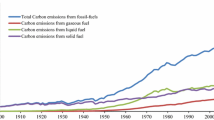
Abstract
In this state-of-the-art review, various adsorbents (i.e., Metal-Organic Framework (MOF), Activated Carbons and their composites, Carbon Molecular Sieve (CMS) and zeolites) for CO2 adsorption both at low and high-pressure applications (i.e., pre- and post-combustion CO2 capture, adsorption heat pumps employing CO2 as refrigerant) are explored. The most suitable candidate, i.e., the various grades of activated carbons (ACs), is identified based on their equilibrium uptake (isotherm data), rate of adsorption (kinetic data), isosteric heat of adsorption and cost. The study presents a comprehensive review on the basis of various models of adsorption isotherms and kinetics, their merits and demerits, and their applicability, especially in the context of CO2-adsorbent pairs. The literature shows that the activated carbons with high surface area, pore volume and better pore network results in higher equilibrium uptake and faster kinetics. A comparative analysis presented in the review work highlights that high-grade activated carbons having higher absolute uptake, also result in higher net uptake, i.e., the deciding factor for selecting the adsorbents for adsorption-based refrigeration and heat transformation applications. The comparative study clearly shows that most of the MOFs with high surface area outperform the best-activated carbons in equilibrium CO2 uptake. However, their high heat of adsorption, slower kinetics and significantly high cost comes in their way of commercialization for high/low-pressure CO2 adsorption applications. One of the notable observations of the review work is that adsorbents that perform better in low-pressure applications may not be a handsome candidate for high-pressure applications, as both mechanisms are different. Various isotherm models are compared based on the R2 value of the fitted data. The comparison clearly demonstrates that some of the models, i.e., (Langmuir, Freundlich), give better predictions at low-pressure conditions while some (Toth, D-A, Modified D-A, and D-R) give a better prediction for high-pressure adsorption. Some isotherm models take care of the surface heterogeneity, hence suitable for AC-CO2 pair. Most importantly, the compiled data for the adsorption isotherms and kinetics will be useful for further analysis and design of adsorption systems and selection of adsorbents, especially for CO2 adsorption systems suitable for green refrigeration/heat pump and carbon capture application.
Graphical Abstract










Similar content being viewed by others
Abbreviations
- A:
-
Polanyi’s coefficient, J mol−1
- a:
-
Henry’s constant in Prausnitz model, Pa−1
- B:
-
Temperature-dependent heat of adsorption in Temkin model, J mol−1
- b :
-
Temkin Parameter, J mol−1
- b0 :
-
Pre-exponential constant in Toth model, Pa−1
- Cp :
-
Specific heat capacity, kJ kg−1 K−1
- E:
-
Adsorbate-adsorbent interaction energy, J mol−1
- Ea :
-
Activation energy, kJ mol−1
- f :
-
Fugacity, Pa
- K:
-
Henry’s constant in Toth model, Pa−1
- k :
-
D-A and Modified D-A parameter for the interaction of adsorbate-adsorbent for estimation of pseudovapour pressures, –
- K, B, b0, Bs, B∞ :
-
Langmuir/Freundlich/Sips/Toth/BET/ Prausnitz isotherm constant, Pa−1
- K1 :
-
Temperature-dependent Sips isotherm parameter, bar−1
- K2 :
-
Temperature-dependent Sips isotherm parameter, K
- K3, K4 :
-
Temperature-dependent Sips isotherm parameter, mol kg−1
- K5 :
-
Temperature-dependent Sips isotherm parameter, –
- K6 :
-
Temperature-dependent Sips isotherm parameter, K
- KD :
-
Modified DA parameter, –
- KT :
-
Temkin constant, cm3 g−1 bar−1
- n :
-
Freundlich/Sips/D-A/Modified DA isotherm constant, –
- P:
-
Pressure, Pa
- Pc :
-
Critical pressure, Pa
- Ps :
-
Saturation pressure, Pa
- Q:
-
Isosteric heat of adsorption, kJ mol−1
- q, C, qe, x :
-
Equilibrium uptake, kg kg−1, mmol g−1, or mol kg−1, g g−1
- qe :
-
Equilibrium adsorption capacity, g of CO2 g−1
- qm, C0, q0, x o :
-
Saturated/ limiting uptake of adsorbate, mmol g−1, or mol kg−1, or g g−1
- qms :
-
Limiting uptake of adsorbate in temperature-dependent Sips model, mmol g−1, or mol kg−1, or g g−1
- r, R:
-
Coefficient of regression, –
- Ru :
-
Universal gas constant, J mol−1 K−1
- T:
-
Temperature, K or °C
- t:
-
Toth/ BET/P isotherm parameter, –
- Tc :
-
Critical temperature of CO2, K or °C
- Tcond :
-
Condenser temperature, °C
- Te :
-
Evaporator temperature, K
- Tg :
-
Generator temperature, K
- Tsat :
-
