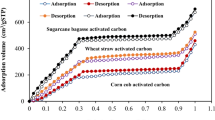
Abstract
The persistent increase in the atmospheric concentration of carbon dioxide (CO2), the primary anthropogenic greenhouse gas contributing to global warming, makes research directed towards carbon capture and storage (CCS) imperative. In the past few years, among the available adsorbents, biochar has drawn significant interest as a promising carbon-based material for low-temperature CO2 capture from flue/fuel gas (such as biogas or gasification-derived syngas) owing to its environmentally friendly nature, cost-effective and facile preparation method, and sustainable adsorption performance. This work provides a review of recent studies on the development of biochar from biomass feedstocks and its subsequent modification through various approaches, including physical, chemical and physicochemical activations for post-combustion CO2 capture. An overview of the factors, including pyrolysis temperature, heating rate and time, and different modification methods, affecting the physicochemical attributes of biochar such as surface area, microporosity, surface properties and functional groups is presented. Biochar with a large micropore volume, a narrow microporosity (0.3–0.8 nm) and basic surface characteristics would be effective in adsorbing CO2 molecules. In this regard, physical modification of biochar is closely related to pore formation, whereas chemical modification emphasizes the creation of oxygen and nitrogen-containing functional groups; hence, they contribute to the enhanced CO2 capture through porosity development and surface chemistry alteration, respectively. Biochar has presented a strong selectivity towards CO2 compared to other gasses and has revealed a sustainable performance in multi-cycles of CO2 adsorption–desorption; these are crucial features to ensure the large-scale application of biochar for CO2 capture.















copyright of RSC license (CC-BY 4.0)
Similar content being viewed by others
References
Bose BK (2010) Global warming: Energy, environmental pollution, and the impact of power electronics. IEEE Ind Electron Mag 4:6–17. https://doi.org/10.1109/MIE.2010.935860
Vaz S, Paula A, De SR, Eduardo B, Baeta L (2022) Technologies for carbon dioxide capture : A review applied to energy sectors. Clean Eng Technol 8:100456. https://doi.org/10.1016/j.clet.2022.100456
(1997) Kyoto Protocol. https://unfccc.int/kyoto_protocol. 13/03/2022. Accessed 13 March 2022.
(2015) Paris Agreement. https://unfccc.int/process-and-meetings/the-paris-. 13/03/2022. Accessed 13 March 2022.
Gielen D, Boshell F, Saygin D, Bazilian MD, Wagner N, Gorini R (2019) The role of renewable energy in the global energy transformation. Energy Strateg Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
Beiyuan J, Awad YM, Beckers F, Tsang DCW, Ok YS, Rinklebe J (2017) Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 178:110–118. https://doi.org/10.1016/j.chemosphere.2017.03.022
Yaumi AL, Bakar MZA, Hameed BH (2017) Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 124:461–480. https://doi.org/10.1016/j.energy.2017.02.053
Lee SY, Park SJ (2015) A review on solid adsorbents for carbon dioxide capture. J Ind Eng Chem 23:1–11. https://doi.org/10.1016/j.jiec.2014.09.001
Creamer AE, Gao B (2016) Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ Sci Technol 50:7276–7289. https://doi.org/10.1021/acs.est.6b00627
Hwang KJ, Choi WS, Jung SH, Kwon YJ, Hong S, Choi C, Lee JW, Shim WG (2018) Synthesis of zeolitic material from basalt rock and its adsorption properties for carbon dioxide. RSC Adv 8:9524–9529. https://doi.org/10.1039/c8ra00788h
Ibrahim GH, Al-Meshragi AM (2020) Experimental study of adsorption on activated carbon for CO2 capture. In: L. A. Frazão, A. M. Silva-Olaya & JCS (ed) CO2 Sequestration. IntechOpen, pp 1–20
Wang J, Huang H, Wang M, Yao L, Qiao W, Long D, Ling L (2015) Direct capture of low-concentration CO2 on mesoporous carbon-supported solid amine adsorbents at ambient temperature. Ind Eng Chem Res 54:5319–5327. https://doi.org/10.1021/acs.iecr.5b01060
Songolzadeh M, Soleimani M, Takht Ravanchi M, Songolzadeh R (2014) Carbon dioxide separation from flue gases: A technological review emphasizing reduction in greenhouse gas emissions. Sci World J 2014:828131. https://doi.org/10.1155/2014/828131
Shao J, Zhang J, Zhang X, Feng Y, Zhang H, Zhang S, Chen H (2018) Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 224:138–146. https://doi.org/10.1016/j.fuel.2018.03.064
Pang S (2019) Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol Adv 37:589–597. https://doi.org/10.1016/j.biotechadv.2018.11.004
Lin Y, Ma X, Peng X, Yu Z, Fang S, Lin Y, Fan Y (2016) Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 181:905–915. https://doi.org/10.1016/j.fuel.2016.05.031
Papageorgiou A, Azzi ES, Enell A, Sundberg C (2021) Biochar produced from wood waste for soil remediation in Sweden: Carbon sequestration and other environmental impacts. Sci Total Environ 776:145953. https://doi.org/10.1016/j.scitotenv.2021.145953
Han T (2020) Properties of biochar from wood and textile. IOP Conf Ser Earth Environ Sci 546:042060. https://doi.org/10.1088/1755-1315/546/4/042060
Manyà JJ, González B, Azuara M, Arner G (2018) Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem Eng J 345:631–639. https://doi.org/10.1016/j.cej.2018.01.092
Zubbri NA, Mohamed AR, Kamiuchi N, Mohammadi M (2020) Enhancement of CO2 adsorption on biochar sorbent modified by metal incorporation. Environ Sci Pollut Res 27:11809–11829. https://doi.org/10.1007/s11356-020-07734-3
Zhang X, Zhang S, Yang H, Shao J, Chen Y, Feng Y, Wang X, Chen H (2015) Effects of hydrofluoric acid pre-deashing of rice husk on physicochemical properties and CO2 adsorption performance of nitrogen-enriched biochar. Energy 91:903–910. https://doi.org/10.1016/j.energy.2015.08.028
Zhang J, Huang B, Chen L, Li Y, Li W, Luo Z (2018) Characteristics of biochar produced from yak manure at different pyrolysis temperatures and its effects on the yield and growth of highland barley. Chem Speciat Bioavailab 30:57–67. https://doi.org/10.1080/09542299.2018.1487774
Rehman A, Nawaz S, Alghamdi HA, Alrumman S, Yan W, Nawaz MZ (2020) Effects of manure-based biochar on uptake of nutrients and water holding capacity of different types of soils. Case Stud Chem Environ Eng 2:100036. https://doi.org/10.1016/j.cscee.2020.100036
Bong CPC, Lim LY, Lee CT, Ong PY, Klemeš JJ, Li C, Gao Y (2020) Lignocellulosic biomass and food waste for biochar production and application: A review. Chem Eng Trans 81:427–432. https://doi.org/10.3303/CET2081072
Igalavithana AD, Choi SW, Dissanayake PD et al (2020) Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption. J Hazard Mater 391:121147. https://doi.org/10.1016/j.jhazmat.2019.121147
Yue Y, Cui L, Lin Q, Li G, Zhao X (2017) Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 173:551–556. https://doi.org/10.1016/j.chemosphere.2017.01.096
Racek J, Sevcik J, Chorazy T, Kucerik J, Hlavinek P (2020) Biochar – Recovery material from pyrolysis of sewage sludge: A review. Waste and Biomass Valorization 11:3677–3709. https://doi.org/10.1007/s12649-019-00679-w
Huang YF, Te SH, Te CP, Lo SL (2016) Co-torrefaction of sewage sludge and Leucaena by using microwave heating. Energy 116:1–7. https://doi.org/10.1016/j.energy.2016.09.102
Islam MS, Kwak JH, Nzediegwu C et al (2021) Biochar heavy metal removal in aqueous solution depends on feedstock type and pyrolysis purging gas. Environ Pollut 281:117094. https://doi.org/10.1016/j.envpol.2021.117094
