Abstract
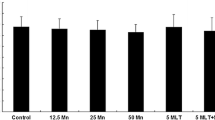
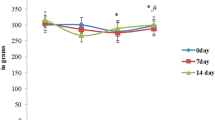
Manganese (Mn) is a trace element involved in many physiological processes. However, excessive Mn exposure leads to neurological complications. Although no precise mechanism(s) has been found for Mn-induced neurotoxicity, oxidative stress and mitochondrial injury seem to play a relevant role in this complication. On the other hand, there is no protective strategy against Mn neurotoxicity so far. Taurine is an amino acid with significant neuroprotective properties. The current study was designed to evaluate the effect of taurine supplementation and its potential mechanism(s) of action in a mouse model of manganism. Animals were treated with Mn (100 mg/kg, s.c) alone and/or in combination with taurine (50, 100, and 500 mg/kg, i.p, for eight consecutive days). Severe locomotor dysfunction along with a significant elevation in brain tissue biomarkers of oxidative stress was evident in Mn-exposed mice. On the other hand, it was revealed that mitochondrial indices of functionality were hampered in Mn-treated animals. Taurine supplementation (50, 100, and 500 mg/kg, i.p) alleviated Mn-induced locomotor deficit. Moreover, this amino acid mitigated oxidative stress biomarkers and preserved brain tissue mitochondrial indices of functionality. These data introduce taurine as a potential neuroprotective agent against Mn neurotoxicity. Antioxidative and mitochondria protecting effects of taurine might play a fundamental role in its neuroprotective properties against Mn toxicity.





Similar content being viewed by others
References
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Asp Med 26:353–362
Santamaria AB (2008) Manganese exposure, essentiality & toxicity. Indian J Med Res 128:484
Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203
Dobson AW, Erikson KM, Aschner M (2004) Manganese neurotoxicity. Ann N Y Acad Sci 1012:115–128
Montes S, Alcaraz-Zubeldia M, Muriel P, Rı́os C (2001) Striatal manganese accumulation induces changes in dopamine metabolism in the cirrhotic rat. Brain Res 891:123–129
Peres TV, Schettinger MRC, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17:57
Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W (2012) Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology 33:881–886
Görg B, Qvartskhava N, Bidmon H-J, Palomero-Gallagher N, Kircheis G, Zilles K, Häussinger D (2010) Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology 52:256–265
Krieger D, Krieger S, Theilmann L, Jansen O, Gass P, Lichtnecker H (1995) Manganese and chronic hepatic encephalopathy. Lancet 346:270–274
Mignarri A, Federico A (2014) From the liver to the brain: manganese matters: focus on cirrhosis-related parkinsonism. Neurol Sci 35:521–522
Rose C, Butterworth RF, Zayed J, Normandin L, Todd K, Michalak A, Spahr L, Huet P–M, Pomier–Layrargues G (1999) Manganese deposition in basal ganglia structures results from both portal-systemic shunting and liver dysfunction. Gastroenterology 117:640–644
Aschner M, Aschner JL (1991) Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev 15:333–340
Erikson KM, Dobson AW, Dorman DC, Aschner M (2004) Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ 334–335:409–416
Olanow CW (2004) Manganese-induced parkinsonism and Parkinson’s disease. Ann N Y Acad Sci 1012:209–223
Rivera-Mancía S, Ríos C, Montes S (2011) Manganese accumulation in the CNS and associated pathologies. BioMetals 24:811–825
Sloot WN, Gramsbergen J-BP (1994) Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res 657:124–132
Verity MA (1999) Manganese neurotoxicity: a mechanistic hypothesis. Neurotoxicology 20:489–497
Butterworth RF, Spahr L, Fontaine S, Layrargues GP (1995) Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis 10:259–267
Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG (2008) Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res Bull 76:361–367
Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiology 7:153–163
Weber S, Dorman DC, Lash LH, Erikson K, Vrana KE, Aschner M (2002) Effects of manganese (mn) on the developing rat brain: oxidative-stress related endpoints. Neurotoxicology 23:169–175
Hamai D, Bondy SC (2004) Oxidative basis of manganese neurotoxicity. Ann N Y Acad Sci 1012:129–141
Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M (2009) Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol 240:219–225
Rao KVR, Norenberg MD (2004) Manganese induces the mitochondrial permeability transition in cultured astrocytes. J Biol Chem 279:32333–32338
Yin Z, Aschner JL, dos Santos AP, Aschner M (2008) Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res 1203:1–11
Zwingmann C, Leibfritz D, Hazell AS (2003) Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab 23:756–771
Gavin CE, Gunter KK, Gunter TE (1992) Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol 115:1–5
Jiao J, Qi Y, Fu J, Zhou Z (2008) Manganese-induced single strand breaks of mitochondrial DNA in vitro and in vivo. Environ Toxicol Pharmacol 26:123–127
Malecki EA (2001) Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull 55:225–228
Gunter TE, Gavin CE, Gunter KK (2012) The role of mitochondrial oxidative stress and ATP depletion in the pathology of manganese toxicity. Metal ion in stroke. Springer Series in Translational Stroke Research. Springer, New York, pp 591–606
Gunter TE (2017) Chapter 32—manganese and mitochondrial function. In: Collins JF (ed) Molecular, genetic, and nutritional aspects of major and trace minerals. Academic Press, Boston, pp 389–396
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Huxtable RJ, Michalk D (2013) Taurine in health and disease, Volume 359 of Advances in Experimental Medicine and Biology, Springer Science & Business Media, 458 p, ISBN: 1489914714, 9781489914712
Oja SS, Saransaari P (2006)Taurine 6: Advances in Experimental Medicine and Biology, Vol. 583 Springer Science & Business Media, 548 p
di Wu Q, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D (1999) Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am J Phys Cell Physiol 277:C1229–C1C38
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87:91–99
Timbrell JA, Seabra V, Waterfield CJ (1995) The in vivo and in vitro protective properties of taurine. Gen Pharmacol 26:453–462
Ghandforoush-Sattari M, Mashayekhi S (2008) Evaluation of taurine as a biomarker of liver damage in paracetamol poisoning. Eur J Pharmacol 581:171–176
Ghandforoush-Sattari M, Mashayekhi SO, Nemati M, Seydi A, Azadi H (2010) Changes in plasma taurine concentration during a period of heroin detoxification. Toxicol Environ Chem 92:1505–1512
Islambulchilar M, Asvadi I, Sanaat Z, Esfahani A, Sattari M (2015) Effect of taurine on febrile episodes in acute lymphoblastic leukemia. Adv Pharm Bull 5:103–108
Chen WQ, Jin H, Nguyen M, Carr J, Lee YJ, Hsu CC, Faiman MD, Schloss JV, Wu JY (2001) Role of taurine in regulation of intracellular calcium level and neuroprotective function in cultured neurons. J Neurosci Res 66:612–619
Idrissi AE (2006) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328
Taranukhin AG, Taranukhina EY, Saransaari P, Podkletnova IM, Pelto-Huikko M, Oja SS (2010) Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J Biomed Sci 17:1–11
Zhou J, Li Y, Yan G, Bu Q, Lv L, Yang Y, Zhao J, Shao X, Deng Y, Zhu R, Zhao Y, Cen X (2011) Protective role of taurine against morphine-induced neurotoxicity in C6 cells via inhibition of oxidative stress. Neurotox Res 20:334–342
Kumari N, Prentice H, Wu J-Y (2013) Taurine and its neuroprotective role. Adv Exp Med Biol 775:19-27
Menzie J, Pan C, Prentice H, Wu J-Y (2012) Taurine and central nervous system disorders. Amino Acids 46:31–46
Wang G-H, Jiang Z-L, Fan X-J, Zhang L, Li X, Ke K-F (2007) Neuroprotective effect of taurine against focal cerebral ischemia in rats possibly mediated by activation of both GABAA and glycine receptors. Neuropharmacology 52:1199–1209