Saturation temperature of CO2, K or °C
- W:
-
Equilibrium volumetric uptake, m3 kg−1
- W0 :
-
Limiting volumetric uptake, m3 kg−1
- ν a, V m :
-
Specific volume of adsorbed phase, m3 kg−1
- λ:
-
Dubinin Radushkevich constant, mol2 J−2
- ω:
-
Polanyi constant in DR model, J mol−1
- κ:
-
Coefficient in 1st and 2nd order kinetic model, 1/min
- AC:
-
Activated carbon
- ACF:
-
Activated Carbon Fibres
- ACS:
-
Activated Carbon Spheres
- ADCS:
-
Adsorption based cooling systems
- APTES:
-
Aminopropyltriethoxysilane
- BET:
-
Brunauer–Emmett–Teller
- CB:
-
Carbon black
- CCS:
-
Carbon capture and storage
- CFCs:
-
Chlorofluorocarbons
- CMS:
-
Carbon molecular sieves
- CNTs:
-
Carbon nanotubes
- CSAC:
-
Coconut shell-based activated carbon
- CVD:
-
Carbon vapour deposition technique
- DES:
-
Deep Eutectic Solvent
- EG:
-
Expanded graphite
- ENG:
-
Expanded natural graphite
- FM:
-
Fine particles of magnetite
- GAC:
-
Granular activated carbon
- GNPs:
-
Graphene nanoplatelets
- HEC:
-
Hydroxyl cellulose
- HMMM:
-
Hexamethoxymethylmelamine
- IL:
-
Ionic liquid
- M:
-
Mangrove
- MOF:
-
Metal Organic Framework
- NBP:
-
Normal boiling point
- OXA-GAC:
-
Ammonia modified activated carbon
- PSA:
-
Pressure swing adsorption
- PVA:
-
Polyvinyl Alcohol
- SBSM:
-
Strong binding site molarity
- SBUs:
-
Secondary binding units
- TCE:
-
Thermal conductivity enhancer
- VBTMA (Ala):
-
Vinylbenzyltrimethyl ammonium alanate
- VCRS:
-
Vapour compression refrigeration systems
- VPSA:
-
Vacuum pressure swing adsorption
- WPT:
-
Waste palm trunk
References
Gautam and Sahoo S 2021 Thermal management and optimization of adsorption vessels for CO2-based green refrigeration systems: A heat and mass transfer approach. Sādhanā 46(4): 1–21
Pariyar P, Kumari K, Jain M K and Jadhao P S 2020 Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Science of the Total Environment 713: 136433
Sarwar A, Ali M, Khoja A H, Nawar A, Waqas A, Liaquat R, Naqvi S R and Asjid M 2021 Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. Journal of CO2 Utilization 46: 101476
Gautam and Sahoo S 2022 A comprehensive thermodynamic analysis and performance evaluation of a transcritical ejector expansion CO2 adsorption refrigeration system integrated with thermoelectric sub-cooler. The Journal of Supercritical Fluids 182: 105517
Gorai B, Sahoo S and Gautam 2022 Comparative Exergy Analysis and Environmental Impact of a Dairy Plant Integrated with a Transcritical Heat Pump System: A Feasibility of Throttle Valve, Expander, and an Ejector as Expansion Devices. Arabian Journal for Science and Engineering. https://doi.org/10.1007/s13369-022-07147-z
Wang J, Pu Q, Ning P and Lu S 2021 Activated carbon-based composites for capturing CO2: a review. Greenhouse Gases: Science and Technology 11(2): 377–393
Rocky K A, Pal A, Rupam T H, Palash M L and Saha B B 2021 Recent advances of composite adsorbents for heat transformation applications. Thermal Science and Engineering Progress 23: 100900
Soni P, Sur A, Gaba V K and Sah R P 2021 Review on improvement of adsorption refrigeration systems performance using composite adsorbent: current state of art. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. https://doi.org/10.1080/15567036.2021.1927252
Rashidi N A and Yusup S 2019 Production of palm kernel shell-based activated carbon by direct physical activation for carbon dioxide adsorption. Environmental Science and Pollution Research 26(33): 33732–33746
Lendzion-Bieluń Z, Czekajło Ł, Sibera D, Moszyński D, Sreńscek-Nazzal J, Morawski A W, Wrobel R J, Michalkiewicz B, Arabczyk W and Narkiewicz U 2018 Surface characteristics of KOH-treated commercial carbons applied for CO2 adsorption. Adsorption Science & Technology 36(1–2): 478–492
Gautam Kumar G and Sahoo S 2020 Performance improvement and comparisons of CO2 based adsorption cooling system using modified cycles employing various adsorbents: a comprehensive study of subcritical and transcritical cycles. International Journal of Refrigeration 112: 136–154
Gautam and Sahoo S 2021 Effects of geometric and heat transfer parameters on adsorption–desorption characteristics of CO2-activated carbon pair. Clean Technologies and Environmental Policy 23: 1065–1085
Samanta A, Zhao A, Shimizu G K, Sarkar P and Gupta R 2012 Post-combustion CO2 capture using solid sorbents: a review. Industrial & Engineering Chemistry Research 51(4): 1438–1463
Yan X F, Fan X R, Wang Q and Shen Y 2017 An adsorption isotherm model for adsorption performance of silver-loaded activated carbon. Thermal Science 21(4): 1645–1649