Wang X, Li X, Liu G, He Y, Chen C, Liu X, Li G, Gu Y, Zhao Y (2019) Mixed heavy metal removal from wastewater by using discarded mushroom-stick biochar: Adsorption properties and mechanisms. Environ Sci Process Impacts 21:584–592. https://doi.org/10.1039/c8em00457a
Deng Y, Li X, Ni F, Liu Q, Yang Y, Wang M, Ao T, Chen W (2021) Synthesis of magnesium modified biochar for removing copper, lead and cadmium in single and binary systems from aqueous solutions: Adsorption mechanism. Water (Switzerland) 13:599. https://doi.org/10.3390/w13050599
Liang M, Lu L, He H, Li J, Zhu Z, Zhu Y (2021) Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustain 13:1–18. https://doi.org/10.3390/su132414041
Dai Y, Wang W, Lu L, Yan L, Yu D (2020) Utilization of biochar for the removal of nitrogen and phosphorus. J Clean Prod 257:120573. https://doi.org/10.1016/j.jclepro.2020.120573
Anthonysamy SI, Lahijani P, Mohammadi M, Mohamed AR (2021) Alkali-modified biochar as a sustainable adsorbent for the low-temperature uptake of nitric oxide. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03617-3
Azwan A, Rahman A, Alias AB, Jaffar NN, Amir MA, Ghani WAWAK (2019) Adsorption of hydrogen sulphide by commercialized Rice Husk Biochar (RHB) & Hydrogel Biochar Composite (RH-HBC). Int J Recent Technol Eng 8:6864–6870. https://doi.org/10.35940/ijrte.d5207.118419
Liu WJ, Jiang H, Yu HQ (2019) Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ Sci 12:1751–1779. https://doi.org/10.1039/C9EE00206E
Lee J, Kim KH, Kwon EE (2017) Biochar as a Catalyst. Renew Sustain Energy Rev 77:70–79. https://doi.org/10.1016/j.rser.2017.04.002
Choi S, Drese JH, Jones CW (2009) Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem 2:796–854. https://doi.org/10.1002/cssc.200900036
Prauchner MJ, da Cunha Oliveira S, Rodríguez-Reinoso F (2020) Tailoring low-cost granular activated carbons intended for CO2 adsorption. Front Chem 8:1–16. https://doi.org/10.3389/fchem.2020.581133
Jo K, Baek Y, Lee C, Yoon J (2019) Effect of hydrophilicity of activated carbon electrodes on desalination performance in membrane capacitive deionization. Appl Sci 9:5055. https://doi.org/10.3390/app9235055
Abd AA, Naji SZ, Hashim AS, Othman MR (2020) Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J Environ Chem Eng 8:104142. https://doi.org/10.1016/j.jece.2020.104142
Millward AR, Yaghi OM (2005) Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J Am Chem Soc 127:17998. https://doi.org/10.1021/ja0570032
Yuan Z, Eden MR, Gani R (2016) Toward the development and deployment of large-scale carbon dioxide capture and conversion processes. Ind Eng Chem Res 55:3383–3419. https://doi.org/10.1021/acs.iecr.5b03277
Sun H, Wu C, Shen B, Zhang X, Zhang Y, Huang J (2018) Progress in the development and application of CaO-based adsorbents for CO2 capture—a review. Mater Today Sustain 1–2:1–27. https://doi.org/10.1016/j.mtsust.2018.08.001
Zhang X, Gao B, Creamer AE, Cao C, Li Y (2017) Adsorption of VOCs onto engineered carbon materials: A review. J Hazard Mater 338:102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Alhashimi HA, Aktas CB (2017) Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour Conserv Recycl 118:13–26. https://doi.org/10.1016/j.resconrec.2016.11.016
Shafawi AN, Mohamed AR, Lahijani P, Mohammadi M (2021) Recent advances in developing engineered biochar for CO2 capture : An insight into the biochar modification approaches. J Environ Chem Eng 9:106869. https://doi.org/10.1016/j.jece.2021.106869
Dulanja P, You S, Deshani A et al (2020) Biochar-based adsorbents for carbon dioxide capture : A critical review. Renew Sustain Energy Rev 119:109582. https://doi.org/10.1016/j.rser.2019.109582
Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W, Jiang S, Li H, Huang H (2021) An overview on engineering the surface area and porosity of biochar. Sci Total Environ 763:144204. https://doi.org/10.1016/j.scitotenv.2020.144204
US Department of Energy (2015) Carbon dioxide capture for natural gas and industrial applications. Chapter 4: Advancing clean electric power technologies. https://www.energy.gov/sites/default/files/2015/12/f27/QTR2015-4D-Carbon-Dioxide-Capture-for-Natural-Gas-and-Industrial-Applications.pdf. Accessed 22 June 2022
United States Department of Energy (2017) Carbon capture opportunities for natural gas fired power systems. Washington DC, USA. https://www.energy.gov/sites/prod/files/2017/01/f34/Carbon%20Capture%20Opportunities%20for%20Natural%20Gas%20Fired%20Power%20Systems_0.pdf. Accessed 22 June 2022
Bains P, Psarras P, Wilcox J (2017) CO2 capture from the industry sector. Prog Energy Combust Sci 63:146–172. https://doi.org/10.1016/j.pecs.2017.07.001
Kanniche M, Gros-Bonnivard R, Jaud P, Valle-Marcos J, Amann JM, Bouallou C (2010) Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl Therm Eng 30:53–62. https://doi.org/10.1016/j.applthermaleng.2009.05.005
Carpenter SM, Long HA (2017) 13-Integration of carbon capture in IGCC systems. In: Wang T, Stiegel G (eds) Integrated Gasification Combined Cycle (IGCC) Technologies. Woodhead Publishing, pp 445–463
Blomen E, Hendriks C, Neele F (2009) Capture technologies: Improvements and promising developments. Energy Procedia 1:1505–1512. https://doi.org/10.1016/j.egypro.2009.01.197
Spigarelli BP, Kawatra SK (2013) Opportunities and challenges in carbon dioxide capture. J CO2 Util 1:69–87. https://doi.org/10.1016/j.jcou.2013.03.002
Padurean A, Cormos CC, Agachi PS (2012) Pre-combustion carbon dioxide capture by gas–liquid absorption for integrated gasification combined cycle power plants. Int J Greenh Gas Control 7:1–11. https://doi.org/10.1016/j.ijggc.2011.12.007
Weiss H (1988) Rectisol wash for purification of partial oxidation gases. Gas Sep Purif 2:171–176. https://doi.org/10.1016/0950-4214(88)80002-1
Osman AI, Hefny M, Abdel Maksoud MIA, Elgarahy AM, Rooney DW (2021) Recent advances in carbon capture storage and utilisation technologies: A review. Environ Chem Lett 19:797–849. https://doi.org/10.1007/s10311-020-01133-3
Chen S, Yu R, Soomro A, Xiang W (2019) Thermodynamic assessment and optimization of a pressurized fluidized bed oxy-fuel combustion power plant with CO2 capture. Energy 175:445–455. https://doi.org/10.1016/j.energy.2019.03.090
Pandey, S. Gupta S, Tomar, A. Kumar, A (2010) Post combustion carbon capture technology. Natl Conf Eco Friendly Manuf Sustain Dev. GLA University Mathura, Paper No. 56
Moioli S, Nagy T, Langé S, Pellegrini LA, Mizsey P (2017) Simulation model evaluation of CO2 capture by aqueous MEA scrubbing for heat requirement analyses. Energy Procedia 114:1558–1566. https://doi.org/10.1016/j.egypro.2017.03.1286
Makertihartha IGBN, Dharmawijaya PT, Zunita M, Wenten IG (2017) Post combustion CO2 capture using zeolite membrane. AIP Conf Proc 1818https://doi.org/10.1063/1.4979941
Gonzalez-Garza D, Rivera-Tinoco R, Bouallou C (2009) Comparison of ammonia, monoethanolamine, diethanolamine and methyldiethanolamine solvents to reduce CO2 greenhouse gas emissions. Chem Eng Trans 18:279–284. https://doi.org/10.3303/CET0918044
Valluri S, Kawatra SK (2021) Use of frothers to improve the absorption efficiency of dilute sodium carbonate slurry for post combustion CO2 capture. Fuel Process Technol 212:106620. https://doi.org/10.1016/j.fuproc.2020.106620
Al-Sudani F (2020) Absorption of carbon dioxide into aqueous ammonia solution using blended promoters (MEA, MEA+PZ, PZ+ArgK, MEA+ArgK). Eng Technol J 38:1359–1372. https://doi.org/10.30684/etj.v38i9a.876
Huang HP, Shi Y, Li W, Chang SG (2001) Dual alkali approaches for the capture and separation of CO2. Energy Fuels 15:263–268. https://doi.org/10.1021/ef0002400