Ward R, Cirkovic-Vellichovia T, Ledeque F, Tirizitis G, Dubars G, Datla K et al (2006) Neuroprotection by taurine and taurine analogues. Adv Exp Med Biol 583:299–306
Wu J-Y, Wu H, Jin Y, Wei J, Sha D, Prentice H, et al. (2009) Mechanism of neuroprotective function of taurine. Adv Exp Med Biol 643:169-79
Heidari R, Babaei H, Eghbal M (2013) Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res Pharm Sci 9:97-105
Heidari R, Babaei H, Eghbal MA (2013) Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arch Ind Hyg Toxicol 64:201–210
Heidari R, Jamshidzadeh A, Niknahad H, Safari F, Azizi H, Abdoli N et al (2016) The hepatoprotection provided by taurine and glycine against antineoplastic drugs induced liver injury in an ex vivo model of normothermic recirculating isolated perfused rat liver. Trends Pharmacol Sci 2:59–76
Jamshidzadeh A, Heidari R, Abasvali M, Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei A, Latifpour Z, Mardani E, Azarpira N, Asadi B, Najibi A (2017) Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother 86:514–520
Boşgelmez İİ, Söylemezoğlu T, Güvendik G (2008) The protective and antidotal effects of taurine on hexavalent chromium-induced oxidative stress in mice liver tissue. Biol Trace Elem Res 125:46–58
Pushpakiran G, Mahalakshmi K, Anuradha CV (2004) Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids 27:91–96
Silva LA, Silveira PCL, Ronsani MM, Souza PS, Scheffer D, Vieira LC, Benetti M, de Souza CT, Pinho RA (2011) Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise. Cell Biochem Funct 29:43–49
Hansen SH, Andersen ML, Birkedal H, Cornett C, Wibrand F (2006) The important role of taurine in oxidative metabolism. Adv Exp Med Biol. 583:129-35
Hansen SH, Andersen ML, Cornett C, Gradinaru R, Grunnet N (2010) A role for taurine in mitochondrial function. J Biomed Sci 17:S23
Hansen SH, Grunnet N (2013) Taurine, glutathione and bioenergetics. In: Idrissi AE, L’Amoreaux WJ (eds) Taurine 8. Advances in experimental medicine and biology. Springer, New York, pp 3–12
Schuller-Levis GB, Park E (2003) Taurine: new implications for an old amino acid. FEMS Microbiol Lett 226:195–202
Dodd CA, Ward DL, Klein BG (2005) Basal Ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. Int J Toxicol 24:389–397
Apelqvist G, Wikell C, Hindfelt B, Bergqvist PB, Andersson G, Bengtsson F (1999) Altered open-field behavior in experimental chronic hepatic encephalopathy after single venlafaxine and citalopram challenges. Psychopharmacology 143:408–416
Kugelberg FC, Apelqvist G, Wikell C, Bengtsson F (2005) Open-field behavioural alterations in liver-impaired and sham-operated rats after acute exposure to the antidepressant venlafaxine. Basic Clin Pharmacol Toxicol 97:155–161
Avraham Y, Grigoriadis NC, Poutahidis T, Vorobiev L, Magen I, Ilan Y, Mechoulam R, Berry EM (2011) Cannabidiol improves brain and liver function in a fulminant hepatic failure-induced model of hepatic encephalopathy in mice. Br J Pharmacol 162:1650–1658
Heidari R, Jamshidzadeh A, Niknahad H, Mardani E, Ommati MM, Azarpira N, Khodaei F, Zarei A, Ayarzadeh M, Mousavi S, Abdoli N, Yeganeh BS, Saeedi A, Najibi A (2016) Effect of taurine on chronic and acute liver injury: focus on blood and brain ammonia. Toxicol Rep 3:870–879
Ommati MM, Jamshidzadeh A, Niknahad H, Mohammadi H, Sabouri S, Heidari R, Abdoli N (2017) N-acetylcysteine treatment blunts liver failure-associated impairment of locomotor activity. PharmaNutrition 5:141–147
Carter RJ, Morton J, Dunnett SB (2001) Motor Coordination and Balance in rodents. Curr Protoc Neurosci: Chapter 8: Unit 8.12. https://doi.org/10.1002/0471142301.ns0812s15
Metz GAS, Merkler D, Dietz V, Schwab ME, Fouad K (2000) Efficient testing of motor function in spinal cord injured rats. Brain Res 883:165–177
