Abstract
Purpose of Review
The objective of this review is to summarize the literature on the prevalence and diagnosis of obesity and its metabolic profile, including bone metabolism, focusing on the main inflammatory and turnover bone mediators that better characterize metabolically healthy obesity phenotype, and to summarize the therapeutic interventions for obesity with their effects on bone health.
Recent Findings
Osteoporosis and fracture risk not only increase with age and menopause but also with metabolic diseases, such as diabetes mellitus. Thus, patients with high BMI may have a higher bone fragility and fracture risk. However, some obese individuals with healthy metabolic profiles seem to be less at risk of bone fracture.
Summary
Obesity has become an alarming disease with growing prevalence and multiple metabolic comorbidities, resulting in a significant burden on healthcare and increased mortality. The imbalance between increased food ingestion and decreased energy expenditure leads to pathological adipose tissue distribution and function, with increased secretion of proinflammatory markers and harmful consequences for body tissues, including bone tissue. However, some obese individuals seem to have a healthy metabolic profile and may not develop cardiometabolic disease during their lives. This healthy metabolic profile also benefits bone turnover and is associated with lower fracture risk.

Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wang YC, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–25.
Okunogbe A, et al. Economic impacts of overweight and obesity: current and future estimates for eight countries. BMJ Glob Health. 2021;6(10):e006351.
Collaboration, N.C.D.R.F. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42.
Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 192 million participants. Lancet. 2016;387(10026):1377–96.
• Bluher M. Obesity: Global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98. This paper summarizes evidence on the concept of metabolically healthy obesity.
Virani SS, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020;141(9):e139–596.
Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):bnaa004.
Ding C, Chan Z, Magkos F. Lean, but not healthy: the 'metabolically obese, normal-weight’ phenotype. Curr Opin Clin Nutr Metab Care. 2016;19(6):408–17.
• Wang B, et al. Prevalence of metabolically healthy obese and metabolically obese but normal weight in adults worldwide: A meta-analysis. Horm Metab Res. 2015;47(11):839–45. Summary of papers evaluating the specific characteristics of metabolically healthy obesity.
Loos RJF, Kilpelainen TO. Genes that make you fat, but keep you healthy. J Intern Med. 2018;284(5):450–63.
World Health Organization. Assessment of osteoporosis at the primary health care level. Summary report of a WHO scientific group. WHO, Geneva. (2007). www.who.int/chp/topics/rheumatic/en/index.html
Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33.
Obesity and Overweight. World Health Organization. Available at: www.who.int/mediacentre/factsheets/fs311/en/. Accessed 19 March 2020, 2016.
• Aung K, et al. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99(2):462–8. Report on the association between metabolically healthy obese individuals and osteoporotic fractures.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Pischon T, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines, O.E.P. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society published by the Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity (Silver Spring). 2014;22 Suppl 2:S5–39.
Britton KA, et al. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62(10):921–5.
Yamaji T, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol. 2009;170(12):1502–11.
Shen W, Chen J. Application of imaging and other noninvasive techniques in determining adipose tissue mass. Methods Mol Biol. 2008;456:39–54.
Cheung AS, et al. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (Lond). 2016;40(8):1325–8.
• Vecchie A, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6–17. This paper describes how two types of obesity have different manifestations in the cardiovascular system.
Lee JH, et al. The role of adipose tissue mitochondria: Regulation of mitochondrial function for the treatment of metabolic diseases. Int J Mol Sci. 2019;20(19):4924.
Mittal B. Subcutaneous adipose tissue & visceral adipose tissue. Indian J Med Res. 2019;149(5):571–3.
Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13.
Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93(1):359–404.
Zhang H, et al. Hepatic fat content is a determinant of metabolic phenotypes and increased carotid intima-media thickness in obese adults. Sci Rep. 2016;6:21894.
McMorrow AM, et al. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: potential impact of dietary fat quality. Proc Nutr Soc. 2015;74(1):67–82.
Wildman RP, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168(15):1617–24.
van Vliet-Ostaptchouk JV, et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr Disord. 2014;14:9.
