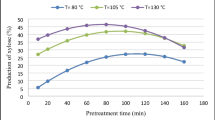
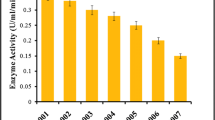
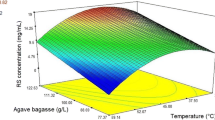
Abstract
Multi-objective optimization using the desirability function is a useful tool, which allows you to select the better conditions to maximize multiple objectives/responses simultaneously. Enzymatic hydrolysis of lignocellulosic residues requires synergistic action of three cellulase enzymes: exoglucanases (FPase), endoglucanases (CMCase) and β-glucosidase (BGL), to the complete conversion of lignocellulosic residue to fermentable sugars. In this work the production of cellulases (FPase, CMCase and BGL) in Aspergillus niger ITV-02 using delignified sugarcane bagasse (DSB), was optimized using a Box–Behnken design, where independent variables were: DSB concentration, Tween 80 concentration and pH. The multi-objective optimization method was applied to maximize the three-enzymatic activities (FPase, CMCase and BGL) simultaneously and find an optimal value between simple and multi-objective optimization. The optimal parameters were 20.69 g/L of DSB, 0.24% v/v of Tween 80 and pH 5.67, obtaining an activity FPase 0.42 U/mL, CMCase 0.35 U/mL and BGL 10.23 U/mL. The activity FPase, CMCase and BGL after optimization increased 47.62, 65 and 47.4%, respectively, with respect to the activity before optimization. Multi-objective or multi-response optimization using the desirability function (d) allowed to obtain an enzymatic extract rich in exoglucanase, endoglucanase and β-glucosidase activity by A. niger ITV-02 using DSB, in order to improve the enzymatic hydrolysis process of lignocellulosic residues and thus the production of 2G biofuels.





Similar content being viewed by others
References
Baskaran, R., and C. Krishnan. 2020. Enhanced production of cellulase from a novel strain Trichoderma gamsii M501 through response surface methodology and its application in biomass saccharification. Process Biochemistry 99: 48–60. https://doi.org/10.1016/j.procbio.2020.08.006.
Castañón-Rodríguez, J.F., J. Welti-Chanes, A.J. Palacios, B. Torrestiana-Sanchez, J.A. Ramírez De León, G. Velázquez, and M.G. Aguilar-Uscanga. 2015. Influence of high pressure processing and alkaline treatment on sugarcane bagasse hydrolysis. CyTA-Journal of Food 13 (4): 613–620.
Chauve, M., H. Mathis, D. Huc, D. Casanave, F. Monot, and N.L. Ferreira. 2010. Comparative kinetic analysis of two fungal β-glucosidases. Biotechnology for Biofuels 3 (1): 1–8. https://doi.org/10.1186/1754-6834-3-3.
de Almeida, M.N., D.L. Falkoski, V.M. Guimarães, and S.T. de Rezende. 2019. Study of Gamba grass as carbon source for cellulase production by Fusarium verticillioides and its application on sugarcane bagasse saccharification. Industrial Crops and Products 133: 33–43. https://doi.org/10.1016/j.indcrop.2019.03.008.
De Castro, A.M., K.C.N.R. Pedro, J.C. Da Cruz, M.C. Ferreira, S.G.F. Leite, and N. Pereira. 2010. Trichoderma harzianum IOC-4038: A promising strain for the production of a cellulolytic complex with significant β-glucosidase activity from sugarcane bagasse cellulignin. Applied Biochemistry and Biotechnology 162 (7): 2111–2122. https://doi.org/10.1007/s12010-010-8986-0.
Derringer, G., and R. Suich. 1980. Simultaneous optimization of several response variables. Journal of Quality Technology 12 (4): 214–219. https://doi.org/10.1080/00224065.1980.11980968.
Ghose, T.K. 1987. Measurement of cellulose activities. Pure and Applied Chemistry 59 (2): 257–268. https://doi.org/10.1351/pac198759020257.
Gottschalk, L.M.F., R.A. Oliveira, and E.P. da Silva Bon. 2010. Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochemical Engineering Journal 51 (1–2): 72–78. https://doi.org/10.1016/j.bej.2010.05.003.
