Abstract
Microbial exopolysaccharides (EPS) provide a broad range of applications. Thus, there is an increasing interest in the production, characterization, and use of EPS derived from various microorganisms. Extremophile polysaccharides have unique properties and applications due to its unique structures. The importance of exopolysaccharides synthesized by a new bacterial strain, Alkalibacillus sp. w3, was highlighted in this study. Alkalibacillus sp. w3, a haloalkalitolerant firmicute that was recovered from a salt lake, was optimized for EPS production, and its biological activities were studied. Exopolysaccharide synthesis was observed in Horikoshi I broth medium. The optimal culture conditions for achieving the highest exopolysaccharide production were a 7-day incubation period, pH 10, and 250 g/L of NaCl. The most effective carbon and nitrogen sources for EPS production were glucose and a combination of yeast extract and peptone. Additionally, Plackett-statistical Burman’s design showed that all factors tested had a favorable impact, with glucose having the greatest significance on the production of EPS. The model’s best predictions for culture conditions resulted in a two-fold improvement in EPS production compared to the original yield before optimization. The recovered EPS contained 65.13% carbohydrates, 30.89% proteins, and 3.98% lipids. Moreover, EPS produced by Alkalibacillus sp. w3 demonstrated anticancer activity against hepatocellular carcinoma (HepG2) and human colon carcinoma (HCT-116) cell lines, with IC50 values as low as 11.8 and 15.5 µg/mL, respectively, besides antibacterial activity against various Gram-positive, Gram-negative bacteria, and yeast. Based on these results, EPS made by Alkalibacillus sp. w3 has many useful properties, which make it suitable for use in the medical field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular polymeric substances (EPSs) are high molecular weight polymers composed mainly of carbohydrate monomers and known as exopolysaccharide and the same is used. The EPS secreted extracellularly by various microorganisms. However, other secreted macromolecules like proteins, lipids, and nucleic acids might also be present to give its heterogeneous structure. These EPSs are either excreted loosely into the environment or attached to the cell surface. EPSs serve in various roles, improving cell integrity and helping in survival in harsh environments either with extreme physical stressors or nutrient scarcity (Ates 2015; Decho and Gutierrez 2017; Almutairi and Helal 2021). Moreover, they provide microenvironments around microbial cells, where genes and metabolites are often exchanged, giving the bacteria the competency to survive in nutrient-poor environments. In other words, the presence of such EPS guards against environmental changes such as temperature and salinity fluctuations, as well as possible predators (Nichols et al. 2005; Squillaci et al. 2016; Rana and Upadhyay 2020) said that the functions of exopolysaccharides depend on the structure of the microorganism and where it lives. Capsular polysaccharides also contributes to vilrulance in pathogenic strains (Deng et al. 2010; Abdella et al. 2017; El-Wazzan et al. 2020).
Competent microorganisms that can survive in harsh environmental conditions, such as high salinity and temperatures, are referred to as an extremophile. Among these fascinating microbes, halophiles live in hypersaline ecosystems like soda lakes and coastal lagoons and are sources of several biomolecules (Ventosa 2006; Naghoni et al. 2017). These biomolecules can be produced by assisting these cells in surviving and shielding them from stress in harsh environments (Parada-Pinilla et al. 2021). Extremophile EPS has drawn attention due to the rising industrial need for natural polymers (Poli et al. 2010; Sahana and Rekha 2020).
Because of their biocompatibility and biodegradability, the macromolecules produced by halophilic bacteria have been used in biomedical and agro-industrial applications (Radchenkova et al. 2013; Corinaldesi et al. 2017). Additionally, they contribute significantly to health, owing to their antiviral, anticancer, and antibacterial capabilities (Yildiz and Karatas 2018; Rajoka et al. 2019). They are water soluble, edible, biocompatible, and biodegradable, which accounts for this (Ates 2015; Liang and Wang 2015; Hao et al. 2019; Riaz Rajoka et al. 2020).
The diverse microbes found in extreme environments hold exciting potential for the discovery of new polysaccharides. In a previous study, several haloalkaliphilic bacterial cultures were isolated from Al Hamra lake, Egypt (Arayes et al. 2021). These isolates were screened for EPS production. One of these bacteria, Alkalibacillus sp. w3, could produce exopolysaccharides. To our knowledge, there are no reports on exopolysaccharide production from the genus Alkalibacillus and this is the first report on the EPS production by Alkalibacillus sp. w3. It has been reported that EPS production is strongly dependent on the types of microbe and fermentation conditions (incubation time, agitation, pH, carbon or nitrogen source, mineral salts). Furthermore, the chemical composition of microbial EPS depends mainly on the composition of the production medium and several physical conditions provided throughout the fermentation process (Ju et al. 2022). Developing industrial fermentation, optimizing culture conditions, and designing media are critical. This research evaluated the EPS production by the haloalkaliphilic Alkalibacillus sp. w3. Exopolysaccharide production by Alkalibacillus sp. w3 was also enhanced by culture conditions and nutritional variables using a one-variable temporal technique and Plackett-Burman statistical design. Ultimately, EPS compositional analyses and potential applications (against germs and cancer) were looked at.