Karelis AD, Brochu M, Rabasa-Lhoret R. Can we identify metabolically healthy but obese individuals (MHO)? Diabetes Metab. 2004;30(6):569–72.
Tsatsoulis A, Paschou SA. Metabolically healthy obesity: Criteria, epidemiology, controversies, and consequences. Curr Obes Rep. 2020;9(2):109–20.
Karelis AD, Rabasa-Lhoret R. Inclusion of C-reactive protein in the identification of metabolically healthy but obese (MHO) individuals. Diabetes Metab. 2008;34(2):183–4.
Li L, et al. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: Data from the BCAMS study. J Clin Endocrinol Metab. 2016;101(4):1816–25.
•• Rask-Andersen M, Johansson A. Illuminating the “healthy obese” phenotype. Nat Metab. 2023;5(2):193–4. Presents evidence for the concept of "healthy obesity".
Phillips CM. Metabolically healthy obesity: Definitions, determinants and clinical implications. Rev Endocr Metab Disord. 2013;14(3):219–27.
Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–56.
Fabbrini E, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145(2):366-74 e1-3.
Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98(10):E1610–9.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–53.
Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29(1):81–90.
Smekal A, Vaclavik J. Adipokines and cardiovascular disease: A comprehensive review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(1):31–40.
Aguilar-Salinas CA, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93(10):4075–9.
Christou KA, et al. The regulation of serum resistin levels in metabolically healthy and unhealthy obese individuals. Hormones (Athens). 2020;19(4):523–9.
Obradovic M, et al. Leptin and obesity: Role and clinical implication. Front Endocrinol (Lausanne). 2021;12: 585887.
Ghantous CM, et al. Differential role of leptin and adiponectin in cardiovascular system. Int J Endocrinol. 2015;2015: 534320.
Labruna G, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an “at-risk” phenotype in young severely obese patients. Obesity (Silver Spring). 2011;19(7):1492–6.
Gomez-Ambrosi J, et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care. 2014;37(10):2813–21.
Jamar G, et al. Leptin as a cardiovascular risk marker in metabolically healthy obese: Hyperleptinemia in metabolically healthy obese. Appetite. 2017;108:477–82.
Kanis JA, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–41.
Scott D, Duque G, Ebeling PR. Does obesity reduce risk for osteoporosis and fractures in older adults? Intern Med J. 2018;48(1):104–5. https://doi.org/10.1111/imj.13655.
Cui LH, et al. Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab. 2007;25(3):165–71.
Lekamwasam S, et al. Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab. 2009;27(1):83–8.
Salamat MR, et al. Relationship between weight, body mass index, and bone mineral density in men referred for dual-energy X-ray absorptiometry scan in Isfahan. Iran J Osteoporos. 2013;2013: 205963.
De Laet C, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–8.
• Compston J. Obesity and fractures in postmenopausal women. Curr Opin Rheumatol. 2015;27(4):414–9. Overview of the association between obesity and fractures.
Felson DT, et al. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J Bone Miner Res. 1993;8(5):567–73.
Berg RM, et al. Positive Association Between Adipose Tissue and Bone Stiffness. Calcif Tissue Int. 2015;97(1):40–9.
Compston JE, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). J Bone Miner Res. 2014;29(2):487–93.
Pirro M, et al. High weight or body mass index increase the risk of vertebral fractures in postmenopausal osteoporotic women. J Bone Miner Metab. 2010;28(1):88–93.
Russell M, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–55.
Bredella MA, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–54.
Brumbaugh DE, et al. Intramyocellular lipid is associated with visceral adiposity, markers of insulin resistance, and cardiovascular risk in prepubertal children: The EPOCH study. J Clin Endocrinol Metab. 2012;97(7):E1099–105.
Ryan AS, et al. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabil Neural Repair. 2011;25(9):865–72.
Bredella MA, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97(4):E584–90.
Leeners B, et al. Ovarian hormones and obesity. Hum Reprod Update. 2017;23(3):300–21.
Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5.
Campos RM, et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine. 2012;42(1):146–56.
Gkastaris K, et al. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact. 2020;20(3):372–81.
de Paula FJ, Rosen CJ. Bone remodeling and energy metabolism: new perspectives. Bone Res. 2013;1(1):72–84.
