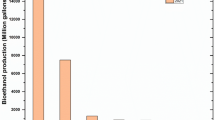
Abstract
One of the limiting stages in the second-generation (2G) bioethanol production process is enzymatic hydrolysis due to the low availability of cellulase enzymes and their high cost. The enzymatic hydrolysis of pretreated sugarcane bagasse is optimized (Box-Behnken design) using an enzymatic extract of Aspergillus niger ITV02 cellulases produced in a bioreactor to obtain glucose. The maximum concentration of glucose (49 g/L) and conversion (70%) was reached using sugarcane bagasse with acid-alkaline pretreatment (SB-AAL) under optimal conditions of enzyme load (18 FPU/g), the concentration of substrate (84.35 g/L) and hydrolysis time (48 h). The enzymatic hydrolysate of A. niger ITV02 from SB-AAL was subsequently fermented by Saccharomyces cerevisiae ITV01 for bioethanol 2G production, reaching a yield (Yp/s) of 0.46 g/g, an efficiency of 90.77%, and 1.6 g/Lh productivity and reaching important values of parameters required for bioethanol 2G production to scale process. The present study shows that cellulases produced by A. niger ITV02 using an available and low-cost substrate such as sugarcane bagasse is efficient in the enzymatic hydrolysis of lignocellulosic residues for bioethanol 2G production.






Similar content being viewed by others
References
Tvaronaviciene M, Baublys J, Raudeliuniene J, Jatautaite D (2020) Global energy consumption peculiarities and energy sources: role of renewables. In Energy Transformation Towards Sustainability. Elsevier, pp 1–49. https://doi.org/10.1016/B978-0-12-817688-7.00001-X
Gielen D, Boshell F, Saygin D, Bazilian MD, Wagner N, Gorini R (2019) The role of renewable energy in the global energy transformation. Energ Strat Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
Carrillo-Nieves D, Alanís MJR, de la Cruz QR, Ruiz HA, Iqbal HM, Parra-Saldívar R (2019) Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew Sustain Energy Rev 102:63–74. https://doi.org/10.1016/j.rser.2018.11.031
Ruiz HA, Martínez A, Vermerris W (2016) Bioenergy potential, energy crops, and biofuel production in Mexico. Bioenerg Res 9:981–984. https://doi.org/10.1007/s12155-016-9802-7
Polaris Market Research (2022) Digital Transformation Market Size Global Report, 2022–2030. Global Market Research Reports and Consulting. https://www.polarismarketresearch.com/industry-analysis/digital-transformation-market. Accessed 11 Jun 2023
Antunes FAF, Rajan K, Djioleu A, Rocha TM, Brumano LP, de Souza Melo YC, da Silva SS (2022) Sustainable second-generation ethanol production from switchgrass biomass via co-fermentation of pentoses and hexoses using novel wild yeasts. BioEnerg Res 15(2):1157–1168. https://doi.org/10.1007/s12155-021-10302-3
Infanzón-Rodríguez MI, del Moral S, Castro-Martínez C, Cano-Sarmiento C, Gómez-Rodríguez J, Aguilar-Uscanga MG (2023) Multi-response optimization using the desirability function of exoglucanases, endoglucanases and β-glucosidases production by Aspergillus niger ITV-02 from delignified sugarcane bagasse. Sugar Tech 25(1):86–98. https://doi.org/10.1007/s12355-022-01191-7
de Almeida MA, Colombo R (2023) Production chain of first-generation sugarcane bioethanol: characterization and value-added application of wastes. Bioenergy Res 16(2):924–939. https://doi.org/10.1007/s12155-021-10301-4
Meunchang S, Panichsakpatana S, Weaver RW (2005) Co-composting of filter cake and bagasse; by-products from a sugar mill. Bioresour Technol 96(4):437–442. https://doi.org/10.1016/j.biortech.2004.05.024