Rashidi N A and Yusup S 2016 An overview of activated carbons utilization for the post-combustion carbon dioxide capture. Journal of CO2 Utilization 13: 1–16
Singh G, Lakhi K S, Sil S, Bhosale S V, Kim I, Albahily K and Vinu A 2019 Biomass derived porous carbon for CO2 capture. Carbon 148: 164–186
Ghanbari T, Abnisa F and Daud W M 2020 A review on production of metal organic frameworks (MOF) for CO2 adsorption. Science of The Total Environment 707: 135090
Shabir F, Sultan M, Miyazaki T, Saha B B, Askalany A, Ali I, Zhou Y, Ahmad R and Shamshiri R R 2020 Recent updates on the adsorption capacities of adsorbent-adsorbate pairs for heat transformation applications. Renewable and Sustainable Energy Reviews 119: 109630
Gautam Chaudhary A and Sahoo S 2023 Experimental investigation on adsorbent composites for CO2 capture application: An attempt to improve the dynamic performance of the parent adsorbent. International Journal of Heat and Mass Transfer 203: 123796
Aimikhe V J, Anyebe M S and Ibezim-Ezeani M 2022 Development of composite activated carbon from mango and almond seed shells for CO2 capture. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-03665-w
Al-Ghouti M A and Da’ana D A 2020 Guidelines for the use and interpretation of adsorption isotherm models: A review. Journal of Hazardous Materials 393: 122383
Ayawei N, Ebelegi A N and Wankasi D 2017 Modelling and interpretation of adsorption isotherms. Journal of Chemistry. https://doi.org/10.1155/2017/3039817
Wang J and Guo X 2020 Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 258: 127279
Dąbrowski A 2001 Adsorption—from theory to practice. Advances in colloid and interface science 93(1–3): 135–224
Thommes M, Kaneko K, Neimark A V, Olivier J P, Rodriguez-Reinoso F, Rouquerol J and Sing K S 2015 Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry 87(9–10): 1051–1069
Ruthven D M 1984 Principles of adsorption and adsorption processes. John Wiley & Sons, USA
Ruthven D M 2006 Fundamentals of Adsorption Equilibrium and Kinetics in Microporous Solids. In: Adsorption and Diffusion. Molecular Sieves (eds) Karge H G and Weitkamp J, vol 7. Springer, Berlin. https://doi.org/10.1007/3829_007
LeVan M D, Carta G and Yon C M 1999 Adsorption and ion exchange. Energy 16:17. Perry’s Chemical Engineers’ Handbook. 7th edn. McGrawHill, New York
Hutson N D and Yang R T 1997 Theoretical basis for the Dubinin-Radushkevitch (DR) adsorption isotherm equation. Adsorption 3: 189–915
Srinivasan K, Saha B B, Ng K C, Dutta P and Prasad M 2011 A method for the calculation of the adsorbed phase volume and pseudo-saturation pressure from adsorption isotherm data on activated carbon. Physical Chemistry Chemical Physics 13(27): 12559–12570
Lowell S, Shields J E, Thomas M A and Thommes M 2012 Characterization of porous solids and powders: surface area, pore size and density. Springer Science & Business Media
Dada A O, Olalekan A P, Olatunya A M and Dada O J 2012 Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR Journal of Applied Chemistry 3(1): 38–45
Ocampo-Pérez R, Leyva-Ramos R, Sanchez-Polo M and Rivera-Utrilla J 2013 Role of pore volume and surface diffusion in the adsorption of aromatic compounds on activated carbon. Adsorption 19(5): 945–957
Younas M, Sohail M, Leong L K, Bashir M J and Sumathi S 2016 Feasibility of CO2 adsorption by solid adsorbents: a review on low-temperature systems. International Journal of Environmental Science and Technology 13(7): 1839–1860
Wang Q, Luo J, Zhong Z and Borgna A 2011 CO2 capture by solid adsorbents and their applications: current status and new trends. Energy & Environmental Science 4(1): 42–55
Rufford T E and Jurcakova D H 2013 Surface functionalities. In: Green carbon materials (ed) Zhu J, CRC Press, Boca Raton, pp 3–13
Bonenfant D, Kharoune M, Niquette P, Mimeault M and Hausler R 2008 Advances in principal factors influencing carbon dioxide adsorption on zeolites. Science and technology of advanced materials 9(1): 013007
Pham T D, Xiong R, Sandler S I and Lobo R F 2014 Experimental and computational studies on the adsorption of CO2 and N2 on pure silica zeolites. Microporous and mesoporous materials 185: 157–166
Uzun A and Keskin S 2014 Site characteristics in metal organic frameworks for gas adsorption. Progress in Surface Science 89(1): 56–79
Yazaydın A O, Snurr R Q, Park T H, Koh K, Liu J, LeVan M D, Benin A I, Jakubczak P, Lanuza M, Galloway D B and Low J J 2009 Screening of metal− organic frameworks for carbon dioxide capture from flue gas using a combined experimental and modeling approach. Journal of the American Chemical Society. 131(51): 18198–18199