Knuutila H, Svendsen HF, Anttila M (2009) CO2 capture from coal-fired power plants based on sodium carbonate slurry; a systems feasibility and sensitivity study. Int J Greenh Gas Control 3:143–151. https://doi.org/10.1016/j.ijggc.2008.06.006
Zhao R, Zhao L, Wang S, Deng S, Li H, Yu Z (2018) Solar-assisted pressure-temperature swing adsorption for CO2 capture: Effect of adsorbent materials. Sol Energy Mater Sol Cells 185:494–504. https://doi.org/10.1016/j.solmat.2018.06.004
Ammendola P, Raganati F, Chirone R, Miccio F (2020) Fixed bed adsorption as affected by thermodynamics and kinetics: Yellow tuff for CO2 capture. Powder Technol 373:446–458. https://doi.org/10.1016/j.powtec.2020.06.075
Kishibayev KK, Serafin J, Tokpayev RR, Khavaza TN, Atchabarova AA, Abduakhytova DA, Ibraimov ZT, Sreńscek-Nazzal J (2021) Physical and chemical properties of activated carbon synthesized from plant wastes and shungite for CO2 capture. J Environ Chem Eng 9:106798. https://doi.org/10.1016/j.jece.2021.106798
Serafin J, Sre´nscek-Nazzal J, Kami´nska A, Paszkiewicz O, Michalkiewicz B (2022) Management of surgical mask waste to activated carbons for CO2 capture. J CO2 Util 59:101970. https://doi.org/10.1016/j.jcou.2022.101970
Voss C (2005) Applications of pressure swing adsorption technology. Adsorption 11:527–529. https://doi.org/10.1007/s10450-005-5979-3
Raganati F, Chirone R, Ammendola P (2020) CO2 capture by temperature swing adsorption: Working capacity as affected by temperature and CO2 Partial pressure. Ind Eng Chem Res 59:3593–3605. https://doi.org/10.1021/acs.iecr.9b04901
Majchrzak-Kucęba I, Sołtysik M (2020) The potential of biocarbon as CO2 adsorbent in VPSA unit. J Therm Anal Calorim 142:267–273. https://doi.org/10.1007/s10973-020-09858-7
Dhoke C, Zaabout A, Cloete S, Amini S (2021) Review on reactor configurations for adsorption-based CO2 capture. Ind Eng Chem Res 60:3779–3798. https://doi.org/10.1021/acs.iecr.0c04547
Pires JCM, Martins FG, Alvim-Ferraz MCM, Simões M (2011) Recent developments on carbon capture and storage: An overview. Chem Eng Res Des 89:1446–1460. https://doi.org/10.1016/j.cherd.2011.01.028
Lei L, Bai L, Lindbråthen A, Pan F, Zhang X, He X (2020) Carbon membranes for CO2 removal: Status and perspectives from materials to processes. Chem Eng J 401:126084. https://doi.org/10.1016/j.cej.2020.126084
Wang Y, Zhao L, Otto A, Robinius M, Stolten D (2017) A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 114:650–665. https://doi.org/10.1016/j.egypro.2017.03.1209
Karimi M, Shirzad M, Silva JAC, Rodrigues AE (2022) Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J CO2 Util 57:. https://doi.org/10.1016/j.jcou.2022.101890
Lai JY, Ngu LH (2021) A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenh Gas Sci Technol 0:1–41. https://doi.org/10.1002/ghg.2112
Ullah R, Salah AH, Saad M, Aparicio S, Atilhan M (2018) Adsorption equilibrium studies of CO2, CH4 and N2on various modified zeolites at high pressures up to 200 bars. Microporous Mesoporous Mater 262:49–58. https://doi.org/10.1016/j.micromeso.2017.11.022
Bae JS, Bhatia SK (2006) High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 20:2599–2607. https://doi.org/10.1021/ef060318y
Ao W, Fu J, Mao X et al (2018) Microwave assisted preparation of activated carbon from biomass: A review. Renew Sustain Energy Rev 92:958–979. https://doi.org/10.1016/j.rser.2018.04.051
Madzaki H, Ghani WAWAK, Rebitanim NZ, Alias AB (2016) Carbon Dioxide Adsorption on Sawdust Biochar. Procedia Eng 148:718–725. https://doi.org/10.1016/j.proeng.2016.06.591
Gargiulo V, Gomis-Berenguer A, Giudicianni P, Ania CO, Ragucci R, Alfè M (2018) Assessing the potential of biochars prepared by steam-assisted slow pyrolysis for CO2 adsorption and separation. Energy Fuels 32:10218–10227. https://doi.org/10.1021/acs.energyfuels.8b01058
Zubbri NA, Mohamed AR, Lahijani P, Mohammadi M (2021) Low temperature CO2 capture on biomass-derived KOH-activated hydrochar established through hydrothermal carbonization with water-soaking pre-treatment. J Environ Chem Eng 9:105074. https://doi.org/10.1016/j.jece.2021.105074
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276–279. https://doi.org/10.1038/46248
Abdi J, Hadavimoghaddam F, Hadipoor M, Hemmati-Sarapardeh A (2021) Modeling of CO2 adsorption capacity by porous metal organic frameworks using advanced decision tree-based models. Sci Rep 11:1–14. https://doi.org/10.1038/s41598-021-04168-w
Williams JL, Piatrik M, Choi S-K, Stannett V (1977) Postdecrystallization rates of grafted fibers and their effect on fiber elasticity. I. Effect of zinc chloride concentration. J Appl Polym Sci 21:1377–1381. https://doi.org/10.1002/app.1977.070210519
Xiang S, He Y, Zhang Z, Wu H, Zhou W, Krishna R, Chen B (2012) Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat Commun 3:954–959. https://doi.org/10.1038/ncomms1956
Mustafa J, Farhan M, Hussain M (2016) CO2 separation from flue gases using different types of membranes. J Membr Sci Technol 6:153. https://doi.org/10.4172/2155-9589.1000153
Shin DW, Hyun SH, Cho CH, Han MH (2005) Synthesis and CO2/N2 gas permeation characteristics of ZSM-5 zeolite membranes. Microporous Mesoporous Mater 85:313–323. https://doi.org/10.1016/j.micromeso.2005.06.035
Brinkmann T, Lillepärg J, Notzke H, Pohlmann J, Shishatskiy S, Wind J, Wolff T (2017) Development of CO2 selective poly(ethylene oxide)-based membranes: From laboratory to pilot plant scale. Engineering 3:485–493. https://doi.org/10.1016/J.ENG.2017.04.004
Papari S, Hawboldt K, Helleur R (2015) Pyrolysis: A theoretical and experimental study on the conversion of softwood sawmill residue to biooil. Ind Eng Chem Res 54:605–611. https://doi.org/10.1021/ie5039456
Adelawon BO, Latinwo GK, Eboibi BE, Agbede OO, Agarry SE (2021) Comparison of the slow, fast, and flash pyrolysis of recycled maize-cob biomass waste, box-benhken process optimization and characterization studies for the thermal fast pyrolysis production of bio-energy. Chem Eng Commun 0:1–31. https://doi.org/10.1080/00986445.2021.1957851
Mazlan MAF, Uemura Y, Osman NB, Yusup S (2015) Characterizations of bio-char from fast pyrolysis of Meranti wood sawdust. J Phys Conf Ser 622:012054. https://doi.org/10.1088/1742-6596/622/1/012054
Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H (2012) Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem 46:73–79. https://doi.org/10.1016/j.soilbio.2011.11.019
Brownsort P (2009) Biomass pyrolysis processes: Performance parameters and their influence on biochar system benefits. Dissertation, University of Edinburgh
Huang YF, Te CP, Shih CH, Lo SL, Sun L, Zhong Y, Qiu C (2015) Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 84:75–82. https://doi.org/10.1016/j.energy.2015.02.026
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: A review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.jclepro.2019.04.282
Nunoura T, Wade SR, Bourke JP, Antal MJ (2006) Studies of the Flash Carbonization Process. 1. Propagation of the Flaming Pyrolysis Reaction and Performance of a Catalytic Afterburner. Ind Eng Chem Res 45:585–599. https://doi.org/10.1021/ie050854y
Panwar NL, Pawar A, Salvi BL (2019) Comprehensive review on production and utilization of biochar. SN Appl Sci 1:1–19. https://doi.org/10.1007/s42452-019-0172-6
Tian H, Hu Q, Wang J, Chen D, Yang Y, Bridgwater AV (2021) Kinetic study on the CO2 gasification of biochar derived from Miscanthus at different processing conditions. Energy 217:119341. https://doi.org/10.1016/j.energy.2020.119341