Materials and methods
Growth medium and cultivation conditions
The haloalkaliphilic Alkalibacillus sp. w3 was isolated from Al Hamra lake (30° 23′ 21” North, 30° 20′ 45′′ East), El-Beheira Governorate, Egypt. Taxonomic identification was done by 16S rRNA and the sequence was deposited in GenBank with an accession number LC164826 (Arayes et al. 2021). The bacterial culture was maintained on modified Horikoshi I agar slants, at 4°C as a working culture, whereas for long-term preservation, 15% glycerol broth was stored at -70°C. The medium Horikoshi I consisted of glucose, 10 g; yeast extract, 5 g; peptone, 5 g; KH2PO4, 1; MgSO4.7H2O, 0.2 g, NaCl 150 g, and agar 20 g dissolved in 1 L distilled water (Horikoshi 1996), pH was adjusted to 9 ± 0.2 with Na2CO3.
EPS production
To produce EPS, Alkalibacillus sp. w3 was first inoculated into a 250 mL Erlenmeyer flask having 50 mL of modified Horikoshi I as seed medium, pH was adjusted after autoclaving to 9 ± 0.2 with sterile Na2CO3 and incubated in an orbital shaker at 150 rpm and 35 °C for 24 h. After cultivation, 2% (v/v) of a seed culture (OD600 of 1 ± 0.1) was inoculated into a 250 mL Erlenmeyer flask having 50 mL of modified Horikoshi I as a production medium and incubated at 35 °C for 7 days in both static and shaked conditions to assess the effect of agitation/aeration. Every 24 h, the optical density of both cultures was evaluated against a sterile uninoculated medium as a blank to check the growth. For the EPS production evaluation, samples of fermentation broth were obtained at the same time intervals in clean and sterile glass tubes (Mehta et al. 2014).
Precipitation and quantification
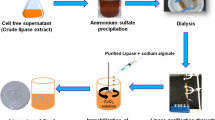
Cell-free supernatant obtained by centrifugation at 15.294 x g (12,000 rpm) for 15 min (Sigma 2-16KL, Germany) was mixed with three volumes of 95% chilled ethanol and stirred vigorously, and kept at 4 °C for 24 h to ensure complete precipitation of EPS. Subsequently, the precipitates were collected by centrifugation at 15.294 x g (12,000 rpm) for 20 min. The formed pellet was dried at 60 °C overnight (Padmanaban et al. 2015). The recovered EPS was figured out gravimetrically by grams per 100 mL culture medium.
Effect of culture conditions on EPS Production
The nutrient components of the EPS production medium were selected by the onefactoratatime (OFAT) approach, based on the yield of the EPS in each formulation. The factors and their respective parameters that were optimized for EPS production were as follows; pH (6 to 11), different carbon sources with concentrations of 10 g/L (glucose, fructose, maltose, galactose, mannitol, sucrose, and lactose), nitrogen sources adjusted to 0.89 g N/L nitrogen equivalent (peptone, beef extract, yeast extract, tryptone, casein, ammonium chloride, sodium nitrate, ammonium nitrate, urea, and ammonium sulfate), and various NaCl concentration (5% 35%). All experiments were performed in triplicate, and the obtained results were expressed as computed mean ± standard error of triplicates.
Optimization of EPS production by Plackett-Burman Design
One-factor-at-a-time approach of the optimization study showed that glucose and a mixture of peptone and yeast extract were the best carbon and nitrogen sources, respectively, for maximum EPS yield. Therefore, it was selected for further optimization experiments. Plackett-Burman design was used as part of a multifactorial design strategy for screening the most influential variable (Plackett and Burman 1946; Mabrouk et al. 2014; Peele et al. 2018). As shown in Table 1, the seven independent variables of the production medium were evaluated at two different levels, high (1) and low (-1). All trials were done in triplicate and the averages of the produced EPS were treated as the response. Table 2 shows how the variable combinations were grouped using the Plackett-Burman design matrix. A multiple regression analysis was used to decide whether variables affected the EPS production, either favorably or adversely. The following equation was used to calculate the main effect for each parameter before continuing.
R (H) = response parameter had the higher quantity of a given parameter, R(L) = response parameter had a lower quantity of a given component, and N = number of assemblies divided by two. The p-value of each variable was calculated for the determination of the significance level. The statistical handling of data obtained for the design of Plackett-Burman design has been achieved by Statistica 10 software. The variables with confidence levels ≥ 85% were considered to significantly influence the EPS production (Abdel-Fattah 2007).
Analytical methods
Total carbohydrate determination
For total carbohydrate determination, the recovered EPS was resolubilized in distilled water and dialyzed against distilled water at 4 °C for 24 h using a 12-kDa cut-off dialysis membrane. Then, it was reprecipitated using chilled acetone and dried at 60 °C overnight (Vijayabaskar et al. 2011; Silva et al. 2020). The carbohydrate content was determined using the method proposed by Dubois et al. (1956). Briefly, 1 mL of dissolved EPS sample (1 mg) was mixed with 0.5 mL of 5% phenol and 3.5 mL of concentrated sulfuric acid and incubated in a hot water bath for 15 min at 40 °C. The absorbance of the mixture was measured at 490 nm (Dubois et al. 1956; Patil and Shirsath 2015). The amount of carbohydrates was determined using a calibration curve with glucose as a standard.
Total protein determination
The protein content was determined by Lowry’s method (Lowry et al. 1951). A standard curve was prepared using bovine serum albumin as the standard (Biswas and Paul 2014).
Total lipid determination
To assess lipid content, the EPS sample (0.2 g) was extracted with a chloroform-methanol (2:1) mixture and agitated vigorously. The solvent phase was recovered by centrifugation at 12,745 x g (10,000 rpm) for 15 min. The extraction was repeated three times. The whole solvent was collected, evaporated, and dried under a vacuum. The lipid content was determined via gravimetric analysis following the method described by Makkar and Cameotra (1998).