Liu Y, Liu J, Chang M and Zheng C 2012 Theoretical studies of CO2 adsorption mechanism on linkers of metal–organic frameworks. Fuel 95: 521–527
Garshasbi V, Jahangiri M and Anbia M 2017 Equilibrium CO2 adsorption on zeolite 13X prepared from natural clays. Applied Surface Science 393: 225–233
Pal A, El-Sharkawy I I, Saha B B, Jribi S, Miyazaki T and Koyama S 2016 Experimental investigation of CO2 adsorption onto a carbon based consolidated composite adsorbent for adsorption cooling application. Applied Thermal Engineering 109: 304–311
Hauchhum L and Mahanta P 2014 Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. International Journal of Energy and Environmental Engineering 5(4): 349–356
Chiang Y C, Hsu W L, Lin S Y and Juang R S 2017 Enhanced CO2 adsorption on activated carbon fibers grafted with nitrogen-doped carbon nanotubes. Materials 10(5): 511
Ma X, Li L, Chen R, Wang C, Li H and Wang S 2018 Heteroatom-doped nanoporous carbon derived from MOF-5 for CO2 capture. Applied Surface Science 435: 494–502
Park Y, Moon D K, Park D, Mofarahi M and Lee C H 2019 Adsorption equilibria and kinetics of CO2, CO, and N2 on carbon molecular sieve. Separation and Purification Technology 212: 952–964
Song X, Ma X and Zeng Y 2017 Adsorption equilibrium and thermodynamics of CO2 and CH4 on carbon molecular sieves. Applied Surface Science 396: 870–878
Sarker A I, Aroonwilas A and Veawab A 2017 Equilibrium and kinetic behaviour of CO2 adsorption onto zeolites, carbon molecular sieve and activated carbons. Energy Procedia 114: 2450–2459
Sarker M A 2012 Equilibrium and mass transfer behaviour of CO2 adsorption on zeolites, carbon molecular sieve, and activated carbons (Doctoral dissertation, Faculty of Graduate Studies and Research, University of Regina)
Rashidi N A, Yusup S and Borhan A 2016 Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Engineering 148: 630–637
Vargas D P, Giraldo L and Moreno-Piraján J C 2012 CO2 adsorption on activated carbon honeycomb-monoliths: a comparison of Langmuir and Toth models. International Journal of Molecular Sciences 13(7): 8388–8397
Goel C, Kaur H, Bhunia H and Bajpai P K 2016 Carbon dioxide adsorption on nitrogen enriched carbon adsorbents: Experimental, kinetics, isothermal and thermodynamic studies. Journal of CO2 Utilization 16:50–63
Singh V K, Kumar E A and Saha B B 2018 Adsorption isotherms, kinetics and thermodynamic simulation of CO2-CSAC pair for cooling application. Energy 160: 1158–1173
Singh V K and Kumar E A 2016 Measurement and analysis of adsorption isotherms of CO2 on activated carbon. Applied Thermal Engineering 97: 77–86
Rashidi N A and Yusup S 2017 Potential of palm kernel shell as activated carbon precursors through single stage activation technique for carbon dioxide adsorption. Journal of Cleaner Production 168: 474–486
Saha B B, Jribi S, Koyama S and El-Sharkawy I I 2011 Carbon dioxide adsorption isotherms on activated carbons. Journal of Chemical & Engineering Data 56(5): 1974–1981
Raganati F, Alfe M, Gargiulo V, Chirone R and Ammendola P 2018 Isotherms and thermodynamics of CO2 adsorption on a novel carbon-magnetite composite sorbent. Chemical Engineering Research and Design 134: 540–552
Shafeeyan M S, Daud W M, Shamiri A and Aghamohammadi N 2015 Adsorption equilibrium of carbon dioxide on ammonia-modified activated carbon. Chemical Engineering Research and Design 104: 42–52
Jribi S, Miyazaki T, Saha B B, Pal A, Younes M M, Koyama S and Maalej A 2017 Equilibrium and kinetics of CO2 adsorption onto activated carbon. International Journal of Heat and Mass Transfer 108: 1941–1946
Berdenova B, Pal A, Muttakin M, Mitra S, Thu K, Saha B B and Kaltayev A 2019 A comprehensive study to evaluate absolute uptake of carbon dioxide adsorption onto composite adsorbent. International Journal of Refrigeration 100: 131–140
Pal A, Uddin K, Saha B B, Thu K, Kil H S, Yoon S H and Miyawaki J 2020 A benchmark for CO2 uptake onto newly synthesized biomass-derived activated carbons. Applied Energy 264: 114720
Singh V K and Kumar E A 2017 Experimental investigation and thermodynamic analysis of CO2 adsorption on activated carbons for cooling system. Journal of CO2 Utilization 17:290–304
Ahmad A L, Loh M M and Aziz J A 2007 Preparation and characterization of activated carbon from oil palm wood and its evaluation on methylene blue adsorption. Dyes and Pigments 75(2): 263–272
