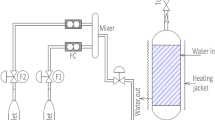
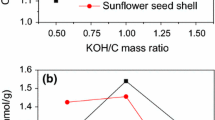
Abstract
The continuous emission of CO2 from anthropogenic activities poses a severe challenge to environmental sustainability. Activated carbon from bio-wastes has evolved as a cost-effect and efficient adsorbent for sequestering harmful gases like CO2. This study investigates the adsorption of CO2 using composite activated carbon from almond and mango seed shells. Three (3) different samples, including the activated almond seed shell (AC-KA), the mango seed shell (AC-KM), and the almond-mango seed shell composite (AC-KAM), were developed using KOH as an activating agent at 380 °C and characterized to determine their suitability as adsorbents. The nitrogen adsorption–desorption isotherms indicate that the activated carbon samples are type I microporous solids. The BET results showed that the AC-KM had the highest specific surface area of 629 m2/g and an average pore diameter of 2.12 nm, followed by the composite AC-KAM sample with a BET surface area and pore diameter of 380 m2/g and 1.78 nm, respectively. The SEM–EDX results showed that the composite AC-KAM had the highest atomic carbon percentage of 59.23%, making it a relatively good source of activated carbon. The FTIR analysis indicated the presence of similar functional groups, especially the primary amine group, with a high affinity for acid gases, in all three samples. The CO2 adsorption experiment results showed that the Langmuir equation adequately modeled the AC-KAM adsorption process, with an adsorption capacity of 1.6245 mmol/g at 26 °C. A Freundlich exponent of n > 1 showed that the adsorption process was favorable. Therefore, the AC-KAM sample can be used as an adsorbent for ambient low-concentration CO2 capture.








Similar content being viewed by others
Data Availability
Not applicable.
References
Spellman F (2016) Carbon capture and sequestration. Sci Renew Energy 2:503–522. https://doi.org/10.1201/b21643-12
Ghorami-Azam A, Balali-Mood M, Riahi-Zanjani B (2016) Effects of air pollution on human health and practical measures for prevention in Iran. J Res Med Sci., 2121–65. https://doi.org/10.4103/1735-1995.189646
Montagnaro F, Silvestre-Albero A, Silvestre-Albero J, Rodríguez-Reinoso F, Erto A, Lancia A, Balsamo M (2015) Post-combustion CO2 adsorption on activated carbons with different textural properties. Microporous and Mesoporous Materials, 209157–164. https://doi.org/10.1016/j.micromeso.2014.09.037
United States Environmental Protection Agency (2019) Inventory of U.S. greenhouse gas emissions and sinks: 1990–2017 https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2017. Accessed 24 Nov 2021
Bernhardsen IM, Knuutila HK (2017) A review of potential amine solvents for CO2 absorption process: absorption capacity, cyclic capacity and pKa. Int J Greenhouse Gas Control 61:27–48. https://doi.org/10.1016/j.ijggc.2017.03.021
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Brown S, Mac Dowell N (2018) Carbon capture and storage (CCS): the way forward. Energy Environ Sci 11:1062–1176. https://doi.org/10.1039/c7ee02342a
Akash S, Savita V (2017) Carbon capture and sequestration-a review. IOP Conf Ser: Earth Environ Sci PAPER 83:012024. https://doi.org/10.1088/1755-1315/83/1/012024+
Leung DYC, Caramanna G, Maroto-valer MM (2014) An overview of current status of carbon dioxide capture and storage technologies. Renew Sustain Energy Rev 39:426–443. https://doi.org/10.1016/j.rser.2014.07.093