Brewer CE, Schmidt-Rohr K, Satrio JA, RCB, (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sustain Energy 28:386–396. https://doi.org/10.1002/ep.10378
Chen WH, Peng J, Bi XT (2015) A state-of-the-art review of biomass torrefaction, densification and applications. Renew Sustain Energy Rev 44:847–866. https://doi.org/10.1016/j.rser.2014.12.039
Demirbas A (2009) Biorefineries: Current activities and future developments. Energy Convers Manag 50:2782–2801. https://doi.org/10.1016/j.enconman.2009.06.035
Xie Y, Wang L, Li H, Westholm LJ, Carvalho L, Thorin E, Yu Z, Yu X, Skreiberg Ø (2022) A critical review on production, modification and utilization of biochar. J Anal Appl Pyrolysis 161:105405. https://doi.org/10.1016/j.jaap.2021.105405
Wang Y, Qiu L, Zhu M, Sun G, Zhang T, Kang K (2019) Comparative evaluation of hydrothermal carbonization and low temperature pyrolysis of Eucommia ulmoides Oliver for the production of solid biofuel. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-38849-4
Peng C, Zhai Y, Zhu Y, Wang T, Xu B, Wang T, Li C, Zeng G (2017) Investigation of the structure and reaction pathway of char obtained from sewage sludge with biomass wastes, using hydrothermal treatment. J Clean Prod 166:114–123. https://doi.org/10.1016/j.jclepro.2017.07.108
Yan W, Hastings JT, Acharjee TC, Coronella CJ, Vásquez VR (2010) Mass and energy balances of wet torrefaction of lignocellulosic biomass. Energy Fuels 24:4738–4742. https://doi.org/10.1021/ef901273n
Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization : Fate of inorganics. Biomass Bioenerg 49:86–94. https://doi.org/10.1016/j.biombioe.2012.12.004
Karthik V, Kumar PS, Vo DVN, Sindhu J, Sneka D, Subhashini B, Saravanan K, Jeyanthi J (2021) Hydrothermal production of algal biochar for environmental and fertilizer applications: A review. Environ Chem Lett 19:1025–1042. https://doi.org/10.1007/s10311-020-01139-x
Zhang B, Heidari M, Regmi B, Salaudeen S, Arku P, Thimmannagari M, Dutta A (2018) Hydrothermal carbonization of fruit wastes: A promising technique for generating hydrochar. Energies 11:1–14. https://doi.org/10.3390/en11082022
Manyà JJ, García-Morcate D, González B (2020) Adsorption performance of physically activated biochars for postcombustion CO2 capture from dry and humid flue gas. Appl Sci 10:1–17. https://doi.org/10.3390/app10010376
Creamer AE, Gao B, Zhang M (2014) Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem Eng J 249:174–179. https://doi.org/10.1016/j.cej.2014.03.105
Lahijani P, Mohammadi M, Mohamed AR (2018) Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J CO2 Util 26:281–293. https://doi.org/10.1016/j.jcou.2018.05.018
Mohamed Noor N, Shariff A, Abdullah N, Mohamad Aziz NS (2019) Temperature effect on biochar properties from slow pyrolysis of coconut flesh waste. Malaysian J Fundam Appl Sci 15:153–158. https://doi.org/10.11113/mjfas.v15n2.1015
Demirbaş A, Arin G (2002) An overview of biomass pyrolysis. Energy Sources 24:471–482. https://doi.org/10.1080/00908310252889979
Shukla N, Sahoo D, Remya N (2019) Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J Clean Prod 235:1073–1079. https://doi.org/10.1016/j.jclepro.2019.07.042
Ng WC, You S, Ling R, Gin KYH, Dai Y, Wang CH (2017) Co-gasification of woody biomass and chicken manure: Syngas production, biochar reutilization, and cost-benefit analysis. Energy 139:732–742. https://doi.org/10.1016/j.energy.2017.07.165
Mustaza MNF, Mizan MN, Yoshida H, Izhar S (2021) Torréfaction of mangrove wood by introducing superheated steam for biochar production. IOP Conf Ser Earth Environ Sci 765:012027. https://doi.org/10.1088/1755-1315/765/1/012027
Roy P, Dutta A, Gallant J (2018) Hydrothermal carbonization of peat moss and herbaceous biomass (miscanthus): A potential route for bioenergy. Energies 11:2794. https://doi.org/10.3390/en11102794
Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T (2014) Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014
Chiang YC, Juang RS (2017) Surface modifications of carbonaceous materials for carbon dioxide adsorption: A review. J Taiwan Inst Chem Eng 71:214–234. https://doi.org/10.1016/j.jtice.2016.12.014
Cetin E, Moghtaderi B, Gupta R, Wall TF (2004) Influence of pyrolysis conditions on the structure and gasification reactivity of biomass chars. Fuel 83:2139–2150. https://doi.org/10.1016/j.fuel.2004.05.008
Chen D, Li Y, Cen K, Luo M, Li H, Lu B (2016) Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature. Bioresour Technol 218:780–788. https://doi.org/10.1016/j.biortech.2016.07.049
Bagreev A, Bandosz TJ, Locke DC (2001) Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage sludge-derived fertilizer. Carbon 39:1971–1979. https://doi.org/10.1016/S0008-622328012900026-4
IUPAC (1972) Manual of symbols and terminology, Appendix 2, Part. 1, colloid and surface chemistry. Pure Appl Chem 31:578–638
Presser V, McDonough J, Yeon SH, Gogotsi Y (2011) Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ Sci 4:3059–3066. https://doi.org/10.1039/c1ee01176f
Deng S, Wei H, Chen T, Wang B, Huang J, Yu G (2014) Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem Eng J 253:46–54. https://doi.org/10.1016/j.cej.2014.04.115
Khandaker T, Hossain MS, Dhar PK, Rahman MS, Hossain MA, Ahmed MB (2020) Efficacies of carbon-based adsorbents for carbon dioxide capture. Processes 8:1–32. https://doi.org/10.3390/PR8060654
Gao X, Yang S, Hu L, Cai S, Wu L, Kawi S (2022) Carbonaceous materials as adsorbents for CO2 capture: Synthesis and modification. Carbon Capture Sci Technol 3:100039. https://doi.org/10.1016/j.ccst.2022.100039
Kloss S, Zehetner F, Dellantonio A, Hamid R, Ottner F, Liedtke V, Schwanninger M, Gerzabek MH, Soja G (2012) Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J Environ Qual 41:990–1000. https://doi.org/10.2134/jeq2011.0070
Zhang H, Chen C, Gray EM, Boyd SE (2017) Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenerg 105:136–146. https://doi.org/10.1016/j.biombioe.2017.06.024
Chatterjee R, Sajjadi B, Chen WY, Mattern DL, Hammer N, Raman V, Dorris A (2020) Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front Energy Res 8:1–18. https://doi.org/10.3389/fenrg.2020.00085
Pallarés J, González-Cencerrado A, Arauzo I (2018) Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenerg 115:64–73. https://doi.org/10.1016/j.biombioe.2018.04.015
Zhang P, Sun H, Yu L, Sun T (2013) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J Hazard Mater 244–245:217–224. https://doi.org/10.1016/j.jhazmat.2012.11.046
Fu P, Hu S, Sun L, Xiang J, Yang T, Zhang A, Zhang J (2009) Structural evolution of maize stalk/char particles during pyrolysis. Bioresour Technol 100:4877–4883. https://doi.org/10.1016/j.biortech.2009.05.009
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon(biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Lua AC, Lau FY, Guo J (2006) Influence of pyrolysis conditions on pore development of oil-palm-shell activated carbons. J Anal Appl Pyrolysis 76:96–102. https://doi.org/10.1016/j.jaap.2005.08.001
Elnour AY, Alghyamah AA, Shaikh HM, Poulose AM, Al-Zahrani SM, Anis A, Al-Wabel MI (2019) Effect of pyrolysis temperature on biochar microstructural evolution, physicochemical characteristics, and its influence on biochar/polypropylene composites. Appl Sci 9:7–9. https://doi.org/10.3390/app9061149
Gai X, Wang H, Liu J, Zhai L, Liu S, Ren T, Liu H (2014) Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE 9:e113888. https://doi.org/10.1371/journal.pone.0113888
Selvarajoo A, Oochit D (2020) Effect of pyrolysis temperature on product yields of palm fibre and its biochar characteristics. Mater Sci Energy Technol 3:575–583. https://doi.org/10.1016/j.mset.2020.06.003