Singh V K and Kumar E A 2017 Thermodynamic analysis of single-stage and single-effect two-stage adsorption cooling cycles using indigenous coconut shell based activated carbon-CO2 pair. International Journal of Refrigeration 84: 238–252
Zhong Y, Critoph R E and Thorpe R 2006 Evaluation of the performance of solid sorption refrigeration systems using carbon dioxide as refrigerant. Applied Thermal Engineering 26(16): 1807–1811
Shen Y, Shi W, Zhang D, Na P and Fu B 2018 The removal and capture of CO2 from biogas by vacuum pressure swing process using silica gel. Journal of CO2 Utilization 27:259–271
Wang K, Shang H, Li L, Yan X, Yan Z, Liu C and Zha Q 2012 Efficient CO2 capture on low-cost silica gel modified by polyethyleneimine. Journal of Natural Gas Chemistry 21(3): 319–323
Oliveira R J, de Conto J F, Oliveira M R, Egues S M, Borges G R, Dariva C and Franceschi E 2019 CO2/CH4 adsorption at high-pressure using silica-APTES aerogel as adsorbent and near infrared as a monitoring technique. Journal of CO2 Utilization 32:232–240
Cazorla-Amorós D, Alcaniz-Monge J and Linares-Solano A 1996 Characterization of activated carbon fibers by CO2 adsorption. Langmuir 12(11): 2820–2824
Qasem N A, Ben-Mansour R and Habib M A 2018 An efficient CO2 adsorptive storage using MOF-5 and MOF-177. Applied energy 210: 317–326
Kazemi S and Safarifard V 2018 Carbon dioxide capture in MOFs: the effect of ligand functionalization. Polyhedron 154: 236–251
Santos K M, Santos R J, Alves M M, De Conto J F, Borges G R, Dariva C, Egues S M, Santana C C and Franceschi E 2019 Effect of high pressure CO2 sorption on the stability of metalorganic framework MOF-177 at different temperatures. Journal of Solid State Chemistry 269: 320–327
Millward A R and Yaghi O M 2005 Metal− organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. Journal of the American Chemical Society 127(51): 17998–17999
Liu Y, Liu J and Lin Y S 2015 Strong binding site molarity of MOFs and its effect on CO2 adsorption. Microporous and Mesoporous Materials 214: 242–245
Saha D and Deng S 2010 Structural stability of metal organic framework MOF-177. The Journal of Physical Chemistry Letters 1(1): 73–78
Glover T G and Mu B Gas Adsorption in Metal-Organic Frameworks: Fundamentals and Applications. CRC Press; 2018
Wahby A, Silvestre-Albero J, Sepúlveda-Escribano A and Rodríguez-Reinoso F 2012 CO2 adsorption on carbon molecular sieves. Microporous and Mesoporous Materials 164: 280–287
Carruthers J D, Petruska M A, Sturm E A and Wilson S M 2012 Molecular sieve carbons for CO2 capture. Microporous and mesoporous materials 154: 62–67
Yin G, Liu Z, Liu Q and Wu W 2013 The role of different properties of activated carbon in CO2 adsorption. Chemical Engineering Journal 230: 133–140
Zulkurnai N Z, Ali U M, Ibrahim N and Manan N A 2017 Carbon dioxide (CO2) adsorption by activated carbon functionalized with deep eutectic solvent (DES). In: IOP Conference Series: Materials Science and Engineering 206(1):012001
Wang L W, Tamainot-Telto Z, Thorpe R, Critoph R E, Metcalf S J and Wang R Z 2011 Study of thermal conductivity, permeability, and adsorption performance of consolidated composite activated carbon adsorbent for refrigeration. Renewable Energy 36(8): 2062–2066
Pal A, Shahrom M S, Moniruzzaman M, Wilfred C D, Mitra S, Thu K and Saha B B 2017 Ionic liquid as a new binder for activated carbon based consolidated composite adsorbents. Chemical Engineering Journal 326: 980–986
Kayal S and Chakraborty A 2018 Activated carbon (type Maxsorb-III) and MIL-101 (Cr) metal organic framework based composite adsorbent for higher CH4 storage and CO2 capture. Chemical Engineering Journal 334: 780–788
Pal A, Uddin K, Thu K and Saha B B 2019 Activated carbon and graphene nanoplatelets based novel composite for performance enhancement of adsorption cooling cycle. Energy conversion and management 180: 134–148
Askalany A A, Henninger S K, Ghazy M and Saha B B 2017 Effect of improving thermal conductivity of the adsorbent on performance of adsorption cooling system. Applied Thermal Engineering 110: 695–702
Cacciola G, Restuccia G and Mercadante L 1995 Composites of activated carbon for refrigeration adsorption machines. Carbon 33(9): 1205–1210
Gautam and Sahoo S 2022 Experimental investigation on different activated carbons as adsorbents for CO2 capture. Thermal Science and Engineering Progress 33: 101339
Jahan I, Rocky K A, Pal A, Rahman M M and Saha B B 2022 A study on activated carbon and carbon nanotube based consolidated composite adsorbents for cooling applications. Thermal Science and Engineering Progress 34: 101388