Madzaki H, Azlina W, Ab W, Ghani K, Choong T, Yaw S, Muda N (2018) Carbon dioxide adsorption on activated carbon hydrothermally treated and impregnated with metal oxides (Penyerapan Karbon Dioksida pada Karbon yang Diaktifkan Secara Rawatan Hidrotermal dan Impregnasi dengan Logam Oksida). Jurnal Kejuruteraan 30:31–38. https://doi.org/10.17576/jkukm-2018-30(1)
Lee SY, Park SJ (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11. https://doi.org/10.1016/j.jiec.2014.09.001
Ghalia MA, Dahman Y (2016) Development and evaluation of zeolites and metal – organic frameworks for carbon dioxide separation and capture. Energ Technol 4:1–18. https://doi.org/10.1002/ente.201600359
Li J, Ma Y, Mccarthy MC, Sculley J, Yu J, Jeong H, Zhou H (2011) Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord Chem Rev 255:1791–1823. https://doi.org/10.1016/j.ccr.2011.02.012
Feng DD, Zhao YD, Wang XQ, Fang DD, Tang J, Fan LM, Yang J (2019) Two novel metal–organic frameworks based on pyridyl-imidazole-carboxyl multifunctional ligand: selective CO 2 capture and multiresponsive luminescence sensor, 48: 10892–10900. Dalton Trans. https://doi.org/10.1039/c9dt01430f
Shao L, Sang Y, Huang J (2019) Imidazole-based hyper-cross-linked polymers derived porous carbons for CO 2 capture. Microporous Mesoporous Mater 275:131–138. https://doi.org/10.1016/j.micromeso.2018.08.025
Zhao H, Luo X, Zhang H, Sun N, Wei W, Sun Y (2018) Carbon-based adsorbents for post-combustion capture: a review. Greenhouse Gases: Sci Technol 8:11–36. https://doi.org/10.1002/ghg.1758
Madzaki H, Azlina W, Ab W, Ghani K, Choong T, Yaw S, Muda N (2018) Carbon dioxide adsorption on activated carbon hydrothermally treated and impregnated with metal oxides (Penyerapan Karbon Dioksida pada Karbon yang Diaktifkan Secara Rawatan Hidrotermal dan Impregnasi dengan Logam Oksida). Jurnal Kejuruteraan 30:31–38. https://doi.org/10.17576/jkukm-2018-30(1)
Aimikhe VJ, Eyankware OE (2019) Adsorbents for noxious gas sequestration: state of the art. J Sci Res Rep 25:1–21. https://doi.org/10.9734/JSRR/2019/v25i1-230176
Boruban C, Esenturk EN (2018) Activated carbon-supported CuO nanoparticles: a hybrid material for carbon dioxide adsorption. J Nanopart Res 20:1–9. https://doi.org/10.1007/s11051-018-4139-0
Boujibar O, Souikny A, Ghamouss F, Achak O, Dahbi M, Chafik T (2018) CO2 capture using N-containing nanoporous activated carbon obtained from argan fruit shells. J Environ Chem Eng 6:1995–2002. https://doi.org/10.1016/j.jece.2018.03.005
Abdeljaoued A, Querejeta N, Durán I, Álvarez-Gutiérrez N, Pevida C, Chahbani MH (2018) Preparation and evaluation of a coconut shell-based activated carbon for CO2/CH4 separation. Energies 11:1748. https://doi.org/10.3390/en11071748
Tadda MA, Ahsan A, Shitu A, ElSergany M, Arunkumar T, Jose B, Razzaque MA, Daud NN (2016) A review on activated carbon: process, application, and prospects. Journal of Advanced Civil Engineering Practice and Research 2(1):7–13. Retrieved from https://www.academia.edu/33372795/A_review_on_activated_carbon_process_application_and_prospects
Singh G, Lakhi KS, Sil S, Bhosale SV, Kim IY, Albahily K, Vinu A (2019) Biomass derived porous carbon for CO2 capture. Carbon 148:164–186. https://doi.org/10.1016/j.carbon.2019.03.050
Sun Y, Li K, Zhao J, Wang J, Tang N, Zhang D, Jin Z (2018) Nitrogen and sulfur Co-doped microporous activated carbon macro-spheres for CO2 capture. J Colloid Interface Sci 526:174–183. https://doi.org/10.1016/j.jcis.2018.04.101