Fourier transform infrared (FT-IR)
Fourier transform infrared was used for the determination of functional groups of EPS. A Bruker Tensor 37 FT-IR (Germany) spectrometer with a mercury cadmium telluride detector chilled with liquid nitrogen was used for the analysis. 2 mg of the dried EPS sample was ground in about 200 mg of spectra-grade KBr (sigma) and crushed into pellets under about 5–6 tons cm− 2 pressure using a hydraulic press. The spectrum measurements were done in transmittance mode in the range 4000 − 400 cm− 1. Using the OPUS 3.1 (Bruker Optics) software, infrared (IR) spectra were analysed.
UV spectroscopy
The purified EPS was diluted in distilled water at a concentration of 1 mg/mL and analyzed using a UV-visible spectrophotometer (Labda /Vis, Perkinelmer, Japan) for the UV spectrum (Sathiyanarayanan et al. 2014; Wang et al. 2015).
Functional characterization of EPS
Antimicrobial activity
The antimicrobial properties of EPS were evaluated using the agar well diffusion method against various human pathogenic bacteria, including Gram-positive (Streptococcus pneumoniae RCMB 010010) and Gram-negative (Pseudomonas aeruginosa RCMB 010043, Escherichia coli RCMB 010052) and yeast (Candida albicans RCMB 05036) (Verma et al. 2015; Viju et al. 2016). Bacterial indicators were cultivated in nutrient broth until the mid-exponential phase, then 100 µl were evenly distributed on nutrient agar plates. Then, wells of 6 mm diameter were cut in the agar using a sterile cork borer and filled with 100 µL (1 mg/mL) pure EPS. The plates were incubated for 1 h at 4 ℃ in a cooled incubator before being incubated for 24 h at 37 ℃ and 72 h at 28 ℃ for bacteria and yeast, respectively. Subsequently, the inhibitory zone was measured in millimeters. Negative controls were employed using sterile deionized water.
Anticancer activity
To assess the anticancer activity, human colon cancer (HCT-116 from ATCC CCL-247) and human hepatocellular carcinoma (HepG2 from ATCC HB-8065) cell lines were used to evaluate the antitumor potential of the EPS. The cells were grown as monolayers in a 96-well microtiter plate on a PRMI-1640 growth medium supplemented with 10% inactivated fetal calf serum and 50 µg/mL gentamicin and incubated for 24 h at 37 °C in a 5% CO2 humidified incubator. The cells grown were then washed with sterile phosphate buffer saline (0.01 M, pH 7.2) and then the cells were treated with 100 µL from different concentrations of the tested sample, Doxorubicin was used as a positive control. The number of surviving cells was determined by staining the cells with crystal violet (Mosmann 1983; Gangadevi and Muthumary 2007) followed by cell lysing using 33% glacial acetic acid and reading the absorbance at 590 nm using a microplate reader (SunRise, TECAN, Inc, USA) after mixing. The percentage of viability is calculated. The 50% inhibitory concentration (IC50) was estimated from graphic plots.
Results
Alkalibacillus sp. w3 growth and EPS production
The production of EPS began after the cells had achieved OD600 ≈ 1. The growth and EPS production increased gradually and reached a maximum on the 6th day of incubation when the cells were stationary. During the stationary phase, the polymer quantity remained constant under shaking circumstances (Fig. 1). Whereas no further increase in EPS production was observed after this period. In contrast, there was a slow growth (OD600 = 0.67), and the EPS was not produced until the 7th day in static incubation (data not shown). These results prove that good growth of Alkalibacillus sp. w3 is a determining factor for the high production of EPS.
Effect of environmental parameters on EPS production
Figure (2a) shows the impact of different pH values on growth and EPS production. The results showed that pH values less than 8 greatly hampered the growth rate of Alkalibacillus sp. w3 and completely inhibited the EPS production, while at pH values from 9 to 11, it showed the maximum growth (OD600 = 2) and EPS yield (14.3 g/L). This result suggests the alkaliphilic nature of the microbe, which confirms our previous work. Moreover, the results show that Alkalibacillus sp. w3 could develop and resist the NaCl concentration up to 25%, but no growth was observed at 30% NaCl concentration (Fig. 2b). It is obvious that EPS was produced only above 5% NaCl and the highest yield (1.5 g EPS/100 mL) was achieved with 15% and 20% NaCl. However, with more increased NaCl concentration, the lowest yield (0.97 g/100 mL) was obtained in media having 25% NaCl. Glucose was shown to be the most effective carbon source for EPS production with a yield of 1.53 g/100 mL, followed by maltose and sucrose (Fig. 2c. As demonstrated in Fig. 2d, organic nitrogen, which is easier to digest, stimulated microbial growth and EPS synthesis more than inorganic nitrogen did. The maximum yield of EPS was produced (1.53 g/100 mL) when peptone and yeast extract were combined.
Statistical optimization and Verification of EPS Production
The influence of medium components on EPS production from Alkalibacillus sp. w3 was studied using the Plackett-Burman design. Table 3 displays the main effect calculation, regression coefficient, p-value, and significance of each variable. All the variables that were examined in the current study had a positive effect on EPS production. A high coefficient value writes down the importance of this variable in EPS production. Among all, glucose had the highest significance for EPS production. Based on the values in Table 3, the other components had confidence scores of less than 85% and were considered insignificant.
The verification experiment was conducted in triplicate using this optimal medium composition (predicted optimum levels of the independent variables) and compared with the basal medium to confirm the results. Under the best conditions, EPS yield increased from 1.45 to 3.0 g/L (2-fold) (Fig. 3b).