Menandro LMS, Cantarella H, Franco HCJ, Kölln OT, Pimenta MTB, Sanches GM, Carvalho JLN (2017) Comprehensive assessment of sugarcane straw: implications for biomass and bioenergy production. Biofuels Bioprod Biorefin 11(3):488–504. https://doi.org/10.1002/bbb.1760
Panakkal EJ, Sriariyanun M, Ratanapoompinyo J, Yasurin P, Cheenkachorn K, Rodiahwati W, Tantayotai P (2022) Influence of sulfuric acid pretreatment and inhibitor of sugarcane bagasse on the production of fermentable sugar and ethanol. Appl Sci Eng Prog 15(1). https://doi.org/10.14416/j.asep.2021.07.006
Niju S, Swathika M (2019) Delignification of sugarcane bagasse using pretreatment strategies for bioethanol production. Biocatal Agric Biotechnol 20:101263. https://doi.org/10.1016/j.bcab.2019.101263
Hu M, Yu H, Li YU, Li AO, Cai Q, Liu P, Xia T (2018) Distinct polymer extraction and cellulose DP reduction for complete cellulose hydrolysis under mild chemical pretreatments in sugarcane. Carbohyd Polym 202:434–443. https://doi.org/10.1016/j.carbpol.2018.08.039
Fu Y, Gao H, Yu H, Yang Q, Peng H, Liu P, Wang Y (2022) Specific lignin and cellulose depolymerization of sugarcane bagasse for maximum bioethanol production under optimal chemical fertilizer pretreatment with hemicellulose retention and liquid recycling. Renew Energ 200:1371–1381. https://doi.org/10.1016/j.renene.2022.10.049
Christopher M, Mathew AK, Kumar MK, Pandey A, Sukumaran RK (2017) A biorefinery-based approach for the production of ethanol from enzymatically hydrolysed cotton stalks. Bioresour Technol 242:178–183. https://doi.org/10.1016/j.biortech.2017.03.190
Saini S, Kuhad RC, Sharma KK (2023) Valorization of rice straw biomass for co-production of bioethanol, biopesticide and biofertilizer following an eco-friendly biorefinery process. Process Saf Environ Prot 173:823–836. https://doi.org/10.1016/j.psep.2023.03.044
Deshavath NN, Sahoo SK, Panda MM, Mahanta S, Goutham DSN, Goud VV, Jetty A (2018) The cost-effective stirred tank reactor for cellulase production from alkaline-pretreated agriculture waste biomass. In: Ghosh S (eds) Utilization and Management of Bioresources. Springer, Singapore, pp 25–35. https://doi.org/10.1007/978-981-10-5349-8_3
Singh S, Mangla J, Singh S (2021) Evaluation of Aspergillus fumigatus NTCC1222 as a source of enzymes for detergent industry. Res Environ Sustain 5:100030. https://doi.org/10.1016/j.resenv.2021.100030
Boggione MJ, Allasia MB, Aguilar CN, Farruggia B (2020) Valorization of corn cob for the obtention and purification of endoglucanase produced by SSF. Process Biochem 88:106–112. https://doi.org/10.1016/j.procbio.2019.09.026
Liu L, Huang WC, Liu Y, Li M (2021) Diversity of cellulolytic microorganisms and microbial cellulases. Int Biodeteriorat Biodegradation 163:105277. https://doi.org/10.1016/j.ibiod.2021.105277
Sharma B, Larroche C, Dussap CG (2020) Comprehensive assessment of 2G bioethanol production. Bioresour Technol 313:123630. https://doi.org/10.1016/j.biortech.2020.123630
Arnau J, Yaver D, Hjort CM (2020) Strategies and challenges for the development of industrial enzymes using fungal cell factories. In: Nevalainen H (eds) Grand Challenges in Fungal Biotechnology. Grand Challenges in Biology and Biotechnology, pp 179–210. Springer, Cham. https://doi.org/10.1007/978-3-030-29541-7_7
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383. https://doi.org/10.1016/S0734-9750(00)00041-0
