Abstract
Objective
To verify if 123I-FP-CIT, DaTSCAN® can differentiate early stages of Parkinson’s disease (PD) as well as patients with Atypical Parkinsonian syndromes (APS) from manifest Parkinson’s disease.
Methods
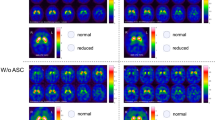
128 consecutive patients were investigated with 123I-FP-CIT SPECT during a 4-year period. All patients were diagnosed according to the established consensus criteria for diagnosis of PD (n = 53) and APS (n = 19). Remaining patients were grouped early PD (before onset of l-DOPA medication), (n = 20), vascular PD (n = 6), and non-PD syndromes (n = 30) and SWEDD (n = 1). SPECT images were analyzed visually according to a predefined ranking scale of dopaminergic nerve cell degeneration, distinguishing a posterior–anterior degeneration pattern (egg shape) from a more global and severe degeneration pattern (burst striatum). Striatum uptake ratios were quantitatively analyzed with the 3D software, EXINI.
Results
In the group of APS patients, the burst striatum pattern was most frequent and found in 61 % (11/18 patients). In PD patients, the egg shape pattern was dominating, especially in early PD where it was present in 95 % (19/20 patients). The positive predictive value for the egg shape pattern to diagnose PD was 92 % in this material (APS and all PD patients) and the specificity 90 % for the burst striatum pattern to exclude APS. The uptake ratios were reduced in both PD and APS patients and closely related to the image ranking.
Conclusion
In this study, we found that in more than half of the patients it was possible to differentiate between PD and APS by visual interpretation only. Similar results were obtained using semi-quantitative uptake ratios. Combining visual assessment with uptake ratios did not add to the discriminating power of DaTSCAN® SPECT in this material.






Similar content being viewed by others
References
Zhang F, Shi JS, Zhou H, Wilson B, Hong JS, Gao HM. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–77.
de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35.
Han M, Nagele E, DeMarshall C, Acharya N, Nagele R. Diagnosis of Parkinson’s disease based on disease-specific autoantibody profiles in human sera. PLoS ONE. 2012;7:e32383.
Nandhagopal R, McKeown MJ, Stoessl AJ. Functional imaging in Parkinson disease. Neurology. 2008;70:1478–88.
Stoessl AJ. Neuroimaging in Parkinson’s disease. Neurotherapeutics. 2011;8:72–81.
Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406.
Brucke T, Djamshidian S, Bencsits G, Pirker W, Asenbaum S, Podreka I. SPECT and PET imaging of the dopaminergic system in Parkinson’s disease. J Neurol. 2000;247(Suppl 4):IV2–7.
Savoiardo M. Differential diagnosis of Parkinson’s disease and atypical parkinsonian disorders by magnetic resonance imaging. Neurol Sci. 2003;24(Suppl 1):S35–7.
Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, Bonifati V, et al. EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol. 2013;20:16–34. doi:10.1111/ene.12022.
Zachrisson H, Fouladiun M, Blomstrand C, Holm J, Volkmann R. Functional assessment of high-grade ICA stenosis with duplex ultrasound and transcranial Doppler. Clin Physiol Funct Imaging. 2012;2012(32):241–6. doi:10.1111/j.475-097X.2011.01118.x.
Papathanasiou N, Rondogianni P, Chroni P, Themistocleous M, Boviatsis E, Pedeli X, et al. Interobserver variability, and visual and quantitative parameters of (123)I-FP-CIT SPECT (DaTSCAN) studies. Ann Nucl Med. 2012;26:234–40.
Berendse HW, Ponsen MM. Diagnosing premotor Parkinson’s disease using a two-step approach combining olfactory testing and DAT SPECT imaging. Parkinsonism Relat Disord. 2009;15(Suppl 3):S26–30.
Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord. 2003;18:1415–23.
Flabeau O, Meissner WG, Tison F. Multiple system atrophy: current and future approaches to management. Ther Adv Neurol Disord. 2010;3:249–63.
Djang DS, Janssen MJ, Bohnen N, Booij J, Henderson TA, Herholz K, et al. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J Nucl Med. 2012;53:154–63.
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of Parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70.
Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, et al. Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord. 2002;17:303–12.
Mo SJ, Linder J, Forsgren L, Larsson A, Johansson L, Riklund K. Pre- and postsynaptic dopamine SPECT in the early phase of idiopathic parkinsonism: a population-based study. Eur J Nucl Med Mol Imaging. 2010;2010(37):2154–64. doi:10.1007/s00259-010-1520-3.
Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord. 2001;16:1023–32.
Kahraman D, Eggers C, Schicha H, Timmermann L, Schmidt M. Visual assessment of dopaminergic degeneration pattern in 123I-FP-CIT SPECT differentiates patients with atypical parkinsonian syndromes and idiopathic Parkinson’s disease. J Neurol. 2012;259:251–60.
Darcourt J, Booij J, Tatsch K, Varrone A, Vander Borght T, Kapucu OL, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging. 2010;37:443–50.
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, et al. Movement disorders society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–86.
Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ. Clinicopathological investigation of vascular Parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–40.
Schapira AH, McDermott MP, Barone P, Comella CL, Albrecht S, Hsu HH, et al. Pramipexole in patients with early Parkinson’s disease (PROUD): a randomised delayed-start trial. Lancet Neurol. 2013;2013(12):747–55. doi:10.1016/S474-4422(13)70117-0.
Jaszczak RJ, Chang LT, Stein NA, Moore FE. Whole-body single-photon emission computed tomography using dual, large-field-of-view scintillation cameras. Phys Med Biol. 1979;24:1123–43.
Booij J, Knol RJ. SPECT imaging of the dopaminergic system in (premotor) Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S425–8.
Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9.
Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301.
Jellinger KA. Post mortem studies in Parkinson’s disease is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29.
Sudmeyer M, Antke C, Zizek T, Beu M, Nikolaus S, Wojtecki L, et al. Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med. 2011;52:733–40.
Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson’s disease. Ann Neurol. 2000;47:493–503.
Tedroff J, Ekesbo A, Rydin E, Langstrom B, Hagberg G. Regulation of dopaminergic activity in early Parkinson’s disease. Ann Neurol. 1999;46:359–65.
Acknowledgments
The authors thank Mats Fredrikson, Linköping Academic Research Centre (LARC), Linköping University for statistical advice.
Conflict of interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidsson, A., Georgiopoulos, C., Dizdar, N. et al. Comparison between visual assessment of dopaminergic degeneration pattern and semi-quantitative ratio calculations in patients with Parkinson’s disease and Atypical Parkinsonian syndromes using DaTSCAN® SPECT. Ann Nucl Med 28, 851–859 (2014). https://doi.org/10.1007/s12149-014-0878-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-014-0878-x