Chemical composition and characterization of purified EPS
The obtained EPS had a chemical composition of 65.13% carbohydrate, 30.89% protein, and 3.98% lipid. The FT-IR spectrum of the purified Alkalibacillus sp. w3 EPS (Fig. 4) showed distinctive functional groups such as large stretching in the 3439 cm− 1 area, matching the stretching vibration of the hydroxyl groups of polysaccharides and the (N-H) stretch of amines (Nanda and Raghavan 2014; Gugliandolo et al. 2015). The absorption peak at 2972 cm− 1 is attributed to the asymmetrical C-H stretching vibration of the methyl group (Vasanthakumari et al. 2015). The peak around 1643 cm− 1 indicates the C = O stretch of amides corresponding to amide C = O and C-N bending of protein and peptide amines that indicates the characteristic IR absorption of polysaccharides and this absorption peak concluded that there is an amino sugar (Qiang et al. 2013). The C = C stretching vibration at 1643.27 cm− 1 might indicate a phenyl ring or the presence of conjugated carbonyl groups (Pal et al. 2015). The absorption peak at 1414.02 cm− 1 confirms the presence of O = H bend of esters that could be assigned to C = O of the COO and C-O bond from COO- (Helm 1995). The 621 cm− 1 band indicates the acetylenic C-H bend of alkynes (Zhang et al. 2013; Nisha and Thangavel 2014).
Figure 5 shows the UV spectrum of EPS generated by Alkalibacillus sp. w3, which exhibits functional groups such as carboxyl, carbonyl, amine, and ester, with maximal absorption at 200–230 nm (Singh et al. 2011; Sathiyanarayanan et al. 2014). A modest peak was noticed at roughly 280 nm, which is typical of π-π* transitions in aromatic or poly-aromatic compounds found in the most conjugated molecules, such as proteins (Sathiyanarayanan et al. 2014).
Functional characterization of EPS
Antimicrobial activity
EPS was assessed for antimicrobial activities against various microorganisms. Figure 6 shows that at a dose of 1 mg/mL, EPS was effective against Gram-positive and negative bacteria and yeast. Candida albicans has the largest inhibitory zone (17.8 mm), followed by Streptococcus pneumoniae (13.8 mm).
Anticancer activities
As the concentration of EPS was increased, the proportion of viable cancer cells dropped (Fig. 7). EPS from Alkalibacillus sp. w3 was evaluated for anticancer efficacy against hepatocellular carcinoma (HepG2) and human colon carcinoma (HCT-116) cell lines (IC50 = 11.8 and 15.5 µg/mL, respectively).
Discussion
The results of the current study demonstrated a relationship between cell growth and EPS production by Alkalibacillus sp. w3. Besides, agitation was essential for the organism to grow and synthesize materials as efficiently as possible, this is because the fact that it maintains the oxygen diffusion rate and keeps the mixture homogenous, which influences mass transfer and, in turn, metabolite concentrations (Yadav et al. 2014). Earlier studies reported that both biomass and curdlan formation by Pseudoalteromonas sp. and Paenibacillus polymyxa increased with an increase in the agitation speed to 150 rpm (Al-Nahas 2011; Rafigh et al. 2014). The length of the fermentation period also affects how much EPS is made in the culture. Our results corroborate those of prior research showing that EPS was formed by Pseudoalteromonas sp. and Enterobacter cloacae MBB8 isolated from marine water throughout both the exponential and stationary phases (Al- Nahas 2011; Prakash Shyam et al. 2021). Additionally, EPS synthesis occurred during the stationary phase, which may have been caused by a lack of nutrients. Since it might affect cell growth, nutrient absorption, and EPS production, the initial pH of the culture medium is crucial (Manivasagan et al. 2013). Increasing the pH from 9 to 11 had no effect on production, suggesting that pH 9 is the bare minimum for EPS synthesis by Alkalibacillus sp. w3. Bacillus amyloliquefaciens, according to Rao et al. (2013), has an optimum pH of 9, whereas Vagococcus carniphilus, according to Joshi and Kanekar (2011), has an optimum pH of 10 to generate the maximum EPS value (560 mg/L) after 1 week.
Our results evidence the concept that EPS may aid microorganisms in surviving in harsh conditions such as very high salinity and low food availability by surrounding the cells and their proximity (Finore et al. 2014). Cultures of Planococcus rifietoensis in a medium containing 8.8 to 11.8% NaCl demonstrated the largest increase in EPS synthesis (Qurashi and Sabri 2012). The growth and production of EPS by Pseudoalteromonas agarovorans were similarly stimulated by an increase in salt concentration from 10 to 40 g/L (Choi et al. 2009).
Alkalibacillus needed a carbon source to produce EPS, and it was found that glucose was the best alternative. Sphingomonas sp. (Xu et al. 2015) and Halomonas sp. also prefer this carbon source (Karuppiah et al. 2015).
The kind of nitrogen source, concentration, and availability impacted EPS production. According to our studies, the optimal nitrogen source is totally organic. This might be because certain essential amino acids cannot be synthesized from inorganic nitrogen molecules, restricting growth and resulting in decreased EPS output (Abdul Razack et al. 2013). According to our findings, peptone was essential for EPS production and this was in accordance with previous reports. For instance, peptone was the best nitrogen source for Pseudomonas fluorescens WR-1 EPS synthesis (Raza et al. 2012). Moreover, Bacillus amyloliquefaciens produced the most EPS (18.5 g/L) using yeast extract (Rao et al. 2013). Yeast extract has also been identified as the most effective source of gellan gum from Sphingomonas paucimobilis (Bajaj et al. 2006; Raghunandan et al. 2018). This is due to the higher concentrations of amino acids, vitamins, short peptides, and growth promoters.