Zhang Y, Chen K, Lv C, Wu T, Wen Y, He H, Yu S and Wang L A 2021 Adsorption Separation of CO2/CH4 from Landfill Gas by Ethanolamine-Modified Silica Gel. Water, Air, & Soil Pollution 232(2): 1–1
Abdi J, Hadavimoghaddam F, Hadipoor M and Hemmati-Sarapardeh A 2021 Modeling of CO2 adsorption capacity by porous metal organic frameworks using advanced decision tree-based models. Scientific Reports 11(1): 1–4
Choe J H, Kim H and Hong C S 2021 MOF-74 type variants for CO2 capture. Materials Chemistry Frontiers 5(14): 5172–5185
Arellano I H and Pendleton P 2016 Phenomenological analyses of carbon dioxide adsorption kinetics on supported zinc-functionalized ionic liquid hybrid sorbents. Chemical Engineering Journal 288: 255–263
Querejeta N, Rubiera F and Pevida C 2022 Experimental Study on the Kinetics of CO2 and H2O Adsorption on Honeycomb Carbon Monoliths under Cement Flue Gas Conditions. ACS Sustainable Chemistry & Engineering 10(6): 2107–2124
Singh V K and Kumar E A 2016 Comparative studies on CO2 adsorption kinetics by solid adsorbents. Energy Procedia 90: 316–325
Xiao G, Li Z, Saleman T L and May E F 2017 Adsorption equilibria and kinetics of CH4 and N2 on commercial zeolites and carbons. Adsorption 23(1): 131–147
Nugent P, Belmabkhout Y, Burd S D, Cairns A J, Luebke R, Forrest K, Pham T, Ma S, Space B, Wojtas L and Eddaoudi M 2013 Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495(7439): 80–84
Zagho M M, Hassan M K, Khraisheh M, Al-Maadeed M A and Nazarenko 2021 A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chemical Engineering Journal Advances 6: 100091
Cheung O, Bacsik Z, Fil N, Krokidas P, Wardecki D and Hedin N 2020 Selective Adsorption of CO2 on Zeolites NaK-ZK-4 with Si/Al of 1.8–2.8. ACS Omega 5(39): 25371–25380
Karka S, Kodukula S, Nandury S V and Pal U 2019 Polyethylenimine-modified zeolite 13X for CO2 capture: adsorption and kinetic studies. ACS Omega 4(15): 16441–16449
Zhang Z, Zhang W, Chen X, Xia Q and Li Z 2010 Adsorption of CO2 on zeolite 13X and activated carbon with higher surface area. Separation Science and Technology 45(5): 710–719
Seabra R, Ribeiro A M, Gleichmann K, Ferreira A F and Rodrigues A E 2019 Adsorption equilibrium and kinetics of carbon dioxide, methane and nitrogen on binderless zeolite 4A adsorbents. Microporous and Mesoporous Materials 277: 105–114
Khajeh Amiri M, Ghaemi A and Arjomandi H 2019 Experimental, Kinetics and Isotherm Modeling of Carbon Dioxide Adsorption with 13X Zeolite in a fixed bed column. Iranian Journal of Chemical Engineering (IJChE) 16(1): 54–64
Farha O K, Eryazici I, Jeong N C, Hauser B G, Wilmer C E, Sarjeant A A, Snurr R Q, Nguyen S T, Yazaydın A O and Hupp J T 2012 Metal–organic framework materials with ultrahigh surface areas: is the sky the limit? Journal of the American Chemical Society 134(36): 15016–15021
Grünker R, Bon V, Müller P, Stoeck U, Krause S, Mueller U, Senkovska I and Kaskel S 2014 A new metal–organic framework with ultra-high surface area. Chemical Communications 50(26): 3450–3452
Ding L and Yazaydin A O 2013 Hydrogen and methane storage in ultrahigh surface area Metal-Organic Frameworks. Microporous and Mesoporous Materials 182: 185–190
Elhenawy S E, Khraisheh M, AlMomani F and Walker G 2020 Metal-organic frameworks as a platform for CO2 capture and chemical processes: Adsorption, membrane separation, catalytic-conversion, and electrochemical reduction of CO2. Catalysts 10(11): 1293
Saha D, Bao Z, Jia F and Deng S 2010 Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environmental science & technology 44(5): 1820–1826
Martell J D, Milner P J, Siegelman R L and Long J R 2020 Kinetics of cooperative CO2 adsorption in diamine-appended variants of the metal–organic framework Mg2(dobpdc). Chemical Science 11(25): 6457–6471
Ojwang D O, Grins J and Svensson 2018 The adsorption kinetics of CO2 on copper hexacyanoferrate studied by thermogravimetric analysis. Microporous and Mesoporous Materials 272: 70–78
Landaverde-Alvarado C, Morris A J and Martin S M 2017 Gas sorption and kinetics of CO2 sorption and transport in a polymorphic microporous MOF with open Zn (II) coordination sites. Journal of CO2 Utilization 19:40–48
Wu X, Yuan B, Bao Z and Deng S 2014 Adsorption of carbon dioxide, methane and nitrogen on an ultramicroporous copper metal–organic framework. Journal of Colloid and Interface Science 430: 78–84