Krings A, et al. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52.
Bredella MA, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36.
Yeung DK, et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85.
•• Rosen CJ, Bouxsein ML. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. Presents current evidence on the role of marrow fat in osteoporosis.
Ko DS, et al. Altered physiology of mesenchymal stem cells in the pathogenesis of adolescent idiopathic scoliosis. World J Clin Cases. 2020;8(11):2102–10.
Moorthi RN, et al. Bone marrow fat is increased in chronic kidney disease by magnetic resonance spectroscopy. Osteoporos Int. 2015;26(6):1801–7.
Halade GV, et al. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21(12):1162–9.
Shu L, et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int. 2015;96(4):313–23.
Mutt SJ, et al. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;5:228.
Zhou S, LeBoff MS, Glowacki J. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology. 2010;151(1):14–22.
Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2). Exp Gerontol. 2004;39(3):333–8.
Duque G, et al. 1,25(OH)2D3 acts as a bone-forming agent in the hormone-independent senescence-accelerated mouse (SAM-P/6). Am J Physiol Endocrinol Metab. 2005;288(4):E723–30.
Shockley KR, et al. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106(2):232–46.
Ichida F, et al. Reciprocal roles of MSX2 in regulation of osteoblast and adipocyte differentiation. J Biol Chem. 2004;279(32):34015–22.
Ilich JZ, et al. Interrelationship among muscle, fat, and bone: Connecting the dots on cellular, hormonal, and whole body levels. Ageing Res Rev. 2014;15:51–60.
Kang S, et al. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–24.
Horowitz MC, et al. Bone marrow adipocytes. Adipocyte. 2017;6(3):193–204.
Singh L, et al. Good, bad, or ugly: the biological roles of bone marrow fat. Curr Osteoporos Rep. 2018;16(2):130–7.
Cadenas-Sanchez C, et al. Differences in specific abdominal fat depots between metabolically healthy and unhealthy children with overweight/obesity: the role of cardiorespiratory fitness. Scand J Med Sci Sports. 2023;33(8):1462–72.
Bredella MA, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring). 2011;19(1):49–53.
Shen W, et al. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18(5):641–7.
Bredella MA, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97(11):4115–22.
Fischer V, et al. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep. 2017;7(1):7223.
•• Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39(6):938–59. Presents mechanistic evidence for the impact of leptin on bone.
Cornish J, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15.
Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8.
Bjornholm M, et al. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest. 2007;117(5):1354–60.
Shi Y, et al. Dissociation of the neuronal regulation of bone mass and energy metabolism by leptin in vivo. Proc Natl Acad Sci U S A. 2008;105(51):20529–33.
Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207.
Pasco JA, et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86(5):1884–7.
Yamauchi M, et al. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf). 2001;55(3):341–7.
Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–903.
Odabasi E, et al. Plasma leptin concentrations in postmenopausal women with osteoporosis. Eur J Endocrinol. 2000;142(2):170–3.
Jurimae J, Jurimae T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos Int. 2007;18(9):1253–9.
Jurimae J, et al. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23.
Zoico E, et al. Relation between adiponectin and bone mineral density in elderly post-menopausal women: role of body composition, leptin, insulin resistance, and dehydroepiandrosterone sulfate. J Endocrinol Invest. 2008;31(4):297–302.
Basurto L, et al. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160(2):289–93.
Kontogianni MD, et al. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. J Bone Miner Res. 2004;19(4):546–51.
Fasshauer M, et al. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290(3):1084–9.
Lee S, et al. Effect of adipokine and ghrelin levels on BMD and fracture risk: an updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1044039.
Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–11.
Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion Diabetologia. 1980;19(5):475–81.
Suchacki KJ, Thomas BJ, Ikushima YM, Chen KC, Fyfe C, Tavares AAS, Sulston RJ, Lovdel A, Woodward HJ, Han X, Mattiucci D, Brain EJ, Alcaide-Corral CJ, Kobayashi H, Gray GA, Whitfield PD, Stimson RH, Morton NM, Johnstone AM, Cawthorn WP. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. Elife. 2023;12:e88080.
