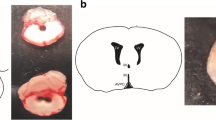
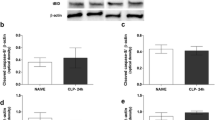
Abstract
Sepsis promotes an inflammatory state in the central nervous system (CNS) that may cause autonomic, cognitive, and endocrine changes. Microglia, a resident immune cell of the CNS, is activated in several brain regions during sepsis, suggesting its participation in the central alterations observed in this disease. In this study, we aimed to investigate the role of microglial activation in the neuroendocrine system functions during systemic inflammation. Wistar rats received an intracerebroventricular injection of the microglial activation inhibitor minocycline (100 μg/animal), shortly before sepsis induction by cecal ligation and puncture. At 6 and 24 h after surgery, hormonal parameters, central and peripheral inflammation, and markers of apoptosis and synaptic function in the hypothalamus were analyzed. The administration of minocycline decreased the production of inflammatory mediators and the expression of cell death markers, especially in the late phase of sepsis (24 h). With respect to the endocrine parameters, microglial inhibition caused a decrease in oxytocin and an increase in corticosterone and vasopressin plasma levels in the early phase of sepsis (6 h), while in the late phase, we observed decreased oxytocin and increased ACTH and corticosterone levels compared to septic animals that did not receive minocycline. Prolactin levels were not affected by minocycline administration. The results indicate that microglial activation differentially modulates the secretion of several hormones and that this process is associated with inflammatory mediators produced both centrally and peripherally.






Similar content being viewed by others
Data Availability
Data from this study are available from the corresponding author on reasonable request.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR et al (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810. https://doi.org/10.1001/jama.2016.0287
Gofton TE, Young GB (2012) Sepsis-associated encephalopathy. Nat Rev Neurol 8(10):557–566. https://doi.org/10.1038/nrneurol.2012.183
Mazeraud A, Pascal Q, Verdonk F, Heming N, Chretien F, Sharshar T (2016) Neuroanatomy and physiology of brain dysfunction in Sepsis. Clin Chest Med 37(2):333–345. https://doi.org/10.1016/j.ccm.2016.01.013
Michels M, Steckert AV, Quevedo J, Barichello T, Dal-Pizzol F (2015) Mechanisms of long-term cognitive dysfunction of sepsis: from blood-borne leukocytes to glial cells. Intensive Care Med Exp 3(1):30. https://doi.org/10.1186/s40635-015-0066-x
Calsavara AJC, Nobre V, Barichello T, Teixeira AL (2018) Post-sepsis cognitive impairment and associated risk factors: a systematic review. Aust Crit Care 31(4):242–253. https://doi.org/10.1016/j.aucc.2017.06.001
Kuperberg SJ, Wadgaonkar R (2017) Sepsis-associated encephalopathy: the blood-brain barrier and the sphingolipid rheostat. Front Immunol 8:597. https://doi.org/10.3389/fimmu.2017.00597
Berg RM, Moller K, Bailey DM (2011) Neuro-oxidative-nitrosative stress in sepsis. J Cereb Blood Flow Metab 31(7):1532–1544. https://doi.org/10.1038/jcbfm.2011.48
Polito A, Eischwald F, Maho AL, Polito A, Azabou E, Annane D, Chretien F, Stevens RD et al (2013) Pattern of brain injury in the acute setting of human septic shock. Crit Care 17(5):R204. https://doi.org/10.1186/cc12899
Dal-Pizzol F, Ritter C, Cassol OJ Jr, Rezin GT, Petronilho F, Zugno AI, Quevedo J, Streck EL (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res 35(1):1–12. https://doi.org/10.1007/s11064-009-0043-4
Hotchkiss RS, Tinsley KW, Karl IE (2003) Role of apoptotic cell death in sepsis. Scand J Infect Dis 35(9):585–592
Messaris E, Memos N, Chatzigianni E, Konstadoulakis MM, Menenakos E, Katsaragakis S, Voumvourakis C, Androulakis G (2004) Time-dependent mitochondrial-mediated programmed neuronal cell death prolongs survival in sepsis. Crit Care Med 32(8):1764–1770. https://doi.org/10.1097/01.ccm.0000135744.30137.b4
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominguini D, Steckert A et al (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59. https://doi.org/10.1016/j.bbi.2014.07.002
Cunningham CL, Martínez-Cerdeño V, Noctor SC (2013) Diversity of neural precursor cell types in the prenatal macaque cerebral cortex exists largely within the astroglial cell lineage. PLoS One 8(5):e63848. https://doi.org/10.1371/journal.pone.0063848