Li M, Xiao R (2019) Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Process Technol 186:35–39. https://doi.org/10.1016/j.fuproc.2018.12.015
Mukherjee A, Okolie JA, Abdelrasoul A, Niu C, Dalai AK (2019) Review of post-combustion carbon dioxide capture technologies using activated carbon. J Environ Sci 83:46–63. https://doi.org/10.1016/j.jes.2019.03.014
Sharma P, Sundaram MM, Watcharatharapong T, Jungthawan S, Ahuja R (2021) Tuning the nanoparticle interfacial properties and stability of the core–shell structure in Zn-doped NiMoO4@AWO4. ACS Appl Mater Interfaces 13(47):56116–56130. https://doi.org/10.1021/acsami.1c16287
Alhassan M, Andrew I, Auta M, Umaru M, Garba MU, Isah AG, Alhassan B (2018) Comparative studies of CO2 capture using acid and base modified activated carbon from sugarcane bagasse. Biofuels 9:719–728. https://doi.org/10.1080/17597269.2017.1306680
Fei TX, Bo LS, Guo LY, Ling GY, Ming ZG, Jiang HX, Wang X, Heng LS, Hua JL (2017) Biochar as potential sustainable precursors for activated carbon production: multiple applications in environmental protection and energy storage. Bioresource Technol 227:359–372. https://doi.org/10.1016/j.biortech.2016.12.083
Zulkurnai NZ, Ali UM, Ibrahim N, Manan NA (2017) Carbon dioxide (CO2) adsorption by activated carbon functionalized with deep eutectic solvent (DES). In IOP Conf Ser: Mat Sci Eng. 206:012001. https://doi.org/10.1088/1757-899X/206/1/012001
Wang Z, Jin H, Wang K, Xie Y, Ning J, Tu Y, Zeng H (2019) A two-step method for the integrated removal of HCl, SO2 and NO at low temperature using viscose-based activated carbon fibers modified by nitric acid. Fuel, 272–281. https://doi.org/10.1016/j.fuel.2018.11.002
Li X, Liu Y, Hao J, Wang W (2018) Study of almond shell characteristics. J Mat Sci 11:1782. https://doi.org/10.3390/ma11091782
Mwaurah PW, Kumar S, Kumar N, Panghal A, Attkan AK, Singh VK, Garg MK (2020) Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: comprehensive reviews in food science and food safety. Rev 19:2421–2446. https://doi.org/10.1111/1541-4337.12598
Ebringerová A, Hromádková Z, Košťálová Z, Sasinková V (2008) Chemical valorization of agricultural by-products: isolation and characterization of xylan-based antioxidants from almond shell biomass. BioResources 3:60–70
Toles CA, Marshall WE, Johns MM, Wartelle LH, McAloon A (2000) Acid-activated carbons from almond shells: physical, chemical and adsorptive properties and estimated cost of production. J of Bioresource Tech 71:87–92. https://doi.org/10.1016/S0960-8524(99)00029-2
Deniz F (2013) Dye removal by almond shell residues: studies on biosorption performance and process design. Mat Sci Eng 33:2821–2826. https://doi.org/10.1016/j.msec.2013.03.009
Akpen GD, Nwaogazie IL, Leton TG (2014) Adsorption characteristics of mango (Magnifera indica) seed shell activated carbon for removing phenol from wastewater. J Appl Sci Technol 19:37–42
Kh MK, Xalilov QF, Muhamadiev NQ, Dustov SI, Fazlieva NT (2021) Preparation of activated carbon from almond shells. Cent Asian J Med Nat Sci 2:125–129. https://doi.org/10.47494/cajmns.v2i3.185
Ogwuche EO, Gimba CE, Abechi ES (2015) An evaluation of the adsorptive behaviour of activated carbon derived from Hyphaene thebaica nut shells for the removal of dichlorvos from wastewater. Int J Sci Technoledge 3:274
Ekpete OA, Horsfall M (2011) Preparation and characterization of activated carbon derived from fluted pumpkin stem waste (Telfairia occidentalis Hook F). Res J Chem Sci 1:10–17
