Abstract
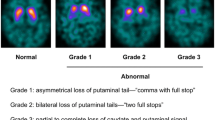
The aim of this study was to investigate whether visual assessment of 123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropan (123I-FP-CIT) single photon emission computed tomography (SPECT) in addition to quantitative analyses can help to differentiate idiopathic Parkinson's disease (PD) from atypical parkinsonian syndromes (APS). From a consecutive series of patients examined with 123I-FP-CIT SPECT (n = 190) over a three-year period we identified 165 patients with a clinical diagnosis of PD (n = 120) or APS (n = 45). 123I-FP-CIT SPECT results were analysed visually and quantitatively and compared for PD and APS and for the subgroup of patients with early PD and APS (disease duration <5 years). According to predefined visual patterns of dopaminergic degeneration the results were graded as normal (grade 5) or abnormal (grade 1–4), distinguishing a posterior-anterior degeneration pattern (“egg shape”) from a global and severe degeneration pattern (“burst striatum”). Visual assessment of 123I-FP-CIT SPECT showed significant different dopaminergic degeneration patterns for PD and APS patients. A grade 1 (“burst striatum”) degeneration pattern was predominantly associated with APS patients. In contrast to that, a grade 2 (egg shape) degeneration pattern was the characteristic finding in PD patients. In a subgroup of patients with early disease, visual assessment with identification of the burst striatum degeneration pattern provided 90% positive predictive value and 99% specificity for the diagnosis of APS. Quantitative analysis of striatal binding ratios failed to depict these different degeneration patterns in PD and APS patients. Visual assessment of the pattern of dopaminergic loss in 123I-FP-CIT SPECT shows different patterns of dopaminergic degeneration for PD and APS patients. Therefore, it could provide valuable information to distinguish APS from PD patients, especially in early stages of disease. Within the first 5 years of disease, the occurrence of a burst striatum degeneration pattern has a high positive predictive value of APS.



Similar content being viewed by others
References
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Goetz CG (1986) Charcot on Parkinson’s disease. Mov Disord 1(1):27–32
Daniel SE, Lees AJ (1993) Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl 39:165–172
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Rajput AH, Rozdilsky B, Rajput A (1991) Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci 18(3):275–278
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125(Pt 4):861–870
Hughes AJ, Daniel SE, Lees AJ (2001) Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57(8):1497–1499
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Litvan I, Agid Y, Goetz C, Jankovic J, Wenning GK, Brandel JP, Lai EC, Verny M, Ray-Chaudhuri K, McKee A, Jellinger K, Pearce RK, Bartko JJ (1997) Accuracy of the clinical diagnosis of corticobasal degeneration: a clinicopathologic study. Neurology 48(1):119–125
Litvan I, Goetz CG, Jankovic J, Wenning GK, Booth V, Bartko JJ, McKee A, Jellinger K, Lai EC, Brandel JP, Verny M, Chaudhuri KR, Pearce RK, Agid Y (1997) What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol 54(8):937–944
Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK (2003) Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 18(5):467–486
Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, Poewe W, Jellinger K, Ray Chaudhuri K, D’Olhaberriague L, Pearce RK (1998) Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry 64(2):184–189
Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP (1997) Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology 48(2):384–393
Santacruz P, Uttl B, Litvan I, Grafman J (1998) Progressive supranuclear palsy: a survey of the disease course. Neurology 50(6):1637–1647
Daniel SE, de Bruin VM, Lees AJ (1995) The clinical and pathological spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain 118(Pt 3):759–770
Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12(2):133–147
Gibb WR, Luthert PJ, Marsden CD (1990) Clinical and pathological features of corticobasal degeneration. Adv Neurol 53:51–54
Gibb WR (1992) Neuropathology of Parkinson’s disease and related syndromes. Neurol Clin 10(2):361–376
Goto S, Hirano A, Matsumoto S (1989) Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration. Ann Neurol 26(6):766–770
Jellinger KA (1999) Post mortem studies in Parkinson’s disease—is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl 56:1–29