Titiladunayo IF, McDonald AG, Fapetu OP (2012) Effect of temperature on biochar product yield from selected lignocellulosic biomass in a pyrolysis process. Waste and Biomass Valorization 3:311–318. https://doi.org/10.1007/s12649-012-9118-6
Mohd Hasan MH, Bachmann RT, Loh SK, Manroshan S, Ong SK (2019) Effect of pyrolysis temperature and time on properties of palm kernel shell-based biochar. IOP Conf Ser Mater Sci Eng 548:012020. https://doi.org/10.1088/1757-899X/548/1/012020
Xu B, Argyle MD, Shi X, Goroncy AK, Rony AH, Tan G, Fan M (2020) Effects of mixture of CO2/CH4 as pyrolysis atmosphere on pine wood pyrolysis products. Renew Energy 162:1243–1254. https://doi.org/10.1016/j.renene.2020.08.069
Parvez AM, Afzal MT, Victor Hebb TG, Schmid M (2020) Utilization of CO2 in thermochemical conversion of biomass for enhanced product properties: A review. J CO2 Util 40:101217. https://doi.org/10.1016/j.jcou.2020.101217
Mellin P, Yu X, Yang W, Blasiak W (2015) Influence of reaction atmosphere (H2O, N2, H2, CO2, CO) on fluidized-bed fast pyrolysis of biomass using detailed tar vapor chemistry in computational fluid dynamics. Ind Eng Chem Res 54:8344–8355. https://doi.org/10.1021/acs.iecr.5b02164
Promraksa A, Rakmak N (2020) Biochar production from palm oil mill residues and application of the biochar to adsorb carbon dioxide. Heliyon 6:e04019. https://doi.org/10.1016/j.heliyon.2020.e04019
Fryda L, Visser R (2015) Biochar for soil improvement: Evaluation of biochar from gasification and slow pyrolysis. Agric 5:1076–1115. https://doi.org/10.3390/agriculture5041076
Guizani C, Escudero Sanz FJ, Salvador S (2014) Effects of CO2 on biomass fast pyrolysis: Reaction rate, gas yields and char reactive properties. Fuel 116:310–320. https://doi.org/10.1016/j.fuel.2013.07.101
Liu R, Liu G, Yousaf B, Abbas Q (2018) Operating conditions-induced changes in product yield and characteristics during thermal-conversion of peanut shell to biochar in relation to economic analysis. J Clean Prod 193:479–490. https://doi.org/10.1016/j.jclepro.2018.05.034
Ertaş M, Hakki Alma M (2010) Pyrolysis of laurel (Laurus nobilis L.) extraction residues in a fixed-bed reactor: Characterization of bio-oil and bio-char. J Anal Appl Pyrolysis 88:22–29. https://doi.org/10.1016/j.jaap.2010.02.006
Igalavithana AD, Lee SE, Lee YH, Tsang DCW, Rinklebe J, Kwon EE, Ok YS (2017) Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 174:593–603. https://doi.org/10.1016/j.chemosphere.2017.01.148
Zhao SX, Ta N, Wang XD (2017) Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 10:1293. https://doi.org/10.3390/en10091293
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Yang X, Kang K, Qiu L, Zhao L, Sun R (2020) Effects of carbonization conditions on the yield and fixed carbon content of biochar from pruned apple tree branches. Renew Energy 146:1691–1699. https://doi.org/10.1016/j.renene.2019.07.148
Zhao B, O’Connor D, Zhang J, Peng T, Shen Z, Tsang DCW, Hou D (2018) Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J Clean Prod 174:977–987. https://doi.org/10.1016/j.jclepro.2017.11.013
Angin D (2013) Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour Technol 128:593–597. https://doi.org/10.1016/j.biortech.2012.10.150
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017) Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 178:466–478. https://doi.org/10.1016/j.chemosphere.2017.03.072
Zhang J, Liu J, Liu R (2015) Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour Technol 176:288–291. https://doi.org/10.1016/j.biortech.2014.11.011
Al-wabel MI, Al-omran A, El-naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from Conocarpus wastes. Bioresour Technol 131:374–379. https://doi.org/10.1016/j.biortech.2012.12.165
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Gaffar S, Dattamudi S, Baboukani AR, Chanda S, Novak JM, Watts DW, Wang C, Jayachandran K (2021) Physiochemical characterization of biochars from six feedstocks and their effects on the sorption of atrazine in an organic soil. Agronomy 11:716. https://doi.org/10.3390/agronomy11040716
Hossain MK, Strezov Vladimir V, Chan KY, Ziolkowski A, Nelson PF (2011) Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manage 92:223–228. https://doi.org/10.1016/j.jenvman.2010.09.008
Chen H, Guo Y, Du Y, Xu X, Su C, Zeng Z, Li L (2021) The synergistic effects of surface functional groups and pore sizes on CO2 adsorption by GCMC and DFT simulations. Chem Eng J 415:128824. https://doi.org/10.1016/j.cej.2021.128824
Petrovic B, Gorbounov M, Masoudi Soltani S (2021) Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater 312:110751. https://doi.org/10.1016/j.micromeso.2020.110751
Boehm HP (1966) Chemical identification of surface groups. Adv Catal 16:179–274. https://doi.org/10.1016/S0360-0564(08)60354-5
Montes-Morán MA, Suárez D, Menéndez JA, Fuente E (2004) On the nature of basic sites on carbon surfaces: An overview. Carbon 42:1219–1225. https://doi.org/10.1016/j.carbon.2004.01.023
Biniak S, Świątkowski A, Pakuła M (2001) Electrochemical studies of phenomena at active carbon- electrolyte solution interfaces. In: Radovic LR (ed) Chemistry and physics of carbon: A series of advances. Marcel Dekker Inc, New York, pp 126–216
Shen W, Fan W (2013) Nitrogen-containing porous carbons: Synthesis and application. J Mater Chem A 1:999–1013. https://doi.org/10.1039/c2ta00028h
Guo T, Ma N, Pan Y, Bedane AH, Xiao H, Eić M, Du Y (2018) Characteristics of CO2 adsorption on biochar derived from biomass pyrolysis in molten salt. Can J Chem Eng 96:2352–2360. https://doi.org/10.1002/cjce.23153
Yaumi AL, Bakar MZA, Hameed BH (2018) Melamine-nitrogenated mesoporous activated carbon derived from rice husk for carbon dioxide adsorption in fixed-bed. Energy 155:46–55. https://doi.org/10.1016/j.energy.2018.04.183
Brewer CE, Unger R, Schmidt-Rohr K, Brown RC (2011) Criteria to select biochars for field studies based on biochar chemical properties. Bioenergy Res 4:312–323. https://doi.org/10.1007/s12155-011-9133-7
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301. https://doi.org/10.1021/es903140c
Spokas KA (2010) Review of the stability of biochar in soils: Predictability of O: C molar ratios. Carbon Manag 1:289–303. https://doi.org/10.4155/cmt.10.32
Enders A, Hanley K, Whitman T, Joseph S, Lehmann J (2012) Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol 114:644–653. https://doi.org/10.1016/j.biortech.2012.03.022
Shen Y, Linville JL, Ignacio-de Leon PAA, Schoene RP, Urgun-Demirtas M (2016) Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J Clean Prod 135:1054–1064. https://doi.org/10.1016/j.jclepro.2016.06.144
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143. https://doi.org/10.1021/es8002684
Antal MJ, Grønli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42:1619–1640. https://doi.org/10.1021/ie0207919
Ok YS, Yang JE, Zhang YS, Kim SJ, Chung DY (2007) Heavy metal adsorption by a formulated zeolite-Portland cement mixture. J Hazard Mater 147:91–96. https://doi.org/10.1016/j.jhazmat.2006.12.046
Imam T, Capareda S (2012) Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J Anal Appl Pyrolysis 93:170–177. https://doi.org/10.1016/j.jaap.2011.11.010
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72:243–248. https://doi.org/10.1016/j.jaap.2004.07.003
Zhao Z, Wu Q, Nie T, Zhou W (2019) Quantitative evaluation of relationships between adsorption and partition of atrazine in biochar-amended soils with biochar characteristics. RSC Adv 9:4162–4171. https://doi.org/10.1039/C8RA08544G