Sugumaran P, Susan VP, Ravichandran P, Seshadri S (2012) Production and characterization of activated carbon from banana empty fruit bunch and Delonix regia fruit pod. J Sustainable Energy and Env 3:125–132
Shoaib AG, El-Sikaily A, El Nemr A, Mohamed AED, Hassan AA (2020) Preparation and characterization of highly surface area activated carbons followed type IV from marine red alga (Pterocladia capillacea) by zinc chloride activation. Biomass Conversion and Biorefinery, 1-13. https://doi.org/10.1007/s13399-020-00760-8
ASTM E 1755, Standard test method for ash in biomass. (2001), American Society for Testing and Materials, ASTM International, West Conshohocken, PA 19428–2959, United States
ASTM E 1756, Standard test method for determination of total solids in biomass (2008). American Society for Testing and Materials, ASTM International, West Conshohocken, PA 19428–2959, United States
ASTM E 872, Standard test method for volatile matter in the analysis of particulate wood fuels (1998), American Society for Testing and Materials, ASTM International, West Conshohocken, PA 19428- 2959, United States
ASTM Designation: D4607-94 (1999) Standard test method for determination of iodine number of activated carbon. https://mazraehgroup.webs.com/Downloads/ASTM%20D4607-94%20Iodine%20test%20method.pdf. Accessed 3 April 2022
Stadie NP (2013). Appendix A. Experimental Adsorption Measurements. Accessed on 13th July 2020 from https://thesis.library.caltech.edu/7198/77/Stadie_N_2013_Appendices.pdf
Voskuilen T, Zheng Y, Pourpoint T (2010) Development of a Sievert apparatus for characterization of high-pressure hydrogen sorption materials. Int J Hydrogen Energy 35(19):10387–10395. https://doi.org/10.1016/j.ijhydene.2010.07.169
Policicchio A, Maccallini E, Kalantzopoulos G, Cataldi U, Abate S et al (2013) Volumetric apparatus for hydrogen adsorption and diffusion measurements: sources of systematic error and impact of their experimental resolutions. Rev Sci Instrum 84:103907. https://doi.org/10.1063/1.4824485
Aimikhe VJ, Eyankware OE (2021) Design, fabrication, and validation of a flow loop for CO2 adsorption studies. Pet Coal 63(3):824–932
Kumar A, Jena HM (2015) High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. J Applied Surface Sci 356:753–761. https://doi.org/10.1016/j.apsusc.2015.08.074
Muchan P, Saiwan C, Narku-Tetteh J, Idem R, Supap T, Tontiwachwuthikul P (2017) Screening tests of aqueous alkanolamine solutions based on primary, secondary, and tertiary structure for blended aqueous amine solution selection in post-combustion CO2 capture. Chem Eng Sci 170:574–582. https://doi.org/10.1016/j.ces.2017.02.031
Khalil HPSA, Firoozian P, Bakare IO, Akil HM, Noor AM (2010) Exploring biomass-based carbon black as filler in epoxy composites: flexural and thermal properties. Mater Des 31:3419. https://doi.org/10.1016/j.matdes.2010.01.044
Wickramaarachchi W, Minakshi M, Gao X, Dabare R, Wong K (2021) Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem Eng J Adv 8:100158. https://doi.org/10.1016/j.ceja.2021.100158
Prahas D, Kartika Y, Indraswati N, Ismadji S (2008) Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem Eng J 140:32. https://doi.org/10.1016/j.cej.2007.08.032
Hui TS, Zaini MA (2015) Potassium hydroxide activation of activated carbon: a commentary. Carbon Letters 16(4):275–280. https://doi.org/10.5714/CL.2015.16.4.275