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME et al (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74(4):691–705. https://doi.org/10.1016/j.neuron.2012.03.026
Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16(5):543–551. https://doi.org/10.1038/nn.3358
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91(2):461–553. https://doi.org/10.1152/physrev.00011.2010
Lively S, Schlichter LC (2018) Microglia responses to pro-inflammatory stimuli (LPS, IFNgamma+TNFalpha) and reprogramming by resolving cytokines (IL-4, IL-10). Front Cell Neurosci 12:215. https://doi.org/10.3389/fncel.2018.00215
Kaushik DK, Thounaojam MC, Kumawat KL, Gupta M, Basu A (2013) Interleukin-1beta orchestrates underlying inflammatory responses in microglia via Kruppel-like factor 4. J Neurochem 127(2):233–244. https://doi.org/10.1111/jnc.12382
Michels M, Abatti MR, Avila P, Vieira A, Borges H, Carvalho Junior C, Wendhausen D, Gasparotto J et al (2019) Characterization and modulation of microglial phenotypes in an animal model of severe sepsis. J Cell Mol Med 24:88–97. https://doi.org/10.1111/jcmm.14606
Wahab F, Atika B, Oliveira-Pelegrin GR, Rocha MJ (2013) Recent advances in the understanding of sepsis-induced alterations in the neuroendocrine system. Endocr Metab Immune Disord Drug Targets 13(4):335–347
Romanov RA, Alpar A, Hokfelt T, Harkany T (2019) Unified classification of molecular, network, and endocrine features of hypothalamic neurons. Annu Rev Neurosci 42:1–26. https://doi.org/10.1146/annurev-neuro-070918-050414
Buller KM (2001) Role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic-pituitary-adrenal axis. Clin Exp Pharmacol Physiol 28(7):581–589. https://doi.org/10.1046/j.1440-1681.2001.03490.x
Glass MJ, Chan J, Pickel VM (2017) Ultrastructural characterization of tumor necrosis factor alpha receptor type 1 distribution in the hypothalamic paraventricular nucleus of the mouse. Neuroscience 352:262–272. https://doi.org/10.1016/j.neuroscience.2017.03.044
Diana A, Van Dam AM, Winblad B, Schultzberg M (1999) Co-localization of interleukin-1 receptor type I and interleukin-1 receptor antagonist with vasopressin in magnocellular neurons of the paraventricular and supraoptic nuclei of the rat hypothalamus. Neuroscience 89(1):137–147. https://doi.org/10.1016/s0306-4522(98)00274-7
Vallieres L, Rivest S (1999) Interleukin-6 is a needed proinflammatory cytokine in the prolonged neural activity and transcriptional activation of corticotropin-releasing factor during endotoxemia. Endocrinology 140(9):3890–3903. https://doi.org/10.1210/endo.140.9.6983
Langouche L, Van den Berghe G (2006) The dynamic neuroendocrine response to critical illness. Endocrinol Metab Clin N Am 35(4):777–791, ix. https://doi.org/10.1016/j.ecl.2006.09.007
Maxime V, Siami S, Annane D (2007) Metabolism modulators in sepsis: the abnormal pituitary response. Crit Care Med 35(9 Suppl):S596–S601. https://doi.org/10.1097/01.CCM.0000279097.67263.52
Santos-Junior NN, Costa LHA, Catalao CHR, Kanashiro A, Sharshar T, Rocha MJA (2017) Impairment of osmotic challenge-induced neurohypophyseal hormones secretion in sepsis survivor rats. Pituitary 20(5):515–521. https://doi.org/10.1007/s11102-017-0812-z
da Costa LHA, Junior N, Catalao CHR, Sharshar T, Chretien F, da Rocha MJA (2017) Vasopressin impairment during sepsis is associated with hypothalamic intrinsic apoptotic pathway and microglial activation. Mol Neurobiol 54(7):5526–5533. https://doi.org/10.1007/s12035-016-0094-x
Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG (2004) Humane endpoints in shock research. Shock 21(1):17–25. https://doi.org/10.1097/01.shk.0000101667.49265.fd
Oliveira-Pelegrin GR, de Azevedo SV, Yao ST, Murphy D, Rocha MJ (2010) Central NOS inhibition differentially affects vasopressin gene expression in hypothalamic nuclei in septic rats. J Neuroimmunol 227(1–2):80–86. https://doi.org/10.1016/j.jneuroim.2010.06.019
Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, Rocha MJ (2015) Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrine 49(1):215–221. https://doi.org/10.1007/s12020-014-0452-2
Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP (2016) Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia 64(2):300–316. https://doi.org/10.1002/glia.22930