Sun X, Shan R, Li X, Pan J, Liu X, Deng R, Song J (2017) Characterization of 60 types of Chinese biomass waste and resultant biochars in terms of their candidacy for soil application. GCB Bioenergy 9:1423–1435. https://doi.org/10.1111/gcbb.12435
Kameyama K, Miyamoto T, Iwata Y (2019) The preliminary study of water-retention related properties of biochar produced from various feedstock at different pyrolysis temperatures. Materials (Basel) 12:1732. https://doi.org/10.3390/ma12111732
Bamdad H, Hawboldt K, MacQuarrie S, Papari S (2019) Application of biochar for acid gas removal: experimental and statistical analysis using CO2. Environ Sci Pollut Res 26:10902–10915. https://doi.org/10.1007/s11356-019-04509-3
Anthonysamy SI, Lahijani P, Mohammadi M, Mohamed AR (2020) Low temperature adsorption of nitric oxide on cerium impregnated biomass-derived biochar. Korean J Chem Eng 37:130–140. https://doi.org/10.1007/s11814-019-0405-9
Dubinin MM (1989) Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 27:457–467. https://doi.org/10.1016/0008-6223(89)90078-X
Barrett EP, Joyner LG, Halenda PP (1951) The Determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Mao J, Cao X, Chen N (2013) Characterization of biochars using advanced solid-state 13C nuclear magnetic resonance spectroscopy. In: Lee JW (ed) Advanced Biofuels and Bioproducts. Springer, New York, New York, pp 47–55
Tangsathitkulchai C, Naksusuk S, Wongkoblap A, Phadungbut P (2021) Equilibrium and kinetics of CO2 adsorption by coconut shell activated carbon impregnated with sodium hydroxide. Processes 9:1–23. https://doi.org/10.3390/pr9020201
Igalavithana AD, Choi SW, Shang J, Hanif A, Dissanayake PD, Tsang DCW, Kwon JH, Lee KB, Ok YS (2020) Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci Total Environ 739:139845. https://doi.org/10.1016/j.scitotenv.2020.139845
Shafeeyan MS, Wan Daud WMA, Houshmand A, Arami-Niya A (2012) The application of response surface methodology to optimize the amination of activated carbon for the preparation of carbon dioxide adsorbents. Fuel 94:465–472. https://doi.org/10.1016/j.fuel.2011.11.035
Lim G, Lee KB, Ham HC (2016) Effect of N-containing functional groups on CO2 adsorption of carbonaceous materials: A Density Functional Theory approach. J Phys Chem C 120:8087–8095. https://doi.org/10.1021/acs.jpcc.5b12090
Xing W, Liu C, Zhou Z, Zhou J, Wang G, Zhuo S, Xue Q (2014) Oxygen-containing functional group-facilitated CO2 capture by carbide-derived carbons. Nanoscale Res Lett 9:189
Liu Y, Wilcox J (2012) Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons. Environ Sci Technol 46:1940–1947. https://doi.org/10.1021/es204071g
Zhang X, Wu J, Yang H, Shao J, Wang X, Chen Y, Zhang S, Chen H (2016) Preparation of nitrogen-doped microporous modified biochar by high temperature CO2-NH3 treatment for CO2 adsorption: Effects of temperature. RSC Adv 6:98157–98166. https://doi.org/10.1039/c6ra23748g
Liu WJ, Jiang H, Tian K, Ding YW, Yu HQ (2013) Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ Sci Technol 47:9397–9403. https://doi.org/10.1021/es401286p
Chiang YC, Yeh CY, Weng CH (2019) Carbon dioxide adsorption on porous and functionalized activated carbon fibers. Appl Sci 9:1977. https://doi.org/10.3390/app9101977
Fan X, Zhang L, Zhang G, Shu Z, Shi J (2013) Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 61:423–430. https://doi.org/10.1016/j.carbon.2013.05.026
Zhang C, Song W, Sun G et al (2013) CO2 capture with activated carbon grafted by nitrogenous functional groups. Energy Fuels 27:4818–4823. https://doi.org/10.1021/ef400499k
Songolzadeh M, Ravanchi MT, Soleimani M (2012) Carbon dioxide capture and storage : A general review on adsorbents. Int J Chem Mol Nucl Mater Metall Eng 6:900–907. https://doi.org/10.5281/zenodo.1076265
Igalavithana AD, Mandal S, Niazi NK et al (2017) Advances and future directions of biochar characterization methods and applications. Crit Rev Environ Sci Technol 47:2275–2330. https://doi.org/10.1080/10643389.2017.1421844
Sajjadi B, Chen WY, Egiebor NO (2019) A comprehensive review on physical activation of biochar for energy and environmental applications. Rev Chem Eng 35:735–776. https://doi.org/10.1515/revce-2017-0113
Dalai AK, Azargohar R (2007) Production of activated carbon from biochar using chemical and physical activation: Mechanism and modeling. ACS Symp Ser 954:463–476. https://doi.org/10.1021/bk-2007-0954.ch029
Hagemann N, Spokas K, Schmidt HP, Kägi R, Böhler MA, Bucheli TD (2018) Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water (Switzerland) 10:1–19. https://doi.org/10.3390/w10020182
Ahmed MB, Zhou JL, Ngo HH, Guo W, Chen M (2016) Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour Technol 214:836–851. https://doi.org/10.1016/j.biortech.2016.05.057
Budinova T, Ekinci E, Yardim F, Grimm A, Björnbom E, Minkova V, Goranova M (2006) Characterization and application of activated carbon produced by H3PO4 and water vapor activation. Fuel Process Technol 87:899–905. https://doi.org/10.1016/j.fuproc.2006.06.005
Mestre AS, Pires J, Nogueira JMF, Carvalho AP (2007) Activated carbons for the adsorption of ibuprofen. Carbon 45:1979–1988. https://doi.org/10.1016/j.carbon.2007.06.005
Santos RM, Santos AO, Midori E, Nascimento JS, Lima ÁS, Freitas LS (2015) Pyrolysis of Mangaba seed : Production and characterization of bio-oil. Bioresour Technol 196:43–48. https://doi.org/10.1016/j.biortech.2015.07.060
Rajapaksha AU, Vithanage M, Ahmad M, Seo DC, Cho JS, Lee SE, Lee SS, Ok YS (2015) Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 290:43–50. https://doi.org/10.1016/j.jhazmat.2015.02.046
Rodríguez-Reinoso F, Molina-Sabio M, González MT (1995) The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 33:15–23. https://doi.org/10.1016/0008-6223(94)00100-E
Feng D, Zhang Y, Zhao Y, Sun S, Gao J (2018) Improvement and maintenance of biochar catalytic activity for in-situ biomass tar reforming during pyrolysis and H2O/CO2 gasification. Fuel Process Technol 172:106–114. https://doi.org/10.1016/j.fuproc.2017.12.011
Lussier MG, Zhang Z, Miller DJ (1998) Characterizing rate inhibition in steam/hydrogen gasification via analysis of adsorbed hydrogen. Carbon 36:1361–1369. https://doi.org/10.1016/S0008-6223(98)00123-7
Aworn A, Thiravetyan P, Nakbanpote W (2008) Preparation and characteristics of agricultural waste activated carbon by physical activation having micro- and mesopores. J Anal Appl Pyrolysis 82:279–285. https://doi.org/10.1016/j.jaap.2008.04.007
Maroto-Valer MM, Fauth DJ, Kuchta ME, Zhang Y, Andrésen JM (2005) Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process Technol 86:1627–1645. https://doi.org/10.1016/j.fuproc.2005.01.017
Fan M (2004) Steam activation of chars produced from oat hulls and corn stover. Bioresour Technol 93:103–107. https://doi.org/10.1016/j.biortech.2003.08.016
Zhang YJ, Xing ZJ, Duan ZK, Li M, Wang Y (2014) Effects of steam activation on the pore structure and surface chemistry of activated aarbon derived from Bamboo waste. Appl Surf Sci 315:279–286. https://doi.org/10.1016/j.apsusc.2014.07.126
Thommes M (2010) Physical adsorption characterization of nanoporous materials. Chem-Ing-Tech 82:1059–1073. https://doi.org/10.1002/cite.201000064
Ogungbenro AE, Quang DV, Al-Ali KA, Vega LF, Abu-Zahra MRM (2018) Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J Environ Chem Eng 6:4245–4252. https://doi.org/10.1016/j.jece.2018.06.030
Sevilla M, Al-Jumialy ASM, Fuertes AB, Mokaya R (2018) Optimization of the pore structure of biomass-based carbons in relation to their use for CO2 capture under low- and high-pressure regimes regimes. ACS Appl Mater Interfaces 10:1623–1633. https://doi.org/10.1021/acsami.7b10433