Abechi SE, Gimba CE, Uzairu A, Dallatu YA (2013) Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res J Chem Sci 3:54
Taha MF, Kiat CF, Shaharun MS, Ramli A (2011) Removal of Ni(II), Zn(II), and Pb(II) ions from single metal aqueous solution using activated carbon prepared from rice husk. World Academy of Science, Eng Technol 5:1473
Okman I, Karagöz S, Tay T, Erdem M (2014) Activated carbons from grape seeds by chemical activation with potassium carbonate and potassium hydroxide. Appl Surf Sci 293:138. https://doi.org/10.1016/j.apsusc.2013.12.117
Adibfar M, Kaghazchi T, Asasian N, Soleimani M (2014) Conversion of poly(ethylene terephthalate) waste into activated carbon: chemical activation and characterization. Chem Eng Technol 37:979. https://doi.org/10.1002/ceat.201200719
Yu Q, Li M, Ning P, Yi H, Tang X (2014) Preparation and phosphine adsorption of activated carbon prepared from walnut shells by KOH chemical activation. Sep Sci Technol 49:2366. https://doi.org/10.1080/01496395.2014.917326
Linares-Solano A, Lillo-Ródenas M, Marco-Lozar JP, Kunowsky M, Romero-Anaya AJ (2012) NaOH and KOH for preparing activated carbons used in energy and environmental applications. Int J Energy Environ Econ 20(4):59–91
Nedjai R, Kabbashi NA, Alam MZ, Al-Khatib MFR (2021) Production and characterization of activated carbon from baobab fruit shells by chemical activation using ZnCl2, H3PO4 and KOH. Journal of Physics: Conference Series. 2129:012009. https://doi.org/10.1088/1742-6596/2129/1/012009. (IOP Publishing)
Heidarinejada Z, Rahmanianb O, Heidar M (2019) Production of KOH-activated carbon from date press cake: effect of the activating agent on its properties and Pb(II) adsorption potential. Desalin Water Treat 165(2019):232–243. https://doi.org/10.5004/dwt.2019.24501
Li XF, Xu Q, Fu Y, Guo QX (2014) Preparation and characterization of activated carbon from Kraft lignin via KOH activation. Environ Prog Sustain Energy 33:519. https://doi.org/10.1002/ep.11794
Idris-Hermann KT, Raoul TTD, Giscard D, Gabche AS (2018) Preparation and characterization of activated carbons from bitter kola (Garcinia kola) nut shells by chemical activation method using H3PO4; KOH and ZnCl2. Chem Sci Int J 23:1–15. https://doi.org/10.9734/CSJI/2018/43411
Mojoudi N, Mirghafari N, Soleimani M, Shariatmadari S, Belver C, Bedia J (2019) Phenol adsorption on high microporous activated carbons prepared from oily sludge: equilibrium, kinetic and thermodynamic studies. Sci Rep 9:19352. https://doi.org/10.1038/s41598-019-55794-4
Jing J, Zhao Z, Zhang X, Feng J, Li W (2022) CO2 capture over activated carbon derived from pulverized semi-coke. Separations 9:174. https://doi.org/10.3390/separations9070174
Presser V, McDonough J, Yeon SH, Gogotsi Y (2011) Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ Sci 4:3059–3066
Machrouhia A, Farnanea M, Tounsadib H, Kadmic Y, Favierd L, Qourzale S, Abdennouria M, Barkaa N (2019) Activated carbon from Thapsia transtagana stems: central composite design (CCD) optimization of the preparation conditions and efficient dyes removal. Desalin Water Treat 166:259–278. https://doi.org/10.5004/dwt.2019.24471
Vunain E, Kenneth D, Biswick T (2017) Synthesis and characterization of low-cost activated carbon prepared from Malawian baobab fruit shells by H3PO4 activation for removal of Cu(II) ions: equilibrium and kinetics studies. Appl Water Sci 7(8):4301–4319. https://doi.org/10.1007/s13201-017-0573-x
