; Xing, L. D.; Xu, M. Q.; Li, X. P.; Li, W. S. Surface natures of conductive carbon materials and their contributions to charge/discharge performance of cathodes for lithium ion batteries. J. Power Sources 2015, 287, 276–282.
Sun, L. S.; Wang, C. L.; Wang, X. X.; Wang, L. M. Morphology evolution and control of Mo-polydopamine coordination complex from 2D single nanopetal to hierarchical microflowers. Small 2018, 14, 1800090.
Leung, P. K.; Ponce-de-León, C.; Low, C. T. J.; Walsh, F. C. Zinc deposition and dissolution in methanesulfonic acid onto a carbon composite electrode as the negative electrode reactions in a hybrid redox flow battery. Electrochim. Acta 2011, 56, 6536–6546.
Banik, S. J.; Akolkar, R. Suppressing dendrite growth during zinc electrodeposition by PEG-200 additive. J. Electrochem. Soc. 2013, 160, D519–D523.
Liu, Z. H.; Du, Y.; Zhang, P. F.; Zhuang, Z. C.; Wang, D. S. Bringing catalytic order out of chaos with nitrogen-doped ordered mesoporous carbon. Matter 2021, 4, 3161–3194.
Cheng, Y.; Sun, Y.; Chu, C. T.; Chang, L. M.; Wang, Z. M.; Zhang, D. Y.; Liu, W. Q.; Zhuang, Z. C.; Wang, L. M. Stabilizing effects of atomic Ti doping on high-voltage high-nickel layered oxide cathode for lithium-ion rechargeable batteries. Nano Res. 2022, 15, 4091–4099.
Zhuang, Z. C.; Li, Y. H.; Yu, R. H.; Xia, L. X.; Yang, J. R.; Lang, Z. Q.; Zhu, J. X.; Huang, J. Z.; Wang, J. O.; Wang, Y. et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat. Catal. 2022, 5, 300–310.
Gao, Y.; Liu, Y. W.; Chen, S. L. A theoretical consideration of ion size effects on the electric double layer and voltammetry of nanometer-sized disk electrodes. Faraday Discuss. 0016, 193, 251–263.
Guo, S.; Liang, S. Q.; Zhang, B. S.; Fang, G. Z.; Ma, D.; Zhou, J. Cathode interfacial layer formation via in situ electrochemically charging in aqueous zinc-ion battery. ACS Nano 2019, 13, 13456–13464.
Zhuang, Z. C.; Li, Y.; Li, Y. H.; Huang, J. Z.; Wei, B.; Sun, R.; Ren, Y. J.; Ding, J.; Zhu, J. X.; Lang, Z. Q. et al. Atomically dispersed nonmagnetic electron traps improve oxygen reduction activity of perovskite oxides. Energy Environ. Sci. 2021, 14, 1016–1028.
Abdallah, M. Ethoxylated fatty alcohols as corrosion inhibitors for dissolution of zinc in hydrochloric acid. Corros. Sci. 2003, 45, 2705–2716.
Tan, H.; Zhou, Y.; Qiao, S. Z.; Fan, H. J. Metal organic framework (MOF) in aqueous energy devices. Mater. Today 2021, 48, 270–284.
Ballesteros, J. C.; Díaz-Arista, P.; Meas, Y.; Ortega, R.; Trejo, G. Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochim. Acta 2007, 52, 3686–3696.
Yang, Q.; Liang, G. J.; Guo, Y.; Liu, Z. X.; Yan, B. X.; Wang, D. H.; Huang, Z. D.; Li, X. L.; Fan, J.; Zhi, C. Y. Do zinc dendrites exist in neutral zinc batteries: A developed electrohealing strategy to in situ rescue in-service batteries. Adv. Mater. 2019, 31, 1903778.
Zheng, J. X.; Cao, Z.; Ming, F. W.; Liang, H. F.; Qi, Z. B.; Liu, W. Q.; Xia, C.; Chen, C. X.; Cavallo, L.; Wang, Z. C. et al. Preferred orientation of TiN coatings enables stable zinc anodes. ACS Energy Lett. 2021, 7, 197–203.
Pei, A.; Zheng, G. Y.; Shi, F. F.; Li, Y. Z.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2017YFE0198100), the National Natural Science Foundation of China (No. 21975250), the Open Funds of the State Key Laboratory of Rare Earth Resource Utilization (Nos. RERU2021004 and RERU2021006), and the Open Project Program of Key Laboratory of Preparation and Application of Environmental Friendly Materials (Jilin Normal University), Ministry of Education, China (No. 2021007).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4458_MOESM1_ESM.pdf
Anti-catalytic and zincophilic layers integrated zinc anode towards efficient aqueous batteries for ultra-long cycling stability
Rights and permissions
About this article
Cite this article
Wang, C., Gao, Y., Sun, L. et al. Anti-catalytic and zincophilic layers integrated zinc anode towards efficient aqueous batteries for ultra-long cycling stability. Nano Res. 15, 8076–8082 (2022). https://doi.org/10.1007/s12274-022-4458-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4458-4