Bellaver B, Dos Santos JP, Leffa DT, Bobermin LD, Roppa PHA, da Silva Torres IL, Goncalves CA, Souza DO et al (2018) Systemic inflammation as a driver of brain injury: the astrocyte as an emerging player. Mol Neurobiol 55(3):2685–2695. https://doi.org/10.1007/s12035-017-0526-2
Moraes CA, Santos G, Spohr TC, D'Avila JC, Lima FR, Benjamim CF, Bozza FA, Gomes FC (2014) Activated microglia-induced deficits in excitatory synapses through IL-1β: implications for cognitive impairment in Sepsis. Mol Neurobiol 52:653–663. https://doi.org/10.1007/s12035-014-8868-5
Goncharova ND (2013) Stress responsiveness of the hypothalamic-pituitary-adrenal axis: age-related features of the vasopressinergic regulation. Front Endocrinol (Lausanne) 4:26. https://doi.org/10.3389/fendo.2013.00026
Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Vanwijngaerden YM, Spriet I et al (2013) Reduced cortisol metabolism during critical illness. N Engl J Med 368(16):1477–1488. https://doi.org/10.1056/NEJMoa1214969
Venkatesh B, Imeson L, Kruger P, Cohen J, Jones M, Bellomo R, Group AaNZICSCT, Investigators ST (2015) Elevated plasma-free cortisol concentrations and ratios are associated with increased mortality even in the presence of statin therapy in patients with severe sepsis. Crit Care Med 43(3):630–635. https://doi.org/10.1097/CCM.0000000000000750
Vardas K, Ilia S, Sertedaki A, Charmandari E, Briassouli E, Goukos D, Apostolou K, Psarra K et al (2017) Increased glucocorticoid receptor expression in sepsis is related to heat shock proteins, cytokines, and cortisol and is associated with increased mortality. Intensive Care Med Exp 5(1):10. https://doi.org/10.1186/s40635-017-0123-8
Flierl MA, Rittirsch D, Weckbach S, Huber-Lang M, Ipaktchi K, Ward PA, Stahel PF (2011) Disturbances of the hypothalamic-pituitary-adrenal axis and plasma electrolytes during experimental sepsis. Ann Intensive Care 1:53. https://doi.org/10.1186/2110-5820-1-53
Carlson DE, Chiu WC, Fiedler SM, Hoffman GE (2007) Central neural distribution of immunoreactive Fos and CRH in relation to plasma ACTH and corticosterone during sepsis in the rat. Exp Neurol 205(2):485–500. https://doi.org/10.1016/j.expneurol.2007.03.015
Yamashita H, Ishikawa M, Inoue T, Usami M, Usami Y, Kotani J (2017) Interleukin-18 reduces blood glucose and modulates plasma corticosterone in a septic mouse model. Shock 47(4):455–462. https://doi.org/10.1097/SHK.0000000000000747
Bernardini R, Kamilaris TC, Calogero AE, Johnson EO, Gomez MT, Gold PW, Chrousos GP (1990) Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology 126(6):2876–2881. https://doi.org/10.1210/endo-126-6-2876
Gaillard RC, Turnill D, Sappino P, Muller AF (1990) Tumor necrosis factor alpha inhibits the hormonal response of the pituitary gland to hypothalamic releasing factors. Endocrinology 127(1):101–106. https://doi.org/10.1210/endo-127-1-101
Gjerstad JK, Lightman SL, Spiga F (2018) Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21(5):403–416. https://doi.org/10.1080/10253890.2018.1470238
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651
Eggen BJ, Raj D, Hanisch UK, Boddeke HW (2013) Microglial phenotype and adaptation. J NeuroImmune Pharmacol 8(4):807–823. https://doi.org/10.1007/s11481-013-9490-4
Soldani C, Scovassi AI (2002) Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 7(4):321–328. https://doi.org/10.1023/a:1016119328968
Bethin KE, Vogt SK, Muglia LJ (2000) Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A 97(16):9317–9322
Song DK, Im YB, Jung JS, Yan JJ, Huh SO, Suh HW, Kim YH (2000) Central injection of nitric oxide synthase inhibitors increases peripheral interleukin-6 and serum amyloid A: involvement of adrenaline from adrenal medulla. Br J Pharmacol 130(1):41–48. https://doi.org/10.1038/sj.bjp.0703273
Elorza-Avila AR, Reyes-Lagos JJ, Hadamitzky M, Pena-Castillo MA, Echeverria JC, Ortiz-Pedroza MD, Luckemann L, Schedlowski M et al (2017) Oxytocin’s role on the cardiorespiratory activity of endotoxemic rats. Respir Physiol Neurobiol 236:19–22. https://doi.org/10.1016/j.resp.2016.10.008
Reyes-Lagos JJ, Hadamitzky M, Pena-Castillo MA, Echeverria JC, Bosche K, Luckemann L, Schedlowski M, Pacheco-Lopez G (2016) Exogenous oxytocin reduces signs of sickness behavior and modifies heart rate fluctuations of endotoxemic rats. Physiol Behav 165:223–230. https://doi.org/10.1016/j.physbeh.2016.07.013