Wang J, Nie P, Ding B, Dong S, Hao X, Dou H, Zhang X (2017) Biomass derived carbon for energy storage devices. J Mater Chem A 5:2411–2428. https://doi.org/10.1039/c6ta08742f
Shahkarami S, Azargohar R, Dalai AK, Soltan J (2015) Breakthrough CO2 adsorption in bio-based activated carbons. J Environ Sci (China) 34:68–76. https://doi.org/10.1016/j.jes.2015.03.008
Wu X, Yu Y, Qin Z, Zhang Z (2014) The advances of post-combustion CO2 capture with chemical solvents: review and guidelines. Energy Procedia 63:1339–1346. https://doi.org/10.1016/j.egypro.2014.11.143
Creamer AE, Gao B, Wang S (2016) Carbon dioxide capture using various metal oxyhydroxide–biochar composites. Chem Eng J 283:826–832. https://doi.org/10.1016/j.cej.2015.08.037
Mu J, Perlmutter DD (1982) Thermal decomposition of metal nitrates and their hydrates. Thermochim Acta 56:253–260. https://doi.org/10.1016/0040-6031(82)87033-0
Guo Y, Tan C, Sun J, Li W, Zhang J, Zhao C (2020) Biomass ash stabilized Mgo adsorbents for CO2 Capture application. Fuel 259:116298. https://doi.org/10.1016/j.fuel.2019.116298
Shafeeyan MS, Daud WMAW, Houshmand A, Shamiri A (2010) A review on surface modification of activated carbon for carbon dioxide adsorption. J Anal Appl Pyrolysis 89:143–151. https://doi.org/10.1016/j.jaap.2010.07.006
Saha D, Kienbaum MJ (2019) Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater 287:29–55. https://doi.org/10.1016/j.micromeso.2019.05.051
Xiong Z, Shihong Z, Haiping Y, Tao S, Yingquan C, Hanping C (2013) Influence of NH3/CO2 modification on the characteristic of biochar and the CO2 capture. Bioenergy Res 6:1147–1153. https://doi.org/10.1007/s12155-013-9304-9
Molavi H, Eskandari A, Shojaei A, Mousavi SA (2018) Enhancing CO2/N2 adsorption selectivity via post-synthetic modification of NH2-UiO-66(Zr). Microporous Mesoporous Mater 257:193–201. https://doi.org/10.1016/j.micromeso.2017.08.043
Sarmah M, Baruah BP, Khare P (2013) A comparison between CO2 capturing capacities of fly ash based composites of MEA/DMA and DEA/DMA. Fuel Process Technol 106:490–497. https://doi.org/10.1016/j.fuproc.2012.09.017
Nie L, Mu Y, Jin J, Chen J, Mi J (2018) Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas. Chinese J Chem Eng 2303–2317https://doi.org/10.1016/j.cjche.2018.07.012
Bandosz TJ, Seredych M, Rodríguez-Castellón E, Cheng Y, Daemen LL, Ramírez-Cuesta AJ (2016) Evidence for CO2 Reactive adsorption on nanoporous S- and N-doped carbon at ambient conditions. Carbon 96:856–863. https://doi.org/10.1016/j.carbon.2015.10.007
Xie W-H, Li H, Yang M, He L-N, Li H-R (2022) CO2 capture and utilization with solid waste. Green Chem Eng. https://doi.org/10.1016/j.gce.2022.01.002
Haleem N, Khattak A, Jamal Y, Sajid M, Shahzad Z, Raza H (2022) Development of poly vinyl alcohol (PVA) based biochar nanofibers for carbon dioxide (CO2) adsorption. Renew Sustain Energy Rev 157:112019. https://doi.org/10.1016/j.rser.2021.112019
Bamdad H, Hawboldt K, Macquarrie S (2018) Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels 32:11742–11748. https://doi.org/10.1021/acs.energyfuels.8b03056
Liu SH, Huang YY (2018) Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J Clean Prod 175:354–360. https://doi.org/10.1016/j.jclepro.2017.12.076
Ghani WAWAK, Rebitanim NZ, Salleh MAM, Alias AB (2015) Carbon Dioxide Adsorption on Coconut Shell Biochar. In: Dincer I, Colpan CO, Kizilkan O, Ezan MA (eds) Progress in Clean Energy, vol 1. Springer International Publishing, Switzerland, pp 683–693
Tan YL, Islam A, Asif M, Hameed BH (2014) Adsorption of carbon dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fixed bed. Energy 77:926–931. https://doi.org/10.1016/j.energy.2014.09.079
Islam MA, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol Environ Saf 138:279–285. https://doi.org/10.1016/j.ecoenv.2017.01.010
Shen F, Wang Y, Li L, Zhang K, Smith RL, Qi X (2018) Porous carbonaceous materials from hydrothermal carbonization and KOH activation of corn stover for highly efficient CO2 capture. Chem Eng Commun 205:423–431. https://doi.org/10.1080/00986445.2017.1367671
Wang R, Wang P, Yan X, Lang J, Peng C, Xue Q (2012) Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl Mater Interfaces 4:5800–5806. https://doi.org/10.1021/am302077c
Huang GG, Liu YF, Wu XX, Cai JJ (2019) Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. Xinxing Tan Cailiao/New Carbon Mater 34:247–257. https://doi.org/10.1016/S1872-5805(19)60014-4
Serafin J, Ouzzine M, Cruz OF, Sreńscek-Nazzal J, Campello Gómez I, Azar FZ, Rey Mafull CA, Hotza D, Rambo CR (2021) Conversion of fruit waste-derived biomass to highly microporous activated carbon for enhanced CO2 capture. Waste Manag 136:273–282. https://doi.org/10.1016/j.wasman.2021.10.025
Yue L, Xia Q, Wang L, Wang L, DaCosta H, Yang J, Hu X (2018) CO2 adsorption at nitrogen-doped carbons prepared by K2CO3 activation of urea-modified coconut shell. J Colloid Interface Sci 511:259–267. https://doi.org/10.1016/j.jcis.2017.09.040
Hu J, Chen Y, Qian K, Yang Z, Yang H, Li Y, Chen H (2017) Evolution of char structure during mengdong coal pyrolysis: influence of temperature and K2CO3. Fuel Process Technol 159:178–186. https://doi.org/10.1016/j.fuproc.2017.01.042
Zhao C, Chen X, Zhao C (2012) K2CO3/Al2O3 for capturing CO2 in flue gas from power plants. part 1: carbonation behaviors of K2CO3/Al2O3. Energy Fuels 26:1401–1405. https://doi.org/10.1021/ef200725z
Park SJ, Jung WY (2002) Effect of KOH activation on the formation of oxygen structure in activated carbons synthesized from polymeric precursor. J Colloid Interface Sci 250:93–98. https://doi.org/10.1006/jcis.2002.8309
Goel C, Bhunia H, Bajpai PK (2015) Synthesis of nitrogen doped mesoporous carbons for carbon dioxide capture. RSC Adv 5:46568–46582. https://doi.org/10.1039/c5ra05684e
Otowa T, Tanibata R, Itoh M (1993) Production and adsorption characteristics of MAXSORB: high-surface-area active carbon. Gas Sep Purif 7:241–245. https://doi.org/10.1016/0950-4214(93)80024-Q
Wang J, Kaskel S (2012) KOH activation of carbon-based materials for energy storage. J Mater Chem 22:23710–23725. https://doi.org/10.1039/C2JM34066F
Zhang C, Song W, Ma Q, Xie L, Zhang X (2016) Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. https://doi.org/10.1021/acs.energyfuels.5b02764
Rostamian R, Heidarpour M, Mousavi SF, Afyuni M (2015) Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J Agric Sci Technol 17:1057–1069
Mistar EM, Alfatah T, Supardan MD (2020) Synthesis and characterization of activated carbon from Bambusa Vulgaris Striata using two-step KOH activation. J Mater Res Technol 9:6278–6286. https://doi.org/10.1016/j.jmrt.2020.03.041
Saad MJ, Chia CH, Zakaria S, Sajab MS, Misran S, Rahman MHA, Chin SX (2019) Physical and chemical properties of the rice straw activated carbon produced from carbonization and KOH activation processes. Sains Malaysiana 48:385–391. https://doi.org/10.17576/jsm-2019-4802-16
Gomez-delgado E, Nunell GV, Cukierman AL, Bonelli PR (2022) Influence of the carbonization atmosphere on the development of highly microporous adsorbents tailored to CO2 capture. J Energy Inst 102:184–189. https://doi.org/10.1016/j.joei.2022.03.003
Li K, Zhang D, Niu X, Guo H, Yu Y, Tang Z, Lin Z, Fu M (2022) Insights into CO2 adsorption on KOH-activated biochars derived from the mixed sewagesludge and pine sawdust. Sci Total Environ 826:154133. https://doi.org/10.1016/j.scitotenv.2022.154133
Ding S, Liu Y (2020) Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 260:116382. https://doi.org/10.1016/j.fuel.2019.116382