Infanzón-Rodríguez MI, Ragazzo-Sánchez JA, Del Moral S, Calderón-Santoyo M, Gutiérrez-Rivera B, Aguilar-Uscanga MG (2020) Optimization of cellulase production by Aspergillus niger ITV 02 from sweet sorghum bagasse in submerged culture using a Box-Behnken design. Sugar Tech 22(2):266–273. https://doi.org/10.1007/s12355-019-00765-2
Pérez-Salazar YI, Peña-Montes C, del Moral S, Aguilar-Uscanga MG (2022) Cellulases production from Aspergillus niger-ITV-02 using corn lignocellulosic residues. Rev Mex de Ing Quím 21:2. https://doi.org/10.24275/rmiq/Alim2772
Michelin M, Mota AM, Silva DP, Ruzene DS, Vicente AA, Teixeira JA (2019) Production of biomass-degrading enzymes by Trichoderma reesei using liquid hot water-pretreated corncob in different conditions of oxygen transfer. Bioenerg Res 12(3):583–592. https://doi.org/10.1007/s12155-019-09991-8
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker DLAP (2008) Determination of structural carbohydrates and lignin in biomass. Lab anal proced. https://www.nrel.gov/biomass/pdfs/42618.pdf
Miller GL (1959) Modified DNS method for reducing sugars. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Singhania RR, Sukumaran RK, Rajasree KP, Mathew A, Gottumukkala L, Pandey A (2011) Properties of a major β-glucosidase-BGL1 from Aspergillus niger NII-08121 expressed differentially in response to carbon sources. Process Biochem 46(7):1521–1524. https://doi.org/10.1016/j.procbio.2011.04.006
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268. https://doi.org/10.1351/pac198759020257
Aftab MN, Iqbal I, Riaz F, Karadag A, Tabatabaei M (2019) Different pretreatment methods of lignocellulosic biomass for use in biofuel production. In: Abomohra AEF (ed) Biomass for bioenergy-recent trends and future challenges. Intechopen, London, pp 15–38. https://doi.org/10.5772/intechopen.84995
Gundupalli MP, Sriariyanun M (2022) Recent trends and updates for chemical pretreatment of lignocellulosic biomass. Appl Sci Eng Prog 16:5842. https://doi.org/10.14416/j.asep.2022.03.002
Jose D, Kitiborwornkul N, Sriariyanun M, Keerthi K (2022) A review on chemical pretreatment methods of lignocellulosic biomass: recent advances and progress. Appl Sci Eng Prog 15:6210. https://doi.org/10.14416/j.asep.2022.08.001
Zhang R, Hu Z, Wang Y, Hu H, Li F, Li M, Peng L (2023) Single-molecular insights into the breakpoint of cellulose nanofibers assembly during saccharification. Nat Commun 14:1100. https://doi.org/10.1038/s41467-023-36856-8
Gupta R, Aswal VK, Saini JK (2020) Sequential dilute acid and alkali deconstruction of sugarcane bagasse for improved hydrolysis: insight from small angle neutron scattering (SANS). Renew Energ 147:2091–2101. https://doi.org/10.1016/j.renene.2019.10.003
Abdella A, Segato F, Wilkins MR (2020) Optimization of process parameters and fermentation strategy for xylanase production in a stirred tank reactor using a mutant Aspergillus nidulans strain. Biotechnol Rep 26:e00457. https://doi.org/10.1016/j.btre.2020.e00457
Buffo MM, Esperança MN, Farinas CS, Badino AC (2020) Relation between pellet fragmentation kinetics and cellulolytic enzymes production by Aspergillus niger in conventional bioreactor with different impellers. Enzyme Microb Technol 139:109587. https://doi.org/10.1016/j.enzmictec.2020.109587
Veiter L, Rajamanickam V, Herwig C (2018) The filamentous fungal pellet—relationship between morphology and productivity. Appl Microbiol Biotechnol 102(7):2997–3006. https://doi.org/10.1007/s00253-018-8818-7