Assen A H, Adil K and Belmabkhout Y 2021 Application of Metal-Organic Frameworks (MOFs) for Capturing CO2: Advancement and Challenges. Austin Chem. Eng. 8(1): 1086
Zulkifli Z I, Lim K L and Teh L P 2022 Metal-Organic Frameworks (MOFs) and their Applications in CO2 Adsorption and Conversion. ChemistrySelect 7(22): e202200572
Rodriguez Acevedo E, Franco C A, Carrasco-Marín F, Pérez-Cadenas A F and Cortés F B 2020 Biomass-Derived Carbon Molecular Sieves Applied to an Enhanced Carbon Capture and Storage Process (e-CCS) for Flue Gas Streams in Shallow Reservoirs. Nanomaterials 10(5): 980
Li L, Liu D, Zhen D, Ge Z, Zhang X, Xiong B, Li Z, Zhang K, Xing T, Xu W and Zhang F 2022 Thermodynamic-Kinetic Synergistic Separation for O2/N2 and CO2/CH4 on Nanoporous Carbon Molecular Sieves. ACS Applied Nano Materials 5: 11414–11422
Reid C R and Thomas K M 1999 Adsorption of gases on a carbon molecular sieve used for air separation: linear adsorptives as probes for kinetic selectivity. Langmuir 15(9): 3206–3218
Ahmad M A, Daud W W and Aroua M K 2008 Adsorption kinetics of various gases in carbon molecular sieves (CMS) produced from palm shell. Colloids and Surfaces A: Physicochemical and Engineering Aspects 312(2–3): 131–135
Shirani B and Eic M 2016 Kinetic behaviours of carbon dioxide and carbon monoxide on carbon molecular sieve. The Canadian Journal of Chemical Engineering 94(10): 2023–2034
Ghazi-MirSaeed M and Matavos-Aramyan S 2021 Gaseous Mixtures Separation via Chemically-Activated Nano Silica-Modified Carbon Molecular Sieves. Silicon 13(5): 1331–1345
Mousavi Z and Bozorgzadeh H R 2017 Preparation of Carbon Molecular Sieves from Pistachio Shell and Walnut Shell for Kinetic Separation of Carbon Monoxide, Hydrogen and Methane. Iran. J. Chem. Chem. Eng. 36(2): 71–80
Balsamo M, Budinova T, Erto A, Lancia A, Petrova B, Petrov N and Tsyntsarski B 2013 CO2 adsorption onto synthetic activated carbon: Kinetic, thermodynamic and regeneration studies. Separation and Purification Technology 116: 214–221
Wei M, Yu Q, Duan W, Hou L, Liu K, Qin Q, Liu S and Dai J 2017 Equilibrium and kinetics analysis of CO2 adsorption on waste ion-exchange resin-based activated carbon. Journal of the Taiwan Institute of Chemical Engineers 77: 161–167
Wei M, Yu Q, Xie H, Zuo Z, Hou L and Yang F 2017 Kinetics studies of CO2 adsorption and desorption on waste ion-exchange resin-based activated carbon. International Journal of Hydrogen Energy 42(44): 27122–27129
Jiao F, Sang H, Guo P, Miao P and Wang X 2022 Efficient adsorption and porous features from activated carbon felts activated by the eutectic of Na2CO3 and K2CO3 with vapor. Chemical Physics Letters 803: 139831
Amer N M, Lahijani P, Mohammadi M and Mohamed A R 2022 Modification of biomass-derived biochar: A practical approach towards development of sustainable CO2 adsorbent. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02905-3
Ramirez-Vidal P, Canevesi R L, Sdanghi G, Schaefer S, Maranzana G, Celzard A and Fierro V 2021 A step forward in understanding the hydrogen adsorption and compression on activated carbons. ACS Applied Materials & Interfaces 13(10): 12562–12574
Canevesi R L, Schaefer S, Izquierdo M T, Celzard A and Fierro V 2022 Roles of surface chemistry and texture of nanoporous activated carbons in CO2 capture. ACS Applied Nano Materials 5(3): 3843–3854
Haghighi Mood S, Pelaez-Samaniego M R and Garcia-Perez M 2022 Perspectives of Engineered Biochar for Environmental Applications: A Review. Energy & Fuels 36(15): 7940–7986
Islam M A, Pal A, Saha B B, Yoon S H and Miyawaki J 2021 Thermophysical Characteristics of Novel Biomass-Derived Activated Carbon as a Function of Synthesis Parameters. Heat Transfer Engineering 43(19): 1694–1707
Akkimaradi B S, Prasad M, Dutta P and Srinivasan K 2002 Effect of packing density and adsorption parameters on the throughput of a thermal compressor. Carbon 40(15): 2855–2859
Do D D 1998 Adsorption analysis: Equilibria and kinetics (with cd containing computer MATLAB programs). World Scientific
Kazi Afzalur Rahman (2011-06-29). Experimental and Theoretical Studies on Adsorbed Natural Gas Storage System Using Activated Carbons. ScholarBank@NUS Repository
Amankwah K A and Schwarz J A 1995 A modified approach for estimating pseudo-vapor pressures in the application of the Dubinin-Astakhov equation. Carbon 33(9): 1313–1319
Himeno S, Komatsu T and Fujita S 2005 High-pressure adsorption equilibria of methane and carbon dioxide on several activated carbons. Journal of Chemical & Engineering Data 50(2): 369–376
