Abstract
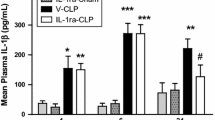
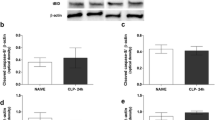
In our previous work, we demonstrated that the intracerebroventricular (i.c.v.) injection of an interleukin-1 receptor antagonist (IL-1ra) prevented the impairment in vasopressin secretion and increased survival rate in septic rats. Additionally, we saw a reduction in nitric oxide (NO) levels in cerebroventricular spinal fluid (CSF), suggesting that the IL-1ra prevents apoptosis that seems to occur in vasopressinergic neurons. Here, we investigated the effect of IL-1ra pre-treatment on the sepsis-induced increase in oxidative stress markers in the hypothalamus of rats. The animals were pre-treated by an i.c.v. injection of IL-1ra (9 nmol) or vehicle (0.01 M PBS) before being subjected to cecal ligation and puncture (CLP) or left as control (sham-operation or naive). After 4, 6, and 24 h, the animals were decapitated (n = 9/group) and the brain removed for hypothalamic tissue collection. Transcript and protein levels of IL-1, inducible nitric oxide synthase (iNOS), caspase-3, and hypoxia-inducible factor 1-alpha (HIF-1α) were measured by quantitative polymerase chain reaction (qPCR) and western blot, respectively. Hypothalamic mRNA levels of all these genes were significantly (P < 0.005) increased at 4, 6, and 24 h CLP, as compared to sham-operated animals. IL-1ra pre-treatment in these CLP animals significantly decreased IL-1 gene expression at all time points and also of iNOS, caspase-3, and HIF-1α at 24 h when compared to vehicle-treated CLP animals. The effect of the pre-treatment on protein expression was most clearly seen for IL-1β and iNOS at 24 h. Our results showed that blocking the IL-1-IL-1r signaling pathway by central administration of an IL-1ra decreases hypothalamic oxidative stress markers during sepsis.


Similar content being viewed by others
References
Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, Rocha MJ (2015) Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrine 49:215–221
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95:1122–1125
Oliveira-Pelegrin GR, Ravanelli MI, Branco LG, Rocha MJ (2009) Thermoregulation and vasopressin secretion during polymicrobial sepsis. Neuroimmunomodulation 16:45–53
Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P et al (2003) Circulating vasopressin levels in septic shock. Crit Care Med 31:1752–1758
Athayde LA, Oliveira-Pelegrin GR, Nomizo A, Faccioli LH, Rocha MJ (2009) Blocking central leukotrienes synthesis affects vasopressin release during sepsis. Neuroscience 160:829–836
Corrêa PB, Pancoto JA, de Oliveira-Pelegrin GR, Cárnio EC, Rocha MJ (2007) Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol 183:17–25
Pancoto JA, Corrêa PB, Oliveira-Pelegrin GR, Rocha MJ (2008) Autonomic dysfunction in experimental sepsis induced by cecal ligation and puncture. Auton Neurosci 138:57–63
Wahab F, Atika B, Oliveira-Pelegrin GR, Rocha MJ (2013) Recent advances in the understanding of sepsis-induced alterations in the neuroendocrine system. Endocr Metab Immune Disord Drug Targets 13:335–347
Oliveira-Pelegrin GR, Basso PJ, Rocha MJ (2014) Cellular bioenergetics changes in magnocellular neurons may affect copeptin expression in the late phase of sepsis. J Neuroimmunol 267:28–34
Oliveira-Pelegrin GR, Basso PJ, Soares AS, Martinez MR, Riester KD, Rocha MJ (2013) Cleaved caspase-3 expression in hypothalamic magnocellular neurons may affect vasopressin secretion during experimental polymicrobial sepsis. J Neuroimmunol 258:10–16
Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J (1997) Interleukin (IL) 1beta, IL-1 receptor antagonist, IL-10, and IL-13 gene expression in the central nervous system and anterior pituitary during systemic inflammation: pathophysiological implications. Proc Natl Acad Sci U S A 94:227–232
Zapelini PH, Rezin GT, Cardoso MR, Ritter C, Klamt F, Moreira JC, Streck EL, Dal-Pizzol F (2008) Antioxidant treatment reverses mitochondrial dysfunction in a sepsis animal model. Mitochondrion 8:211–218
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JC, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349
Bozza FA, D’Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F (2013) Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 1:10–16