Holmes CL, Landry DW, Granton JT (2004) Science review: vasopressin and the cardiovascular system part 2 - clinical physiology. Crit Care 8(1):15–23. https://doi.org/10.1186/cc2338
Oliveira-Pelegrin GR, Saia RS, Cárnio EC, Rocha MJ (2013) Oxytocin affects nitric oxide and cytokine production by sepsis-sensitized macrophages. Neuroimmunomodulation 20(2):65–71. https://doi.org/10.1159/000345044
Zhao L, Brinton RD (2004) Suppression of proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha in astrocytes by a V1 vasopressin receptor agonist: a cAMP response element-binding protein-dependent mechanism. J Neurosci 24(9):2226–2235. https://doi.org/10.1523/JNEUROSCI.4922-03.2004
Athayde LA, Oliveira-Pelegrin GR, Nomizo A, Faccioli LH, Rocha MJ (2009) Blocking central leukotrienes synthesis affects vasopressin release during sepsis. Neuroscience 160(4):829–836. https://doi.org/10.1016/j.neuroscience.2009.03.004
Corrêa PB, Pancoto JA, de Oliveira-Pelegrin GR, Cárnio EC, Rocha MJ (2007) Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol 183(1–2):17–25. https://doi.org/10.1016/j.jneuroim.2006.10.021
Oliveira-Pelegrin GR, Basso PJ, Rocha MJ (2014) Cellular bioenergetics changes in magnocellular neurons may affect copeptin expression in the late phase of sepsis. J Neuroimmunol 267(1–2):28–34. https://doi.org/10.1016/j.jneuroim.2013.12.006
Wahab F, Santos-Junior NN, de Almeida Rodrigues RP, Costa LHA, Catalao CHR, Rocha MJA (2016) Interleukin-1 receptor antagonist decreases hypothalamic oxidative stress during experimental sepsis. Mol Neurobiol 53(6):3992–3998. https://doi.org/10.1007/s12035-015-9338-4
Oliveira-Pelegrin GR, Basso PJ, Soares AS, Martinez MR, Riester KD, Rocha MJ (2013) Cleaved caspase-3 expression in hypothalamic magnocellular neurons may affect vasopressin secretion during experimental polymicrobial sepsis. J Neuroimmunol 258(1–2):10–16. https://doi.org/10.1016/j.jneuroim.2013.02.007
Valtorta F, Pennuto M, Bonanomi D, Benfenati F (2004) Synaptophysin: leading actor or walk-on role in synaptic vesicle exocytosis? Bioessays 26(4):445–453. https://doi.org/10.1002/bies.20012
Rodriguez TT, Biscarde EF, Muniz RF, Amoedo MK, Ramalho MJ (2005) Prolactin secretion in hypothyroid endotoxemic rats: involvement of L-arginine and nitric oxide synthase. Shock 23(5):448–452
Herman AP, Romanowicz K, Tomaszewska-Zaremba D (2010) Effect of LPS on reproductive system at the level of the pituitary of anestrous ewes. Reprod Domest Anim 45(6):e351–e359. https://doi.org/10.1111/j.1439-0531.2009.01577.x
Hollis JH, Lightman SL, Lowry CA (2005) Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol 184(2):393–406. https://doi.org/10.1677/joe.1.05839
Grattan DR (2015) 60 years of neuroendocrinology: the hypothalamo-prolactin axis. J Endocrinol 226(2):T101–T122. https://doi.org/10.1530/JOE-15-0213
Costanza M, Binart N, Steinman L, Pedotti R (2015) Prolactin: a versatile regulator of inflammation and autoimmune pathology. Autoimmun Rev 14(3):223–230. https://doi.org/10.1016/j.autrev.2014.11.005
Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J (2004) Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw 15(2):99–104
Acknowledgments
The authors thank Nadir Fernandes for technical assistance.
Funding
This work was financially supported by Grants #2016/07803-0 and #2018/02854-0, São Paulo Research Foundation (FAPESP) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Contributions
LHAC and MJAR conceived and designed the project. LHAC, NNSJ, and CHRC performed the experiments. LHAC and MJAR analyzed the data. LHAC drafted the manuscript. LHAC, NNSJ, CHRC, and MJAR revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study involves the use of rats. All animal experiments in this study were carried out according to an Institutional Ethics Committee-approved protocol (CEUA protocol number 2016.1.120.58.5) and were conducted in accordance with the Brazilian National Council for Animal Experimentation Control (CONCEA) guidelines and with the Guide for the Care and Use of Laboratory Animals of the National Research Council.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Costa, L.H.A., Santos-Junior, N.N., Catalão, C.H.R. et al. Microglial Activation Modulates Neuroendocrine Secretion During Experimental Sepsis. Mol Neurobiol 58, 2133–2144 (2021). https://doi.org/10.1007/s12035-020-02241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02241-5