Mu B and Walton K S 2011 High-pressure adsorption equilibrium of CO2, CH4, and CO on an impregnated activated carbon. Journal of Chemical & Engineering Data 56(3): 390–397
Furukawa H, Ko N, Go Y B, Aratani N, Choi S B, Choi E, Yazaydin A Ö, Snurr R Q, O’Keeffe M, Kim J and Yaghi O M 2010 Ultrahigh porosity in metal-organic frameworks. Science 329(5990): 424–428
Liu J, Wang Y, Benin A I, Jakubczak P, Willis R R and LeVan M D 2010 CO2/H2O adsorption equilibrium and rates on metal− organic frameworks: HKUST-1 and Ni/DOBDC. Langmuir 26(17): 14301–14307
Gaikwad S, Kim Y, Gaikwad R and Han S 2021 Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. Journal of Environmental Chemical Engineering 9(4): 105523
Ahmed M B, Johir M A, Zhou J L, Ngo H H, Nghiem L D, Richardson C, Moni M A and Bryant M R 2019 Activated carbon preparation from biomass feedstock: clean production and carbon dioxide adsorption. Journal of Cleaner Production 225: 405–413
Chuah C Y and Laziz A M 2022 Recent Progress in Synthesis and Application of Activated Carbon for CO2 Capture. C 8(2):29
Serafin J and Cruz O F Jr 2022 Promising activated carbons derived from common oak leaves and their application in CO2 storage. Journal of Environmental Chemical Engineering 10(3): 107642
Silvestre-Albero J, Wahby A, Sepúlveda-Escribano A, Martínez-Escandell M, Kaneko K and Rodríguez-Reinoso F 2011 Ultrahigh CO2 adsorption capacity on carbon molecular sieves at room temperature. Chemical Communications 47(24): 6840–6842
PaláSingh A 1995 Sorption isotherms of methane, ethane, ethene and carbon dioxide on NaX, NaY and Na-mordenite zeolites. Journal of the Chemical Society, Faraday Transactions 91(17): 2935–2944
Singh G, Lakhi K S, Ramadass K, Sathish C I and Vinu A 2019 High-performance biomass-derived activated porous biocarbons for combined pre-and post-combustion CO2 capture. ACS Sustainable Chemistry & Engineering 7(7): 7412–7420
Zeng H, Qu X, Xu D and Luo Y 2022 Porous Adsorption Materials for Carbon Dioxide Capture in Industrial Flue Gas. Frontiers in Chemistry 10: 939701
Petrovic B, Gorbounov M and Soltani S M 2021 Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous and Mesoporous Materials. 312: 110751
Rocky KA, Jahan I, Pal A and Saha B B 2021 Activated carbon and carbon nanotube based composite adsorbents for adsorption cooling systems. In Sixth International Conference on Polygeneration-ICP: 556-560
Pal A, Uddin K, Rocky K A, Thu K and Saha B B 2019 CO2 adsorption onto activated carbon–graphene composite for cooling applications. International Journal of Refrigeration 106: 558–569
Sabouni and Rana 2013, "Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)" (2013). Electronic Thesis and Dissertation Repository. Paper 1472., The University of Western Ontario
Serafin J, Dziejarski B, Junior O F C and Sreńscek-Nazzal J 2023 Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 201: 633–647
Serafin J, Baca M, Biegun M, Mijowska E, Kaleńczuk R J, Sreńscek-Nazzal J and Michalkiewicz B 2019 Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Applied Surface Science 497: 143722
Serafin J, Narkiewicz U, Morawski AW, Wróbel R J and Michalkiewicz B 2017 Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. Journal of CO2 Utilization 18:73–79
Serafin J, Sreńscek-Nazzal J, Kamińska A, Paszkiewicz O and Michalkiewicz B 2022 Management of surgical mask waste to activated carbons for CO2 capture. Journal of CO2 Utilization 59:101970
Serafin J, Kiełbasa K and Michalkiewicz B 2022 The new tailored nanoporous carbons from the common polypody (Polypodium vulgare): The role of textural properties for enhanced CO2 adsorption. Chemical Engineering Journal 429: 131751
Jansen D, Gazzani M, Manzolini G, van Dijk E and Carbo M 2015 Pre-combustion CO2 capture. International Journal of Greenhouse Gas Control 40: 167–187
Pal A, Rocky K A and Saha B B 2021 Thermodynamic analysis of promising biomass-derived activated carbons/CO2 based adsorption cooling systems. Journal of CO2 Utilization 46:101457
Acknowledgements
This work is supported by the Department of Science and Technology (Science and Engineering Research Board), Govt. of India [Grant No. ECR/2018/000141].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gautam, Sah, R.P. & Sahoo, S. A review on adsorption isotherms and kinetics of CO2 and various adsorbent pairs suitable for carbon capture and green refrigeration applications. Sādhanā 48, 27 (2023). https://doi.org/10.1007/s12046-023-02080-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12046-023-02080-9