Miller KK, et al. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab. 2005;90(2):768–74.
Yakar S, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–81.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Kremer R, et al. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94(1):67–73.
Arunabh S, et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88(1):157–61.
Parikh SJ, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–9.
Karampela I, et al. Vitamin D and obesity: current evidence and controversies. Curr Obes Rep. 2021;10(2):162–80.
Manson JE, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33–44.
Ortega RM, et al. Preliminary data about the influence of vitamin D status on the loss of body fat in young overweight/obese women following two types of hypocaloric diet. Br J Nutr. 2008;100(2):269–72.
Salehpour A, et al. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78.
Wamberg L, et al. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels - results from a randomized trial. Eur J Intern Med. 2013;24(7):644–9.
Zhou J, et al. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. Nutr Metab (Lond). 2010;7:62.
Bolland MJ, et al. Testosterone levels following decreases in serum osteocalcin. Calcif Tissue Int. 2013;93(2):133–6.
Sukumar D, Shapses SA, Schneider SH. Vitamin D supplementation during short-term caloric restriction in healthy overweight/obese older women: effect on glycemic indices and serum osteocalcin levels. Mol Cell Endocrinol. 2015;410:73–7.
Gilsanz V, et al. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95(4):1595–601.
Li J, et al. Vitamin D prevents lipid accumulation in murine muscle through regulation of PPARgamma and perilipin-2 expression. J Steroid Biochem Mol Biol. 2018;177:116–24.
Shimizu Y, et al. Serum 25-hydroxyvitamin D level and risk of falls in Japanese community-dwelling elderly women: a 1-year follow-up study. Osteoporos Int. 2015;26(8):2185–92.
Aung K. Review: in postmenopausal women and older men, vitamin D plus calcium reduces some fractures. Ann Intern Med. 2014;161(6):JC5.
Marques Loureiro L, et al. Does the metabolically healthy obese phenotype protect adults with class III obesity from biochemical alterations related to bone metabolism? Nutrients. 2019;11(9):2125.
Wortsman J, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3.
Sukumar D, et al. Can bone-regulating hormones and nutrients help characterize the metabolically healthy obese phenotype. Nutr Health. 2018;24(3):153–62.
Wung CH, et al. Associations between metabolic syndrome and obesity-related indices and bone mineral density T-score in hemodialysis patients. J Pers Med. 2021;11(8):775.
Starup-Linde J, et al. Bone density and structure in overweight men with and without diabetes. Front Endocrinol (Lausanne). 2022;13: 837084.
Nobrega da Silva V, et al. Impact of metabolic syndrome and its components on bone remodeling in adolescents. PLoS One. 2021;16(7):e0253892.
Yamaguchi T, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45(2):174–9.
Gilsanz V, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–93.
Janicka A, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92(1):143–7.
Choi HS, et al. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int. 2010;87(3):218–25.
Pollock NK, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25(12):2760–9.
•• Mirzababaei A, et al. Metabolically healthy/unhealthy components may modify bone mineral density in obese people. Arch Osteoporos. 2017;12(1):95. Presents the main components linking healthy and unhealthy obesity with bone.
Wang Y, et al. Association between forearm bone mineral density and metabolic obesity in a northern Chinese population. Metab Syndr Relat Disord. 2020;18(5):251–9.
Ubago-Guisado E, et al. Differences in areal bone mineral density between metabolically healthy and unhealthy overweight/obese children: the role of physical activity and cardiorespiratory fitness. Pediatr Res. 2020;87(7):1219–25.
Li X, Gong X, Jiang W. Abdominal obesity and risk of hip fracture: A meta-analysis of prospective studies. Osteoporos Int. 2017;28(10):2747–57.
Nguyen ND, et al. Abdominal fat and hip fracture risk in the elderly: The Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord. 2005;6:11.
Bachmann KN, et al. Vertebral strength and estimated fracture risk across the BMI spectrum in women. J Bone Miner Res. 2016;31(2):281–8.