Luong TN, Carlisle HJ, Southwell A, Patterson PH (2011) Assessment of motor balance and coordination in mice using the balance beam. J Visualized Exp : JoVE. https://doi.org/10.3791/2376
Tchekalarova J, Kubova H, Mareš P (2005) Postnatal caffeine exposure: effects on motor skills and locomotor activity during ontogenesis. Behav Brain Res 160:99–106
Chander K, Vaibhav K, Ejaz Ahmed M, Javed H, Tabassum R, Khan A, Kumar M, Katyal A, Islam F, Saeed Siddiqui M (2014) Quercetin mitigates lead acetate-induced behavioral and histological alterations via suppression of oxidative stress, Hsp-70, Bak and upregulation of Bcl-2. Food Chem Toxicol 68:297–306
Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, Freret T (2009) The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat Protoc 4:1560–1564
Gupta R, Dubey DK, Kannan GM, Flora SJS (2007) Concomitant administration of Moringa oleifera seed powder in the remediation of arsenic-induced oxidative stress in mouse. Cell Biol Int 31:44–56
Jamshidzadeh A, Niknahad H, Heidari R, Azadbakht M, Khodaei F, Arabnezhad MR, Farshad O (2017) Propylthiouracil-induced mitochondrial dysfunction in liver and its relevance to drug-induced hepatotoxicity. Pharm Sci 23:95–102
Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW (1999) Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol 155:109–117
Meeks RG, Harrison S (1991) Hepatotoxicology. CRC Press, 712 p, ISBN: 0849388104, 9780849388101, Pages 303-304
Truong DH, Eghbal MA, Hindmarsh W, Roth SH, O’Brien PJ (2006) Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev 38:733–744
Heidari R, Babaei H, Roshangar L, Eghbal MA (2014) Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv Pharm Bull 4:21–28
Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol C Toxicol Pharmacol 140:47–52
Niknahad H, Jamshidzadeh A, Heidari R, Abdoli N, Ommati MM, Jafari F, Zarei M, Asadi B (2016) The postulated hepatotoxic metabolite of methimazole causes mitochondrial dysfunction and energy metabolism disturbances in liver. Pharm Sci 22:217–226
Ommati MM, Heidari R, Jamshidzadeh A, Zamiri MJ, Sun Z, Sabouri S, Wang J, Ahmadi F, Javanmard N, Seifi K, Mousapour S, Yeganeh BS (2018) Dual effects of sulfasalazine on rat sperm characteristics, spermatogenesis, and steroidogenesis in two experimental models. Toxicol Lett 284:46–55
Heidari R, Moezi L, Asadi B, Ommati MM, Azarpira N (2017) Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis. PharmaNutrition 5:109–117
Alía M, Horcajo C, Bravo L, Goya L (2003) Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 23:1251–1267
Tarohda T, Yamamoto M, Amamo R (2004) Regional distribution of manganese, iron, copper, and zinc in the rat brain during development. Anal Bioanal Chem 380:240–246
Kihira T, Mukoyama M, Ando K, Yase Y, Yasui M (1990) Determination of manganese concentrations in the spinal cords from amyotrophic lateral sclerosis patients by inductively coupled plasma emission spectroscopy. J Neurol Sci 98:251–258
Caro AA, Adlong LW, Crocker SJ, Gardner MW, Luikart EF, Gron LU (2012) Effect of garlic-derived organosulfur compounds on mitochondrial function and integrity in isolated mouse liver mitochondria. Toxicol Lett 214:166–174
Zhao P, Kalhorn TF, Slattery JT (2002) Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology 36:326–335
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Niknahad H, Heidari R, Alzuhairi AM, Najibi A (2015) Mitochondrial dysfunction as a mechanism for pioglitazone-induced injury toward HepG2 cell line. Pharm Sci 20:169–174
Held P (2006) Luminescent determination of ATP concentrations using the Clarity™ luminescence microplate reader. Nat Methods. https://doi.org/10.1038/an1705
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ommati MM, Jamshidzadeh A, Heidari R, Sun Z, Zamiri MJ, Khodaei F, Mousapour S, Ahmadi F, Javanmard N, Shirazi Yeganeh B (2018) Carnosine and histidine supplementation blunt lead-induced reproductive toxicity through antioxidative and mitochondria-dependent mechanisms. Biol Trace Elem Res:1–12. https://doi.org/10.1007/s12011-018-1358-2