Fadzilah K, Mashitah MD (2010) Cellulases production in palm oil mill effluent: Effect of aeration and agitation. J Appl Sci 10(24):3307–3312. https://scialert.net/abstract/?doi=jas.2010.3307.3312
Sukumaran RK, Singhania RR, Mathew GM, Pandey A (2009) Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew energy 34(2):421–424. https://doi.org/10.1016/j.renene.2008.05.008
Rabelo SC, Andrade RR, Maciel Filho R, Costa AC (2014) Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 136:349–357. https://doi.org/10.1016/j.fuel.2014.07.033
Delfin-Ruíz ME, Calderón-Santoyo M, Ragazzo-Sánchez JA, Gómez-Rodríguez J, Aguilar-Uscanga MG (2021) Ethanol production from enzymatic hydrolysates optimized of Agave tequilana Weber var. azul and Agave karwinskii bagasses. Bioenergy Res 14(3):785–798. https://doi.org/10.1007/s12155-020-10196-7
Nochebuena-Morando LE, Dominguez GC, Lopez Zamora L, Aguilar Uscanga MG (2014) Statistical optimization of alkaline hydrogen peroxide pretreatment of sugarcane bagasse for enzymatic saccharification with Tween 80 using response surface methodology. Biomass Conv Bioref 4:15–23. https://doi.org/10.1007/s13399-013-0091-5
Castañón-Rodríguez JF, Welti-Chanes J, Palacios AJ, Torrestiana-Sanchez B, Ramírez de León JA, Velázquez G, Aguilar-Uscanga MG (2015) Influence of high-pressure processing and alkaline treatment on sugarcane bagasse hydrolysis. CyTA-J Food 13(4):613–620. https://doi.org/10.1080/19476337.2015.1029523
Partida-Sedas G, Montes-García N, Carvajal-Zarrabal O, López-Zamora L, Gómez-Rodríguez J, Aguilar-Uscanga MG (2017) Optimization of hydrolysis process to obtain fermentable sugars from sweet sorghum bagasse using a Box-Behnken design. Sugar Tech 19(3):317–325. https://doi.org/10.1007/s12355-016-0461-y
Gottschalk LMF, Oliveira RA, da Silva Bon EP (2010) Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem Eng J 51(1–2):72–78. https://doi.org/10.1016/j.bej.2010.05.003
Infanzón-Rodríguez MI, Ragazzo-Sánchez JA, Del Moral S, Calderón-Santoyo M, Aguilar-Uscanga MG (2021) Enzymatic hydrolysis of lignocellulosic biomass using native cellulase produced by Aspergillus niger ITV02 under liquid state fermentation. Biotechnol Appl Biochem 69(1):198–208. https://doi.org/10.1002/bab.2097
Prajapati BP, Jana UK, Suryawanshi RK, Kango N (2020) Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renew energy 152:653–663. https://doi.org/10.1016/j.renene.2020.01.063
Acknowledgements
The authors thank the National Council of Science and Technology (CONACYT) for the grant-supported postdoctoral fellowship for M. I. Infanzón-Rodriguez and also Juan Ernesto Gomez-Aguilar for the critical review of this manuscript.
Author information
Authors and Affiliations
Contributions
M.I. Infanzón-Rodriguez (MIIR) and M.G. Aguilar-Uscanga (MGAU) contributed to the study conception and design. Material preparation and experiment operation were performed by MIIR, Sandra del Moral (SdM), and MGAU. Data collection and analysis were performed by MIIR, SdM, MGAU, Faife-Pérez E (FPE), and Javier Gomez-Rodriguez (JGR). The draft of the manuscript was written and commented on by all authors on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Research Involving Human and Animal Participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Infanzón-Rodríguez, M.I., del Moral, S., Gómez-Rodríguez, J. et al. Second-Generation Bioethanol Production and Cellulases of Aspergillus niger ITV02 Using Sugarcane Bagasse as Substrate. Bioenerg. Res. 17, 160–172 (2024). https://doi.org/10.1007/s12155-023-10640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10640-4