Dal-Pizzol F, Ritter C, Cassol-Jr OJ, Rezin GT, Petronilho F, Zugno AI, Quevedo J, Streck EL (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochem Res 35:1–12
Rittirsh D, Hoesel LM, Ward PA (2007) The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81:137–143
Vincent JL, Korkut HA (2008) Defining sepsis. Clin Chest Med 29:585–590
Annane D, Bellissant E, Cavaillon JM (2005) Septic shock. Lancet 365:63–78
Kovács KJ (2002) Neurohypophyseal hormones in the integration of physiological responses to immune challenges. Prog Brain Res 139:127–146
McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V (2000) The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann N Y Acad Sci 917:4–18
Wong ML, Rettori V, al-Shekhlee A, Bongiorno PB, Canteros G, McCann SM, Gold PW, Licinio J (1996) Inducible nitric oxide synthase gene expression in the brain during systemic inflammation. Nat Med 2:581–584
Parrillo JE (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 328:1471–1477
Oliveira-Pelegrin GR, Aguila FA, Basso PJ, Rocha MJ (2010) Role of central NO-cGMP pathway in vasopressin and oxytocin gene expression during sepsis. Peptides 31:1847–1852
Oliveira-Pelegrin GR, de Azevedo SV, Yao ST, Murphy D, Rocha MJ (2010) Central NOS inhibition differentially affects vasopressin gene expression in hypothalamic nuclei in septic rats. J Neuroimmunol 227:80–86
Gabellec MM, Griffais R, Fillion G, Haour F (1995) Expression of interleukin 1 alpha, interleukin 1 beta and interleukin 1 receptor antagonist mRNA in mouse brain: regulation by bacterial lipopolysaccharide (LPS) treatment. Brain Res Mol Brain Res 31:122–130
Brown GC, Cooper CE (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 356:295–298
Erusalimsky JD, Moncada S (2007) Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol 27:2524–2531
Ghafourifar P, Asbury ML, Joshi SS, Kincaid ED (2005) Determination of mitochondrial nitric oxide synthase activity. Methods Enzymol 396:424–444
Sharp FR, Bernaudin M (2004) HIF1 and oxygen sensing in the brain. Nat Rev Neurosci 5:437–448
Bruick RK (2000) Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci U S A 97:9082–9087
Rivest S, Lacroix S, Vallières L, Nadeau S, Zhang J, Laflamme N (2000) How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223:22–38
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, Orlikowski D, Raphael JC, Gajdos P, Annane D (2003) Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet 362:1799–1805
Comim CM, Barichello T, Grandgirard D, Dal-Pizzol F, Quevedo J, Leib SL (2013) Caspase-3 mediates in part hippocampal apoptosis in sepsis. Mol Neurobiol 47:394–398
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominquini D, Steckert A, Schuck PF, Quevedo J, Petronilho F, Dal-Pizzol F (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59
da Silva FP, Machado MC, Sallet PC, Zampieri FG, Goulart AC, Torggler Filho F, Velasco IT, da Cruz Neto LM, de Souza HP (2014) Neuropeptide downregulation in sepsis. Inflammation 37:142–145
Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, Wada E, Wada K, Zacharias S, Mohanasundaram UM, Faix JD, Abrink M, Pejler G, Pearl RG, Tsai M, Galli SJ (2008) Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med 14:392–398
Acknowledgments
We thank Nadir Martins for the technical assistant and Klaus Hartfelder for providing the infrastructure for the qPCR analysis and reviewing a prior version of the manuscript.
Funding
Financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) is gratefully acknowledged. Fazal Wahab was a recipient of a FAPESP post-doctoral scholarship.
Conflict of Interest
The authors declare that they have no competing interests.
Compliance with Ethical Standards
This study involves the use of rats. All animal experiments in this study were carried out according to an Institutional Ethics Committee-approved protocol (CEUA protocol number: 12.1.1205.53.0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wahab, F., Santos-Junior, N.N., de Almeida Rodrigues, R.P. et al. Interleukin-1 Receptor Antagonist Decreases Hypothalamic Oxidative Stress During Experimental Sepsis. Mol Neurobiol 53, 3992–3998 (2016). https://doi.org/10.1007/s12035-015-9338-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9338-4