Ghezelbash F, et al. Obesity and obesity shape markedly influence spine biomechanics: a subject-specific risk assessment model. Ann Biomed Eng. 2017;45(10):2373–82.
Gandham A, et al. Incidence and predictors of fractures in older adults with and without obesity defined by body mass index versus body fat percentage. Bone. 2020;140: 115546.
Walsh JS, Vilaca T. Obesity, type 2 diabetes and bone in adults. Calcif Tissue Int. 2017;100(5):528–35.
Hamer M, et al. Stability of metabolically healthy obesity over 8 years: The English Longitudinal Study of Ageing. Eur J Endocrinol. 2015;173(5):703–8.
Schroder H, et al. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr. 2014;53(6):1345–53.
Langlois JA, et al. Weight loss from maximum body weight among middle-aged and older white women and the risk of hip fracture: The NHANES I epidemiologic follow-up study. Osteoporos Int. 2001;12(9):763–8.
Ensrud KE, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90(4):1998–2004.
Bleicher K, et al. The role of fat and lean mass in bone loss in older men: findings from the CHAMP study. Bone. 2011;49(6):1299–305.
Pop LC, et al. Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr. 2015;101(3):659–67.
Seimon RV, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO Diet randomized clinical trial. JAMA Netw Open. 2019;2(10): e1913733.
•• Tencerova M, Duque G, Beekman KM, Corsi A, Geurts J, Bisschop PH, Paccou J. The impact of interventional weight loss on bone marrow adipose tissue in people living with obesity and its connection to bone metabolism. Nutrients. 2023;15(21):4601. Comprehensive review on the impact of weight loss on marrow fat.
Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res. 2019;16(2):118–27.
Velho S, et al. Metabolically healthy obesity: Different prevalences using different criteria. Eur J Clin Nutr. 2010;64(10):1043–51.
Shah K, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26(12):2851–9.
Emerenziani GP, et al. Effects of body weight loss program on parameters of muscle performance in female obese adults. J Sports Med Phys Fitness. 2019;59(4):624–31.
Xie B, et al. The impact of glucagon-like peptide 1 receptor agonists on bone metabolism and its possible mechanisms in osteoporosis treatment. Front Pharmacol. 2021;12: 697442.
Cai TT, et al. Effects of GLP-1 receptor agonists on bone mineral density in patients with type 2 diabetes mellitus: a 52-week clinical study. Biomed Res Int. 2021;2021:3361309.
Kong QX, et al. Evaluation of the risk of fracture in type 2 diabetes mellitus patients with incretins: an updated meta-analysis. Endokrynol Pol. 2021;72(4):319–28.
Hartman ML, et al. Effects of novel dual gip and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–5.
Ding KH, et al. Impact of glucose-dependent insulinotropic peptide on age-induced bone loss. J Bone Miner Res. 2008;23(4):536–43.
Felipe LA, et al. Effects of Roux-en-Y gastric bypass on the metabolic profile and systemic inflammatory status of women with metabolic syndrome: randomized controlled clinical trial. Diabetol Metab Syndr. 2023;15(1):19.
Yu EW, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100(4):1452–9.
Mele C, et al. Bone response to weight loss following bariatric surgery. Front Endocrinol (Lausanne). 2022;13: 921353.
Rousseau C, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354: i3794.
Yu EW, et al. Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017;32(6):1229–36.
Murai IH, et al. Exercise mitigates bone loss in women with severe obesity after Roux-en-Y gastric bypass: a randomized controlled trial. J Clin Endocrinol Metab. 2019;104(10):4639–50.
Author information
Authors and Affiliations
Contributions
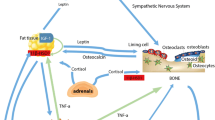
E.G., R.K., and G.D. wrote the main manuscript text and E.G. and G.D. prepared figure 1. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
EG, RK, and GD have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Ethical Approval
This review article does not present any previously unpublished original research, and ethical approval is therefore not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gruneisen, E., Kremer, R. & Duque, G. Fat as a Friend or Foe of the Bone. Curr Osteoporos Rep (2024). https://doi.org/10.1007/s11914-024-00864-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11914-024-00864-4