Based on its chemical makeup, Alkalibacillus w3 EPS is mostly made up of carbohydrates (65.13%), followed by proteins (30.89%) and lipid (3.98%).This is consistent with the EPS properties and classification reviewed by López-Ortega et al. (2021). In a similar report, Pal and Paul (2013) showed that the EPS made by the rhizobacterium Cupriavidus pauculus KPS 201 has protein, lipid plus uronic acid, and nucleic acid. Which supprprt the concept of the heterogeneity of the the EPS from microbibes.
In hepatocellular carcinoma (HepG2) and human colon cancer (HCT-116) cell lines, the pure EPS had IC50 values of 11.8 and 15.5 µg/mL, respectively. The anticancer properties of microbial EPS have been thoroughly recognized and supported by research. For instance, Halomonas smyrnensis strain’s levan shown anticancer effectiveness in vitro against human lung (A549), liver (HepG2/C3A), gastric (AGS), and breast (MCF-7) cancer cell lines (Kazak Sarilmiser and Toksoy Oner 2014). Similarly, clavan, an L-fucose-containing polysaccharide, may help out in the inhibiting of lung carcinoma cell colonization (Patel and Prajapati 2013). Furthermore, Lactobacillus helveticus MB2-1 in vitro investigations revealed that c-EPS successfully reduced the proliferation of HepG2 and HT-29 cancer cells (Li et al. 2015); Lactobacillus kefri MSR101 demonstrated good anticancer activity (44.1%) on colon cancer cells (HT-29) (Rajoka et al. 2019). In mice with hepatoma 22 (H22) and Ehrlich ascites carcinoma, glucan generated by an isolated Chinese Rhizobium sp. demonstrated immunological potentiating and anticancer activity in vivo research (Zhao et al. 2010).
Furthermore, it has previously been noted that EPS may have antibacterial action, which is consistent with our findings. The EPS generated by Alkalibacillus sp. w3 showed antibacterial activity against the yeast Candida albicans, Gram-positive bacteria, and Gram-negative. The antibacterial properties of EPS produced by microorganisms have been previously reported. For example, crude EPS generated by Lysinibacillus fusiformis showed antibacterial activity against E. coli, Staphylococcus aureus, Proteus sp., B. subtilis, P. aeruginosa, and Klebsiella sp., according to Mahendran et al. (2013). The formation of an external barrier that prevents nutrient and metal uptake from the medium, deterioration of cell membrane integrity via ionic interactions, and inhibition of DNA replication and mRNA synthesis by binding of EPS to enzymes or genetic elements may all contribute to the antimicrobial mechanism of microbial EPS (Tokura et al. 1997; Rajoka et al. 2019).
In conclusion, a haloalkalitolerant Alkalibacillus sp. was isolated and evaluated to produce exopolysaccharide, and various environmental parameters such as salt concentrations, pH value, and different carbon and nitrogen sources were investigated. The best formula to produce EPS from Alkalibacillus sp. was (g/L): glucose, 25; peptone, 7; yeast extract, 7; KH2PO4, 1.5; MgSO4, 0.3; NaCl, 25% (w/v); and inoculum volume, 3% (v/v). The ideal environment doubled the produced quantity of EPS, from 15 to 30 g/L. Finally, future research should focus on extending the investigation into the activity of EPS produced by Alkalibacillus sp. w3 as an antimicrobial and anticancer drug against other microbial cells and cancer cell lines coming from various malignancies, as well as examining the mode of action and moving the study further to animal models.
References
Abdel-Fattah YR (2007) Application of Fractional Factorial Design for the Development of Production Media for the Pikromycin Macrolide Family by Streptomyces venezuelae Trends Appl Sci Res 2:472–482. https://doi.org/10.3923/tasr.2007.472.482
Abdul Razack S, Velayutham V, Thangavelu V (2013) Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turkish J Biol 37:280–288. https://doi.org/10.3906/biy-1206-50
Al-Nahas (2011) Characterization of an exopolysaccharide-producing marine bacterium, isolate Pseudoalteromonas sp. AM. Afr J Microbiol Res 5. https://doi.org/10.5897/AJMR11.757
Almutairi MH, Helal MMI (2021) Exopolysaccharide production from isolated Enterobacter sp. strain ACD2 from the northwest of Saudi Arabia. J King Saud Univ - Sci 33:101318. https://doi.org/10.1016/j.jksus.2020.101318
Arayes MA, Mabrouk MEM, Sabry SA, Abdella B (2021) Diversity and characterization of culturable haloalkaliphilic bacteria from two distinct hypersaline lakes in northern Egypt. Biol (Bratisl) 76:751–761. https://doi.org/10.2478/s11756-020-00609-5
Ates O (2015) Systems Biology of Microbial Exopolysaccharides Production. Front Bioeng Biotechnol 3:1–16. https://doi.org/10.3389/fbioe.2015.00200
Abdella B, El-Wazzan E, El-Sersy NA, Sabry SA, El-Helow ER (2017) Pathogenicity and antibiotic susceptibility of two bacterial pathogens associated with the clam Tapes decussatus in some Egyptian fisheries. Ege J Fish Aquat Sci 34(4):383–389. https://doi.org/10.12714/egejfas.2017.34.4.04