Caro AA, Cederbaum AI (2001) Synergistic toxicity of iron and arachidonic acid in HepG2 cells overexpressing CYP2E1. Mol Pharmacol 60:742–752
Heidari R, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Niknahad H (2018) Mitochondria protecting amino acids: Application against a wide range of mitochondria-linked complications. PharmaNutrition 6:180-190
Klos KJ, Chandler M, Kumar N, Ahlskog JE, Josephs KA (2006) Neuropsychological profiles of manganese neurotoxicity. Eur J Neurol 13:1139–1141
Zhang S, Zhou Z, Fu J (2003) Effect of manganese chloride exposure on liver and brain mitochondria function in rats. Environ Res 93:149–157
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26:232–236
Gavin CE, Gunter KK, Gunter TE (1999) Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology 20:445–453
Zhang S, Fu J, Zhou Z (2004) In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol in Vitro 18:71–77
Heidari R, Abdoli N, Ommati MM, Jamshidzadeh A, Niknahad H (2018) Mitochondrial impairment induced by chenodeoxycholic acid: the protective effect of taurine and carnosine supplementation. Trends Pharmaceut Sci 4:99–108
Niknahad H, Jamshidzadeh A, Heidari R, Zarei M, Ommati MM (2017) Ammonia-induced mitochondrial dysfunction and energy metabolism disturbances in isolated brain and liver mitochondria, and the effect of taurine administration: relevance to hepatic encephalopathy treatment. Clin Exp Hepatol 3:141–151
Chen K, Zhang Q, Wang J, Liu F, Mi M, Xu H, Chen F, Zeng K (2009) Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res 1279:131–138
Sun M, Gu Y, Zhao Y, Xu C (2011) Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids 40:1419–1429
Chtourou Y, Fetoui H, Sefi M, Trabelsi K, Barkallah M, Boudawara T, Kallel H, Zeghal N (2010) Silymarin, a natural antioxidant, protects cerebral cortex against manganese-induced neurotoxicity in adult rats. BioMetals 23:985–996
Chtourou Y, Trabelsi K, Fetoui H, Mkannez G, Kallel H, Zeghal N (2011) Manganese induces oxidative stress, redox state unbalance and disrupts membrane bound atpases on murine neuroblastoma cells in vitro: protective role of silymarin. Neurochem Res 36:1546–1557
Lu C-L, Tang S, Meng Z-J, He Y-Y, Song L-Y, Liu Y-P, Ma N, Li XY, Guo SC (2014) Taurine improves the spatial learning and memory ability impaired by sub-chronic manganese exposure. J Biomed Sci 21:51
Ahmadi N, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Heidari R (2018) Taurine prevents mitochondrial membrane permeabilization and swelling upon interaction with manganese: implication in the treatment of cirrhosis-associated central nervous system complications. J Biochem Mol Toxicol 0:e22217. https://doi.org/10.1002/jbt.22216
Fordahl SC, Anderson JG, Cooney PT, Weaver TL, Colyer CL, Erikson KM (2010) Manganese exposure inhibits the clearance of extracellular GABA and influences taurine homeostasis in the striatum of developing rats. NeuroToxicology 31:639–646
Lipe GW, Duhart H, Newport GD, Jr WS, Ali SF (1999) Effect of manganese on the concentration of amino acids in different regions of the rat brain. J Environment Sci Health Part B 34:119–132
Funding
This work was financially supported by Pharmaceutical Sciences Research Center and Vice Chancellor of Research Office of Shiraz University of Medical Sciences (Grant 15295/13477/14210).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All the experiments were performed in conformity with the guidelines for care and use of experimental animals which were approved by the ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (#15295/13477/14210).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ommati, M.M., Heidari, R., Ghanbarinejad, V. et al. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res 190, 384–395 (2019). https://doi.org/10.1007/s12011-018-1552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1552-2