Elliott CG, Colby TV, Kelly TM, Hicks HG (1989) Charcoal lung: Bronchiolitis obliterans after aspiration of activated charcoal. Chest 96:672–674
Arriagada R, Garcia R, Molina-Sabio M, Rodriguez-Reinoso F (1997) Effect of steam activation on the porosity and chemical nature of activated carbons from Eucalyptus globules and peach stones. Microporous Mater 8:123–130. https://doi.org/10.1016/S0927-6513(96)00078-8
Yang T, Lua AC (2006) Textural and chemical properties of zinc chloride activated carbons prepared from pistachio-nut shells. Mater Chem Phys 100:438–444. https://doi.org/10.1016/j.matchemphys.2006.01.039
Tangjuank S, Insuk N, Udeye V, Tontrakoon J (2009) Chromium (III) sorption from aqueous solutions using activated carbon prepared from cashew nut shells. Int J Phys Sci 4:412–417. https://doi.org/10.5897/IJPS.9000118
Zhao Y, Liu X, Yao KX, Zhao L, Han Y (2012) Superior capture of CO2 achieved by introducing extra-framework cations into N-doped microporous carbon. Chem Mater 24:4725–4734
Rashidi NA, Yusup S, Hameed BH (2013) Kinetic studies on carbon dioxide capture using lignocel-lulosic based activated carbon. Energy 61:440–446
Shahkarami S (2017). CO2 capture from gases using activated carbon. A Thesis Submitted to the College of Graduate and Postdoctoral Studies In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy In the Department of Chemical and Biological Engineering University of Saskatchewan Saskatoon, Canada
Fiuza-Jr RA, Andrade RC, Andrade HM (2016) CO2 capture on KOH-activated carbons derived from yellow mombin fruit stones, Journal of Environmental Chemical Engineering. Part A 4(4):4229–4236. https://doi.org/10.1016/j.jece.2016.09.025
Wang R, Wang P, Yan X, Lang J, Peng C, Xue Q (2012) Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl Mater Interfaces 4:5800–5806
Abuelnoor N, AlHajaj A, Khaleel M, Vega LF, Abu Zahra M (2021). Single step synthesis and characterization of activated carbon from date seeds for CO2 capture. In Proceedings of the 15th International Conference on Greenhouse Gas Control Technologies, Abu Dhabi, United Arab Emirates, 15–18 March 2021
Sarwar A, Ali M, Khoja AH, Nawar A, Waqas A, Liaquat R, Naqvi SR, Asjid M (2021) Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J CO2 Util 46:101476
He S, Chen G, Xiao H, Shi G, Ruan C, Ma Y, Dai H, Yuan B, Chen X, Yang X (2021) Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J Colloid Interface Sci 582:90–101
Heidari A, Younesi H, Rashidi A, Ghoreyshi A (2014) Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: effect of chemical activation. J Taiwan Inst Chem Eng 45:579–588
Lee HJ, Ko D, Kim JS, Park Y, Hwang I, Yavuz CT, Choi JW (2021) Cesium ion-mediated microporous carbon for CO2 capture and lithium-ion storage. ChemNanoMat 7:150–157
Author information
Authors and Affiliations
Contributions
All the authors contributed to the final version of this manuscript. Anyebe Moses and Aimikhe Victor did the formal analysis and wrote the first draft. Aimikhe Victor did the research conceptualization, theoretical framework, and reviewed and edited the first draft. Aimikhe Victor and Ibezim-Ezeani Millicent wrote the final manuscript drafts and did the research supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aimikhe, V.J., Anyebe, M.S. & Ibezim-Ezeani, M. Development of composite activated carbon from mango and almond seed shells for CO2 capture. Biomass Conv. Bioref. 14, 4645–4659 (2024). https://doi.org/10.1007/s13399-022-03665-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03665-w