Bajaj IB, Saudagar PS, Singhal RS, Pandey A (2006) Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J Biosci Bioeng 102:150–156. https://doi.org/10.1263/jbb.102.150
Biswas J, Paul AK (2014) Production of Extracellular Polymeric Substances by Halophilic Bacteria of Solar Salterns. Chin J Biol 2014:1–12. https://doi.org/10.1155/2014/205731
Choi D, Piao YL, Shin W-S, Cho H (2009) Production of Oligosaccharide from Alginate Using Pseudoalteromonas agarovorans Appl Biochem Biotechnol 159:438–452. https://doi.org/10.1007/s12010-008-8514-7
Corinaldesi C, Barone G, Marcellini F et al (2017) Marine Microbial-Derived Molecules and Their Potential Use in Cosmeceutical and Cosmetic Products. Mar Drugs 15:118. https://doi.org/10.3390/md15040118
Decho AW, Gutierrez T (2017) Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol 8:1–28. https://doi.org/10.3389/fmicb.2017.00922
Deng LL, Alexander AA, Lei S, Anderson JS (2010) The cell wall teichuronic acid synthetase (tuas) is an enzyme complex located in the cytoplasmic membrane of Micrococcus luteus. Biochem Res Int 2010:1–8. https://doi.org/10.1155/2010/395758
DuBois M, Gilles KA, Hamilton JK et al (1956) Colorimetric Method for Determination of Sugars and Related Substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
El-Wazzan E, Ghareeb DA, Abdella B (2020) Pre-induction of Hsp70 expression to protect the grooved carpet shell clam, Ruditapes decussatus, against Micrococcus luteus: A trained immunity strategy. Egypt J Aquat Res 46(1):79–84. https://doi.org/10.1016/j.ejar.2019.10.004
Finore I, Di Donato P, Mastascusa V et al (2014) Fermentation Technologies for the Optimization of Marine Microbial Exopolysaccharide Production. Mar Drugs 12:3005–3024. https://doi.org/10.3390/md12053005
Gangadevi V, Muthumary J (2007) Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr J Biotechnol 6:1382–1386. https://doi.org/10.5897/AJB2007.000-2194
Gugliandolo C, Spanò A, Maugeri T et al (2015) Role of Bacterial Exopolysaccharides as Agents in Counteracting Immune Disorders Induced by Herpes Virus. Microorganisms 3:464–483. https://doi.org/10.3390/microorganisms3030464
Hao L, Liu W, Liu K et al (2019) Isolation, Optimization of Fermentation Conditions, and Characterization of an Exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar Drugs 17:703. https://doi.org/10.3390/md17120703
Helm D (1995) Identification of some bacterial cell components by FT-IR spectroscopy. FEMS Microbiol Lett 126:75–79. https://doi.org/10.1016/0378-1097(94)00529-Z
Horikoshi K (1996) Alkaliphiles — from an industrial point of view. FEMS Microbiol Rev 18:259–270. https://doi.org/10.1016/0168-6445(96)00017-4
Joshi AA, Kanekar PP (2011) Production of exopolysaccharide by Vagococcus carniphilus MCM B-1018 isolated from alkaline Lonar Lake, India. Ann Microbiol 61:733–740. https://doi.org/10.1007/s13213-010-0189-y
Ju Y, Shan K, Liu W et al (2022) Effect of Different Initial Fermentation pH on Exopolysaccharides Produced by Pseudoalteromonas agarivorans Hao 2018 and Identification of Key Genes Involved in Exopolysaccharide Synthesis via Transcriptome Analysis. Mar Drugs 20:89. https://doi.org/10.3390/md20020089
Karuppiah P, Venkatasamy V, Viswaprakash N, Ramasamy T (2015) A statistical approach on optimization of exopolymeric substance production by Halomonas sp. S19 and its emulsification activity. Bioresour Bioprocess 2:48. https://doi.org/10.1186/s40643-015-0077-1
Kazak Sarilmiser H, Toksoy Oner E (2014) Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem Eng J 92:28–34. https://doi.org/10.1016/j.bej.2014.06.020
Li W, Xia X, Tang W et al (2015) Structural Characterization and Anticancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus helveticus MB2-1. J Agric Food Chem 63:3454–3463. https://doi.org/10.1021/acs.jafc.5b01086
Liang TW, Wang SL (2015) Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar Drugs 13:1847–1863. https://doi.org/10.3390/md13041847
López-Ortega MA, Chavarría-Hernández N, López-Cuellar M, del R, Rodríguez-Hernández AI (2021) A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int J Biol Macromol 177:559–577. https://doi.org/10.1016/j.ijbiomac.2021.02.101
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/0922-338X(96)89160-4
Mabrouk MEM, Arayes MA, Sabry SA (2014) Hexavalent chromium reduction by chromate-resistant haloalkaliphilic Halomonas sp. M-Cr newly isolated from tannery effluent. Biotechnol Biotechnol Equip 28:659–667. https://doi.org/10.1080/13102818.2014.937092
Mahendran S, Saravanan S, Vijayabaskar P, Anandapandian KTK, Shankar T (2013) Antibacterial potential of microbial exopolysaccharide from Ganoderma lucidum and Lysinibacillus fusiformis Int J Recent Sci Res 4:501–505
Makkar RS, Cameotra SS (1998) Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtilis J Ind Microbiol Biotechnol 20:48–52. https://doi.org/10.1038/sj.jim.2900474
Manivasagan P, Sivasankar P, Venkatesan J et al (2013) Production and characterization of an extracellular polysaccharide from Streptomyces violaceus MM72. Int J Biol Macromol 59:29–38. https://doi.org/10.1016/j.ijbiomac.2013.04.012