Goukanapalle, P.K.R., D.K. Kanderi, G. Rajoji, B.S. Shanthi Kumari, and R.R. Bontha. 2020. Optimization of cellulase production by a novel endophytic fungus Pestalotiopsis microspora TKBRR isolated from Thalakona forest. Cellulose 27: 6299–6316. https://doi.org/10.1007/s10570-020-03220-8.
Hossain, S.M.Z., A. Alnoaimi, S.A. Razzak, H. Ezuber, N. Al-Bastaki, M. Safdar, S. Alkaabi, and M.M. Hossain. 2018. Multiobjective optimization of microalgae (Chlorella sp) growth in a photobioreactor using Box-Behnken design approach. The Canadian Journal of Chemical Engineering 96 (9): 1903–1910. https://doi.org/10.1002/cjce.23168.
Infanzón-Rodríguez, M.I., J.A. Ragazzo-Sánchez, S. del Moral, M. Calderón-Santoyo, B. Gutiérrez-Rivera, and M.G. Aguilar-Uscanga. 2020. Optimization of cellulase production by Aspergillus niger ITV 02 from sweet sorghum bagasse in submerged culture using a Box-Behnken design. Sugar Tech 22 (2): 266–273. https://doi.org/10.1007/s12355-019-00765-2.
Infanzón-Rodríguez, M.I., J.A. Ragazzo-Sánchez, S. Del Moral, M. Calderón-Santoyo, and M.G. Aguilar-Uscanga. 2021. Enzymatic hydrolysis of lignocellulosic biomass using native cellulase produced by Aspergillus niger ITV02 under liquid state fermentation. Biotechnology and Applied Biochemistry 69 (1): 198–208. https://doi.org/10.1002/bab.2097.
Jampala, P, S, Tadikamalla, M. Preethi, S. Ramanujam, K, B, Uppuluri. 2017. Concurrent production of cellulase and xylanase from Trichoderma reesei NCIM 1186 enhancement of production by desirability-based multi-objective method. 3 Biotech, 7(1): 1–4. https://doi.org/10.1007/s13205-017-0607-y
Kalantzi, S., D. Kekos, and D. Mamma. 2019. Bioscouring of cotton fabrics by multienzyme combinations: application of box-behnken design and desirability function. Cellulose 26 (4): 2771–2790. https://doi.org/10.1007/s10570-019-02272-9.
Kalsoom, R., S. Ahmed, M. Nadeem, S. Chohan, and M. Abid. 2019. Biosynthesis and extraction of cellulase produced by Trichoderma on agro-wastes. International Journal of Environmental Science and Technology 16 (2): 921–928. https://doi.org/10.1007/s13762-018-1717-8.
Kaschuk, J.J., D. de Alexandria Santos, E. Frollini, F. Canduri, and A.L.M. Porto. 2020. Influence of pH, temperature, and sisal pulp on the production of cellulases from Aspergillus sp CBMAI 1198 and hydrolysis of cellulosic materials with different hemicelluloses content, crystallinity, and average molar mass. Biomass Conversion and Biorefinery 10 (2): 483–494. https://doi.org/10.1007/s13399-019-00440-2.
Kumar, A. 2020. Aspergillus nidulans: A potential resource of the production of the native and heterologous enzymes for industrial applications. International Journal of Microbiology. https://doi.org/10.1155/2020/8894215.
Lee, H., Y.M. Lee, Y.M. Heo, J.H. Hong, S. Jang, B.J. Ahn, S.S. Lee, and J.J. Kim. 2017. Optimization of fungal enzyme production by Trichoderma harzianum KUC1716 through surfactant-induced morphological changes. Mycobiology 45 (1): 48–51. https://doi.org/10.5941/MYCO.2017.45.1.48.
Li, C., Z. Yang, R.H.C. Zhang, D. Zhang, S. Chen, and L. Ma. 2013. Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. Journal of Biotechnology 168 (4): 470–477. https://doi.org/10.1016/j.jbiotec.2013.10.003.
Li, J., S. Shi, L. Kang, W. Wang, and W. Guan. 2019. Cellulase production from kraft hardwood pulp by Trichoderma reesei rut C-30. Biofuels, Bioproducts and Biorefining 13 (5): 1160–1168. https://doi.org/10.1002/bbb.2008.