Kaufman MJ, Madras BK (1991) Severe depletion of cocaine recognition sites associated with the dopamine transporter in Parkinson’s-diseased striatum. Synapse 9(1):43–49
Niznik HB, Fogel EF, Fassos FF, Seeman P (1991) The dopamine transporter is absent in parkinsonian putamen and reduced in the caudate nucleus. J Neurochem 56(1):192–198
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, Speelman JD, Horstink MH, Sips HJ, Dierckx RA, Versijpt J, Decoo D, Van Der Linden C, Hadley DM, Doder M, Lees AJ, Costa DC, Gacinovic S, Oertel WH, Pogarell O, Hoeffken H, Joseph K, Tatsch K, Schwarz J, Ries V (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Mov Disord 15(3):503–510
Vlaar AM, de Nijs T, Kessels AG, Vreeling FW, Winogrodzka A, Mess WH, Tromp SC, van Kroonenburgh MJ, Weber WE (2008) Diagnostic value of 123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur Neurol 59(5):258–266
Plotkin M, Amthauer H, Klaffke S, Kuhn A, Ludemann L, Arnold G, Wernecke KD, Kupsch A, Felix R, Venz S (2005) Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm 112(5):677–692
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pilowsky LS, Pupi A (2002) European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using (123)I-labelled dopamine D(2) transporter ligands. Eur J Nucl Med Mol Imaging 29(10):BP30–BP35
Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16(2):79–84
Koch W, Radau PE, Hamann C, Tatsch K (2005) Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 46(7):1109–1118
Eggers C, Kahraman D, Fink GR, Schmidt M, Timmermann L (2011) Akinetic-rigid and tremor-dominant Parkinson’s disease patients show different patterns of FP-CIT Single photon emission computed tomography. Mov Disord 26(3):416–423
Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15(4):692–698
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318(14):876–880
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS (1990) Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 28(4):547–555
Rajput AH, Voll A, Rajput ML, Robinson CA, Rajput A (2009) Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology 73(3):206–212
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42(6):1142–1146
Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, Lai EC, Wenning G, D’Olhaberriague L, Verny M, Chaudhuri KR, McKee A, Jellinger K, Bartko JJ, Mangone CA, Pearce RK (1996) Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 46(4):922–930
O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131(Pt 5):1362–1372
Wenning GK, Colosimo C, Geser F, Poewe W (2004) Multiple system atrophy. Lancet Neurol 3(2):93–103
Burn DJ, Warren NM (2005) Toward future therapies in progressive supranuclear palsy. Mov Disord 20(Suppl 12):S92–S98
Katzenschlager R, Lees AJ (2002) Treatment of Parkinson’s disease: levodopa as the first choice. J Neurol 249(Suppl 2):II19–II24
Fahn S (2006) Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl 71:1–15
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351(24):2498–2508
Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E (2009) A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 361(13):1268–1278
Van Laere K, Everaert L, Annemans L, Gonce M, Vandenberghe W, Vander Borght T (2008) The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur J Nucl Med Mol Imaging 35(7):1367–1376
Staff RT, Ahearn TS, Wilson K, Counsell CE, Taylor K, Caslake R, Davidson JE, Gemmell HG, Murray AD (2009) Shape analysis of 123I-N-omega-fluoropropyl-2-beta-carbomethoxy-3beta-(4-iodophenyl) nortropane single-photon emission computed tomography images in the assessment of patients with parkinsonian syndromes. Nucl Med Commun 30(3):194–201
Goethals I, Ham H, Dobbeleir A, Santens P, D’Asseler Y (2009) The potential value of a pictorial atlas for aid in the visual diagnosis of 123I FP-CIT SPECT scans. Nuklearmedizin 48(4):173–178
Conflict of interest
Lars Timmermann received honoria for lectures on symposia with sponsoring of GE medical. None of the other authors has any financial disclosures regarding the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Kahraman, C. Eggers, L. Timmermann and M. Schmidt contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kahraman, D., Eggers, C., Schicha, H. et al. Visual assessment of dopaminergic degeneration pattern in 123I-FP-CIT SPECT differentiates patients with atypical parkinsonian syndromes and idiopathic Parkinson's disease. J Neurol 259, 251–260 (2012). https://doi.org/10.1007/s00415-011-6163-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-011-6163-1