Mehta A, Sidhu C, Pinnaka AK, Roy Choudhury A (2014) Extracellular Polysaccharide Production by a Novel Osmotolerant Marine Strain of Alteromonas macleodii and Its Application towards Biomineralization of Silver. PLoS ONE 9:e98798. https://doi.org/10.1371/journal.pone.0098798
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Naghoni A, Emtiazi G, Amoozegar MA et al (2017) Microbial diversity in the hypersaline Lake Meyghan, Iran. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-11585-3
Nanda A, Raghavan CM (2014) Production and characterization of exopolysacharides (EPS) from the bacteria isolated from pharma lab sinks. Int J PharmTech Res 6:1301–1305
Nichols CM, Lardière SG, Bowman JP et al (2005) Chemical Characterization of Exopolysaccharides from Antarctic Marine Bacteria. Microb Ecol 49:578–589. https://doi.org/10.1007/s00248-004-0093-8
Nisha P, Thangavel M (2014) Isolation and characterization of biofilm producing bacteria from Arabian Sea. Res J Recent Sci Res J Recent Sci 3:132–136
Padmanaban S, Balaji N, Muthukumaran C, Tamilarasan K (2015) Statistical optimization of process parameters for exopolysaccharide production by Aureobasidium pullulans using sweet potato based medium. Biotech 5:1067–1073. https://doi.org/10.1007/s13205-015-0308-3
Pal A, Biswas A, Chatterjee S, Paul AK (2015) Optimization of cultural conditions for production of Exopolysaccaride by Halomonas marina HMA 103 under batch-culture. Am J Microbiol 6:31–39. https://doi.org/10.3844/ajmsp.2015.31.39
Pal A, Paul AK (2013) Optimization of Cultural Conditions for Production of Extracellular Polymeric Substances (EPS) by Serpentine Rhizobacterium Cupriavidus pauculus KPS 201. J Polym 2013:1–7. https://doi.org/10.1155/2013/692374
Parada-Pinilla MP, Ferreira MA, Roncallo JC et al (2021) Biopolymer production by halotolerant bacteria isolated from Caatinga biome. Brazilian J Microbiol 52:547–559. https://doi.org/10.1007/s42770-021-00426-1
Patel A, Prajapati j B (2013) Food and health applications of exopolysaccharides produced by lactic acid bacteria. Adv Dairy Res 01. https://doi.org/10.4172/2329-888X.1000107
Patil SP, Shirsath LP (2015) Production of exopolysaccharide by an osmotolerant, thermostable and metal resistant Bacillus subtilis IntJCurrMicrobiolAppSci 4:965–971
Peele A, Krupanidhi S, Reddy ER et al (2018) Plackett-Burman design for screening of process components and their e ff ects on production of lactase by newly isolated Bacillus sp. VUVD101 strain from Dairy effluent. Beni-Suef Univ J Basic Appl Sci 7:543–546. https://doi.org/10.1016/j.bjbas.2018.06.006
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305. https://doi.org/10.2307/2332195
Poli A, Anzelmo G, Nicolaus B (2010) Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar Drugs 8(6):1779–1802
Prakash Shyam K, Rajkumar P, Ramya V, Sivabalan S, Kings AJ, Miriam LRM (2021) Exopolysaccharide production by optimized medium using novel marine Enterobacter cloacae MBB8 isolate and its antioxidant potential. Carbohydr Polym Technol Appl 2:100070. https://doi.org/10.1016/j.carpta.2021.100070
Qiang L, Yumei L, Sheng H et al (2013) Optimization of fermentation conditions and properties of an exopolysaccharide from Klebsiella sp. H-207 and application in adsorption of Hexavalent Chromium. PLoS ONE 8:e53542. https://doi.org/10.1371/journal.pone.0053542
Qurashi AW, Sabri AN (2012) Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. Braz J Microbiol 43:1183–1191. https://doi.org/10.1590/S1517-83822012000300046
Radchenkova N, Vassilev S, Panchev I et al (2013) Production and Properties of Two Novel Exopolysaccharides Synthesized by a Thermophilic Bacterium Aeribacillus pallidus 418. Appl Biochem Biotechnol 171:31–43. https://doi.org/10.1007/s12010-013-0348-2
Rafigh SM, Yazdi AV, Vossoughi M et al (2014) Optimization of culture medium and modeling of curdlan production from Paenibacillus polymyxa by RSM and ANN. Int J Biol Macromol 70:463–473. https://doi.org/10.1016/j.ijbiomac.2014.07.034
Raghunandan K, Kumar A, Kumar S et al (2018) Production of gellan gum, an exopolysaccharide, from biodiesel-derived waste glycerol by Sphingomonas spp. 3 Biotech 8:1–13. https://doi.org/10.1007/s13205-018-1096-3
Rajoka MSR, Mehwish HM, Hayat HF et al (2019) Characterization, the Antioxidant and Antimicrobial Activity of Exopolysaccharide Isolated from Poultry Origin Lactobacilli. Probiotics Antimicrob Proteins 11:1132–1142. https://doi.org/10.1007/s12602-018-9494-8
Rajoka MSR, Wu Y, Mehwish HM et al (2020) Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci Technol 103:36–48. https://doi.org/10.1016/j.tifs.2020.06.003
Rana S, Upadhyay LSB (2020) Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int J Biol Macromol 157:577–583. https://doi.org/10.1016/j.ijbiomac.2020.04.084
Rao BP, Sudharsan K, G RCHS, Mandal AB (2013) Characterization of Exopolysaccharide from Bacillus amyloliquefaciens BPRGS for its Bioflocculant Activity. Int J Sci Eng Res 4:1696–1704