Liu, J, and J, Yang. 2007. Cellulase production by Trichoderma koningii AS3. 4262 in solid-state fermentation using lignocellulosic waste from the vinegar industry. Food Technology and Biotechnology 45 (4): 420–425. https://hrcak.srce.hr/23993
Maeda, R.N., M.M.P. da Silva, L.M.M. Santa Anna, and N. Pereira. 2010. Nitrogen source optimization for cellulase production by Penicillium funiculosum, using a sequential experimental design methodology and the desirability function. Applied Biochemistry and Biotechnology 161 (1): 411–422. https://doi.org/10.1007/s12010-009-8875-6.
Matkar, K., D. Chapla, J. Divecha, A. Nighojkar, and D. Madamwar. 2013. Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. International Biodeterioration and Biodegradation 78: 24–33. https://doi.org/10.1016/j.ibiod.2012.12.002.
Miller, G.L. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31 (3): 426–428. https://doi.org/10.1021/ac60147a030.
Miranda-Sosa, A. 2019. Producción de celulasas de Aspergillus niger ITV-02. In: Masters Thesis. Instituto Tecnologico de Veracruz: México.
Moran-Aguilar, M, G. 2018. Estudio de la sacarificación del bagazo de caña de azúcar y Agave angustifolia para la producción de azúcares fermentables. In: Masters Thesis. Instituto Tecnologico de Veracruz: México.
Comité Nacional para el Desarrollo Sustentable de la Caña de Azúcar. https://www.gob.mx/conadesuca
Nanjundaswamy, A., and B.C. Okeke. 2020. Comprehensive optimization of culture conditions for production of biomass-hydrolyzing enzymes of Trichoderma SG2 in submerged and solid-state fermentation. Applied Biochemistry and Biotechnology 191 (1): 444–462. https://doi.org/10.1007/s12010-020-03258-1.
Nicolás-Santiago, D., C. Regalado-González, B. García-Almendárez, F.J. Fernández, A. Téllez-Jurado, and S. Huerta-Ochoa. 2006. Physiological, morphological, and mannanase production studies on Aspergillus niger uam-gs1 mutants. Electronic Journal of Biotechnology 9 (1): 50–60.
Pardo, A.G. 1996. Effect of surfactants on cellulase production by Nectria catalinensis. Current Microbiology 33: 275–278. https://doi.org/10.1007/s002849900113.
Prajapati, B.P., U.K. Jana, R.K. Suryawanshi, and N. Kango. 2020. Sugarcane bagasse saccharification using Aspergillus tubingensis enzymatic cocktail for 2G bio-ethanol production. Renewable Energy 152: 653–663. https://doi.org/10.1016/j.renene.2020.01.063.
Prasetyo, J., S. Sumita, N. Okuda, and E.Y. Park. 2010. Response of cellulase activity in pH-controlled cultures of the filamentous fungus acremonium cellulolyticus. Applied Biochemistry and Biotechnology 162 (1): 52–61. https://doi.org/10.1007/s12010-009-8826-2.
Rodrigues, P.D.O., B.V. dos Santos, L. Costa, M.A. Henrique, D. Pasquini, and M.A. Baffi. 2017. Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Industrial Crops and Products 95: 453–459. https://doi.org/10.1016/j.indcrop.2016.10.055.
Saini, R., J.K. Saini, M. Adsul, A.K. Patel, A. Mathur, D. Tuli, and R.R. Singhania. 2015. Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresource Technology 188: 240–246. https://doi.org/10.1016/j.biortech.2015.01.048.
Santos, G.B., A. de Sousa Francisco, J.R. da Filho, Silva Rodrigues, and R.R. de Souza. 2022. Cellulase production by Aspergillus niger using urban lignocellulosic waste as substrate: Evaluation of different cultivation strategies. Journal of Environmental Management 305: 114431. https://doi.org/10.1016/j.jenvman.2022.114431.
Singhania, R.R., R.K. Sukumaran, K.P. Rajasree, A. Mathew, L. Gottumukkala, and A. Pandey. 2011. Properties of a major β-glucosidase-BGL1 from Aspergillus niger NII-08121 expressed differentially in response to carbon sources. Process Biochemistry 46 (7): 1521–1524. https://doi.org/10.1016/j.procbio.2011.04.006.