Shao L, Sang Y, Liu N, Liu J, Zhan P, Huang J, Chen J (2020) Selectable microporous carbons derived from poplar wood by three preparation routes for CO2 capture. ACS Omega 5:17450–17462. https://doi.org/10.1021/acsomega.0c01918
Kwiatkowski M, Serafin J, Booth AM, Michalkiewicz B (2021) Computer analysis of the effect of activation temperature on the microporous structure development of activated carbon derived from common polypody. Materials (Basel) 14:2951. https://doi.org/10.3390/ma14112951
Nowrouzi M, Younesi H, Bahramifar N (2018) Superior CO2 capture performance on biomass-derived carbon/metal oxides nanocomposites from Persian ironwood by H3PO4 activation. Fuel 223:99–114. https://doi.org/10.1016/j.fuel.2018.03.035
Varil T, Bergna D, Lahti R, Romar H, Hu T, Lassi U (2017) Activated carbon production from peat using ZnCl2: Characterization and applications. BioResources 12:8078–8092. https://doi.org/10.15376/biores.12.4.8078-8092
Kumar A, Jena HM (2015) High surface area microporous activated carbons prepared from Fox nut ( Euryale ferox ) shell by zinc chloride activation. Appl Surf Sci 356:753–761. https://doi.org/10.1016/j.apsusc.2015.08.074
Heidari A, Younesi H, Rashidi A, Ghoreyshi AA (2014) Adsorptive removal of CO2 on highly microporous activated carbons prepared from Eucalyptus camaldulensis wood: Effect of chemical activation. J Taiwan Inst Chem Eng 45:579–588. https://doi.org/10.1016/j.jtice.2013.06.007
Sevilla M, Mokaya R (2014) Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ Sci 7:1250–1280. https://doi.org/10.1039/c3ee43525c
Thote JA, Iyer KS, Chatti R, Labhsetwar NK, Biniwale RB, Rayalu SS (2010) In situ nitrogen enriched carbon for carbon dioxide capture. Carbon 48:396–402. https://doi.org/10.1016/j.carbon.2009.09.042
Ahmed MB, Hasan Johir MA, Zhou JL, Ngo HH, Nghiem LD, Richardson C, Moni MA, Bryant MR (2019) Activated carbon preparation from biomass feedstock: Clean production and carbon dioxide adsorption. J Clean Prod 225:405–413. https://doi.org/10.1016/j.jclepro.2019.03.342
Soyler N, SelimCeylan YT (2018) CO2 capture analysis of tobacco biochar-AlCl3 composite. Environ Res Technol 5:34–37
Ma Q, Chen W, Jin Z, Chen L, Zhou Q, Jiang X (2021) One-step synthesis of microporous nitrogen-doped biochar for efficient removal of CO2 and H2S. Fuel 289:119932. https://doi.org/10.1016/j.fuel.2020.119932
Xu X, Zheng Y, Gao B, Cao X (2019) doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chem Eng J 368:564–572. https://doi.org/10.1016/j.cej.2019.02.165
Nguyen MV, Lee BK (2016) A novel removal of CO2 using nitrogen doped biochar beads as a green adsorbent. Process Saf Environ Prot 104:490–498. https://doi.org/10.1016/j.psep.2016.04.007
Liu Y, Sajjadi B, Chen WY, Chatterjee R (2019) Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 247:10–18. https://doi.org/10.1016/j.fuel.2019.03.011
Sajjadi B, Broome JW, Chen WY, Mattern DL, Egiebor NO, Hammer N, Smith CL (2019) Urea functionalization of ultrasound-treated biochar: A feasible strategy for enhancing heavy metal adsorption capacity. Ultrason Sonochem 51:20–30. https://doi.org/10.1016/j.ultsonch.2018.09.015
Mohammed Z, Jeelani S, Rangari V (2022) Effective reinforcement of engineered sustainable biochar carbon for 3D printed polypropylene biocomposites. Compos Part C Open Access 7:100221. https://doi.org/10.1016/J.JCOMC.2021.100221
Chatterjee R, Sajjadi B, Mattern DL, Chen WY, Zubatiuk T, Leszczynska D, Leszczynski J, Egiebor NO, Hammer N (2018) Ultrasound cavitation intensified amine functionalization: A feasible strategy for enhancing CO2 capture capacity of biochar. Fuel 225:287–298. https://doi.org/10.1016/j.fuel.2018.03.145
Yang H, Li H, Zhai J, Sun L, Yu H (2014) Simple synthesis of graphene oxide using ultrasonic cleaner from expanded graphite. Ind Eng Chem Res 53:17878–17883. https://doi.org/10.1021/ie503586v
Chatterjee R, Sajjadi B, Chen W-Y, Mattern DL, Egiebor NO, Hammer N, Raman V (2019) Low frequency ultrasound enhanced dual amination of biochar: A nitrogen-enriched sorbent for CO2 capture. Energy Fuels 33:2366–2380. https://doi.org/10.1021/acs.energyfuels.8b03583
Li M, Xiao R (2019) Preparation of a dual pore structure activated carbon from rice husk char as an adsorbent for CO2 capture. Fuel Process Technol 186:35–39. https://doi.org/10.1016/j.fuproc.2018.12.015
Dissanayake PD, Choi SW, Igalavithana AD, Yang X, Tsang DCW, Wang CH, Kua HW, Lee KB, Ok YS (2020) Sustainable gasification biochar as a high efficiency adsorbent for CO2 capture: A facile method to designer biochar fabrication. Renew Sustain Energy Rev 124:109785. https://doi.org/10.1016/j.rser.2020.109785
González B, Manyà JJ (2020) Activated olive mill waste-based hydrochars as selective adsorbents for CO2 capture under postcombustion conditions. Chem Eng Process - Process Intensif 149:107830. https://doi.org/10.1016/j.cep.2020.107830
Younas M, Sohail M, Kong LL, Bashir MJK, Sethupathi S (2016) Erratum to: Feasibility of CO2 adsorption by solid adsorbents: A review on low-temperature systems. Int J Environ Sci Technol 13:1839–1860. https://doi.org/10.1007/s13762-016-1008-1
Cheung O, Hedin N (2014) Zeolites and related sorbents with narrow pores for CO2 separation from flue gas. RSC Adv 4:14480–14494. https://doi.org/10.1039/c3ra48052f
Choi HS, Suh MP (2009) Highly selective CO2 capture in flexible 3d coordination polymer networks. Angew Chemie - Int Ed 48:6865–6869. https://doi.org/10.1002/anie.200902836
Cao L, Zhang X, Xu Y, Xiang W, Wang R, Ding F, Hong P, Gao B (2022) Straw and wood based biochar for CO2 capture: Adsorption performance and governing mechanisms. Sep Purif Technol 287:120592. https://doi.org/10.1016/j.seppur.2022.120592
Kamarudin KSN, Zaini N, Khairuddin NEA (2018) CO2 removal using amine-functionalized kenaf in pressure swing adsorption system. J Environ Chem Eng 6:549–559. https://doi.org/10.1016/j.jece.2017.12.040
Abdullah MO, Tan IAW, Lim LS (2011) Automobile adsorption air-conditioning system using oil palm biomass-based activated carbon: A review. Renew Sustain Energy Rev 15:2061–2072. https://doi.org/10.1016/j.rser.2011.01.012
Lee CS, Ong YL, Aroua MK, Daud WMAW (2013) Impregnation of palm shell-based activated carbon with sterically hindered amines for CO2 adsorption. Chem Eng J 219:558–564. https://doi.org/10.1016/j.cej.2012.10.064
Benson SM, Franklin M, Orr J (2008) Carbon dioxide capture and storage. MRS Bull 33:303–305
Figueiredo JL, Pereira MFR, Freitas MMA, Órfão JJM (1999) Modification of the surface chemistry of activated carbons. Carbon 37:1379–1389. https://doi.org/10.1016/S0008-6223(98)00333-9
Acknowledgements
This work has been funded by the Ministry of Education of Malaysia and Universiti Sains Malaysia under FRGS with Project Code: FRGS/1/2019/TK02/USM/01/3. The authors would like to thank Universiti Malaysia Perlis (UniMAP) for the scholarship granted to the first author.
Author information
Authors and Affiliations
Contributions
Literature review and drafting the original manuscript: Nuradibah Mohd Amer; Critical revision and supervision: Pooya Lahijani; Writing-review and editing: Maedeh Mohammadi; Funding acquisition and review: Abdul Rahman Mohammad.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amer, N.M., Lahijani, P., Mohammadi, M. et al. Modification of biomass-derived biochar: A practical approach towards development of sustainable CO2 adsorbent. Biomass Conv. Bioref. 14, 7401–7448 (2024). https://doi.org/10.1007/s13399-022-02905-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02905-3