Raza W, Yang W, Jun Y et al (2012) Optimization and characterization of a polysaccharide produced by Pseudomonas fluorescens WR-1 and its antioxidant activity. Carbohydr Polym 90:921–929. https://doi.org/10.1016/j.carbpol.2012.06.021
Sahana TG, Rekha PD (2020) A novel exopolysaccharide from marine bacterium Pantoea sp. YU16-S3 accelerates cutaneous wound healing through Wnt/β-catenin pathway. Carbohydr Polym 238:116191. https://doi.org/10.1016/j.carbpol.2020.116191
Sathiyanarayanan G, Vignesh V, Saibaba G et al (2014) Synthesis of carbohydrate polymer encrusted gold nanoparticles using bacterial exopolysaccharide: a novel and greener approach. RSC Adv 4:22817–22827. https://doi.org/10.1039/C4RA01428F
Silva MBF, Azero EG, Teixeira CMLL, Andrade CT (2020) Influence of culture conditions on the production of extracellular polymeric substances (EPS) by Arthrospira platensis Bioresour Bioprocess 7:47. https://doi.org/10.1186/s40643-020-00337-3
Singh RP, Shukla MK, Mishra A et al (2011) Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis Carbohydr Polym 84:1019–1026. https://doi.org/10.1016/j.carbpol.2010.12.061
Squillaci G, Finamore R, Diana P et al (2016) Production and properties of an exopolysaccharide synthesized by the extreme halophilic archaeon Haloterrigena turkmenica Appl Microbiol Biotechnol 100:613–623. https://doi.org/10.1007/s00253-015-6991-5
Tokura S, Ueno K, Miyazaki S, Nishi N (1997) Molecular weight dependent antimicrobial activity by Chitosan. Macromol Symp 120:1–9. https://doi.org/10.1002/masy.19971200103
Vasanthakumari DS, Harikumar S, Beena DJ et al (2015) Physicochemical Characterization of an Exopolysaccharide Produced by a Newly Isolated Weissella cibaria Appl Biochem Biotechnol 176:440–453. https://doi.org/10.1007/s12010-015-1586-2
Ventosa A (2006) Unusual microorganisms from unusual habitats: hypersaline environments. In: Logan NA, Lappin-Scott HM, Oyston PCF (eds) Prokaryotic diversity. Cambridge University Press, Cambridge, pp 223–254
Verma B, Kumar P, karthik L et al (2015) Gas chromatography - Mass spectrometry analysis and antibacterial activity of bluish-green pigment from Pseudomonas sp. JJTBVK (KF836502). Brazilian Arch Biol Technol 58:628–635. https://doi.org/10.1590/S1516-8913201500108
Vijayabaskar P, Babinastarlin S, Shankar T et al (2011) Quantification and characterization of exopolysaccharides from Bacillus subtilis (MTCC 121). Adv Biol Res (Rennes) 5:71–76
Viju N, Satheesh S, Punitha SMJ (2016) Antibiofilm and antifouling activities of extracellular polymeric substances isolated from the bacteria associated with marine gastropod Turbo sp. Oceanol Hydrobiol Stud 45:11–19. https://doi.org/10.1515/ohs-2016-0002
Wang J, Zhao X, Yang Y et al (2015) Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol 74:119–126. https://doi.org/10.1016/j.ijbiomac.2014.12.006
Xu X-Y, Dong S-H, Li S et al (2015) Statistical experimental design optimization of rhamsan gum production by Sphingomonas sp. CGMCC 6833. J Microbiol 53:272–278. https://doi.org/10.1007/s12275-015-3662-2
Yadav KL, Rahi DK, Soni SK (2014) An Indigenous Hyperproductive Species of Aureobasidium pullulans RYLF-10: Influence of Fermentation Conditions on Exopolysaccharide (EPS) Production. Appl Biochem Biotechnol 172:1898–1908. https://doi.org/10.1007/s12010-013-0630-3
Yildiz H, Karatas N (2018) Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem 72:41–46. https://doi.org/10.1016/j.procbio.2018.06.009
Zhang CL, Cui YN, Wang Y (2013) Bioflocculants produced by gram-positive Bacillus xn12 and Streptomyces xn17 for swine wastewater application. Chem Biochem Eng Q 27:245–250
Zhao L, Chen Z, Wang J et al (2010) Synergistic effect of 5-fluorouracil and the flavanoid oroxylin A on HepG2 human hepatocellular carcinoma and on H22 transplanted mice. Cancer Chemother Pharmacol 65:481–489. https://doi.org/10.1007/s00280-009-1053-2
Acknowledgements
The authors want to express their gratitude to the Faculty of Science, Damanhour University, for allowing authors to undertake this research using faculty resources.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SAS, MM designed the study and acquired funding for the study. MA and BA conducted experiments under the supervision of SS & MM. BA wrote the first draft of the manuscript. BA, MM & SAS finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The corresponding author declares that there is no conflict of interest about any financial/commercial problems on behalf of all authors.
Ethics approval
This article does not contain any studies on humans or animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arayes, M.A., Mabrouk, M.E.M., Sabry, S.A. et al. Exopolysaccharide production from Alkalibacillus sp. w3: statistical optimization and biological activity. Biologia 78, 229–240 (2023). https://doi.org/10.1007/s11756-022-01233-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01233-1