Sirohi, R., A. Singh, A. Tarafdar, N.C. Shahi, A.K. Verma, and A. Kushwaha. 2019. Cellulase production from pre-treated Pea Hulls using Trichoderma reesei under submerged fermentation. Waste and Biomass Valorization 10 (9): 2651–2659. https://doi.org/10.1007/s12649-018-0271-4.
Sluiter, C.S.A., B. Hames, R. Ruiz, J. Slui, D.C. Ter, and D. Templeton. 2008. Determination of structural carbohydrates and lignin in biomass: Laboratory Analytical Procedure (LAP); Issue Date: April 2008; Revision Date: July 2011 (Version 07–08–2011) - 42618.pdf, Technical Report NREL/ TP -510 -42618. 1–15. http://www.nrel.gov/biomass/pdfs/42618.pdf.
Sohail, M., R. Siddiqi, A. Ahmad, and S.A. Khan. 2009. Cellulase production from Aspergillus niger MS82: effect of temperature and pH. New Biotechnology 25 (6): 437–441. https://doi.org/10.1016/j.nbt.2009.02.002.
Soni, R., A. Nazir, and B.S. Chadha. 2010. Optimization of cellulase production by a versatile Aspergillus fumigatus fresenius strain (AMA) capable of efficient deinking and enzymatic hydrolysis of Solka floc and bagasse. Industrial Crops and Products 31 (2): 277–283. https://doi.org/10.1016/j.indcrop.2009.11.007.
Srivastava, N., M. Srivastava, P.K. Mishra, V.K. Gupta, G. Molina, S. Rodriguez-Couto, A. Manikanta, and P.W. Ramteke. 2018. Applications of fungal cellulases in biofuel production: Advances and limitations. Renewable and Sustainable Energy Reviews 82: 2379–2386. https://doi.org/10.1016/j.rser.2017.08.074.
Strakowska, J., L. Błaszczyk, and J. Chełkowski. 2014. The significance of cellulolytic enzymes produced by Trichoderma in opportunistic lifestyle of this fungus. Journal of Basic Microbiology 54 (S1): S2–S13. https://doi.org/10.1002/jobm.201300821.
Suwannarangsee, S., J. Arnthong, L. Eurwilaichitr, and V. Champreda. 2014. Production and characterization of multi-polysaccharide degrading enzymes from Aspergillus aculeatus BCC199 for saccharification of agricultural residues. Journal of Microbiology and Biotechnology 24 (10): 1427–1437. https://doi.org/10.4014/jmb.1406.06050.
Teixeira Da Silva, V.D.C., A.L. De Souza Coto, R. De Carvalho Souza, and M.E.G.O. Bertoldi Sanchez Neves. Gomes Bonilla-Rodriguez. 2016. Effect of pH, temperature, and chemicals on the endoglucanases and β-glucosidases from the thermophilic fungus Myceliophthora heterothallica F.2.1.4. Obtained by solid-state and submerged cultivation. Biochemistry Research International. https://doi.org/10.1155/2016/9781216.
Thapa, S., J. Mishra, N. Arora, P. Mishra, H. Li, and J. O′Hair, S. Bhatti, and S. Zhou. 2020. Microbial cellulolytic enzymes: diversity and biotechnology with reference to lignocellulosic biomass degradation. Reviews in Environmental Science and Biotechnology 19: 621–648. https://doi.org/10.1007/s11157-020-09536-y.
Toor, Y., and U. Ilyas. 2014. Optimization of cellulase production by Aspergillus ornatus by the solid state fermentation of Cicer arietinum. American Journal of Research 2: 125–141.
Virgilio, S., and M.C. Bertolini. 2018. Functional diversity in the pH signaling pathway: an overview of the pathway regulation in Neurospora crassa. Current Genetics 64 (3): 529–534. https://doi.org/10.1007/s00294-017-0772-x.
Acknowledgements
The authors acknowledge the economic support from CONACyT by the postdoctoral granted to M.I Infanzon-Rodríguez.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Infanzón-Rodríguez, M.I., del Moral, S., Castro-Martínez, C. et al. Multi-Response Optimization Using the Desirability Function of Exoglucanases, Endoglucanases and β-Glucosidases Production by Aspergillus Niger ITV-02 from Delignified Sugarcane Bagasse. Sugar Tech 25, 86–98 (2023). https://doi.org/10.1007/s12355-022-01191-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01191-7