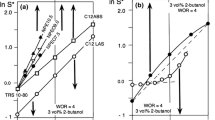
Abstract
In enhanced oil recovery, not only the low-tension performance, but also the robustness at optimum formulation is an important issue. The fourth part of our review series is dedicated to robustness, defined as the width of the zone exhibiting three-phase behavior around the optimum formulation, whatever the scanned variable. It is first corroborated from a screening of the available data in the literature that the tension minimum is inversely proportional to the square of the three-phase range in the HLD scale. However, since there is still an inaccuracy of about a factor 10 in the tension minimum, some significant improvement can be attained in some cases by increasing the three-phase behavior width in two ways. The first approach consists of finding systems that are insensitive to some formulation variable such as temperature, surfactant mixture composition or concentration, and water-to-oil ratio. The second way is to produce an artifact through which the optimum formulation is produced twice in a scan. If the distance between the two events in the scan is reduced down to be zero, their corresponding three-phase behavior zones merge and result in a wider WIII region with a low tension. Several cases of such events are reported: alkaline scans, anionic-nonionic and anionic-cationic mixture changes, linear change in composition in three-surfactant mixture, partial precipitation from a surfactant mixture in a salinity scan, and excessive partitioning of polyethoxylated nonionics. More complex transitions with three effects in a single scan or three concomitantly scanned variables show even more possibilities in practice.






















Similar content being viewed by others
References
Salager JL, Forgiarini AM, Bullón J. How to attain an ultralow interfacial tension and a three-phase behavior with surfactant formulation for enhanced oil recovery—a review. Part 1. Optimum formulation for simple SOW ternary systems. J Surfactants Deterg. 2013;16:449–72.
Bourrel M, Schechter RS. Microemulsions and related systems—formulation, solvency and physical properties. New York: Marcel Dekker; 1988.
Salager JL. Physico-chemical properties of surfactant-ater-oil mixtures: phase behavior, microemulsion formation and interfacial tension. PhD Dissertation, University of Texas at Austin; 1977.
Salager JL, Morgan JC, Schechter RS, Wade WH, Vasquez E. Optimum formulation of surfactant–oil–water systems for minimum tension and phase behavior. Soc Petrol Eng J. 1979;19:107–15.
Bourrel M, Salager JL, Schechter RS, Wade WH. A correlation for phase behavior of nonionic surfactants. J Colloid Interface Sci. 1980;75:451–61.
Wade WH, Morgan J, Schechter RS, Jacobson JK, Salager JL. Interfacial tension and phase behavior of surfactant systems. Soc Petrol Eng J. 1978;18:242–52.
Salager JL. Quantifying the concept of physico-chemical formulation in surfactant–oil–water systems. Progr Colloid Polymer Sci. 1996;100:137–42.
Salager JL, Marquez N, Graciaa A, Lachaise J. Partitioning of ethoxylated octylphenol surfactants in microemulsion–oil–water systems. Influence of temperature and relation between partitioning coefficient and physico-chemical formulation. Langmuir. 2000;16:5534–9.
Antón RE, Garces N, Yajure A. A correlation for three-phase behavior of cationic surfactant–oil–water systems. J Dispers Sci Technol. 1997;18:539–55.
Salager JL, Antón RE, Andérez JM, Aubry JM. Formulation des micro-émulsions par la méthode HLD. Techniques de l’Ingénieur, Vol Génie des Procédés. 2001;J2–157:1–20.
Acosta EJ. The HLD-NAC equation of state for microemulsions formulated with nonionic alcohol ethoxylate and alkylphenol ethoxylate surfactants. Colloids Surf A. 2008;320:193–204.
Cayias JL, Schechter RS, Wade WH. Modeling crude oils for low interfacial tension. Soc Pet Eng J. 1976;16:351–7.
Cash L, Cayias JL, Fournier G, MacAllister D, Schares T, Schechter RS, Wade WH. The application of low interfacial tension scaling rules to binary hydrocarbon mixtures. J Colloid Interface Sci. 1977;59:39–44.
Bourrel M, Verzaro F, Chambu C. Effect of oil type on solubilization by amphiphiles. SPE Reserv Eng. 1987;2:41–53.
Baran JB, Pope GA, Wde WH, Weerasooriya V. Water/chlorocarbon Winsor I ↔ III ↔ II micro-emulsion phase behavior with alkyl glucamide surfactants. Environ Sci Technol. 1996;30:2143–7.
Doe PH, Wade WH, Schechter RS. Alkyl benzene sulfonates for producing low interfacial tensions between hydrocarbons and water. J Colloid Interface Sci. 1977;59:525–31.
Doe PH, El-Emary M, Wade WH. Surfactants for producing low interfacial tension I: linear alkyl benzene sulfonates. J Am Oil Chem Soc. 1977;54:570–7.
Doe PH, El-Emary M, Wade WH, Schechter RS. Surfactants for producing low interfacial tension II: linear alkyl benzene sulfonates with additional alkyl substituents. J Am Oil Chem Soc. 1978;55:505–12.
Doe PH, El-Emary M, Wade WH, Schechter RS. Surfactants for producing low interfacial tension III: Di and Tri n-alkyl benzene sulfonates. J Am Oil Chem Soc. 1978;55:513–20.
Queste S, Salager JL, Strey R, Aubry JM. EACN scale for oil classification revisited thanks to fish diagrams. J Colloid Interface Sci. 2007;312:98–107.
Bouton F, Durand M, Nardello-Rataj V, Borosy AP, Quellet C, Aubry JM. A QSPR model for the prediction of the “fish-tail” temperature of CiE4/water/polar hydrocarbon oil systems. Langmuir. 2010;26:7962–70.
Ontiveros JF, Pierlot C, Catté M, Molinier V, Pizzino A, Salager JL, Aubry JM. Classification of ester oils according to their equivalent alkane carbon number (EACN) and asymmetry of fish diagrams of C10E4/ester oil/water systems. J Colloid Interface Sci. 2013;403:67–76.
Lukowicz T, Benazzouz A, Nardello-Rataj V, Aubry JM. Rationalization and prediction of the equivalent alkane carbon number (EACN) of polar hydrocarbon oils with COMOS-RS σ-moments. Langmuir. 2015;31:11220–6.
Bavière M, Schechter RS, Wade WH. The infuence of alcohols on microemulsion composition. J Colloid Inerface Sci. 1981;81:266–99.
Chiu Y-C. The formulation of high-activity and high-viscosity surfactant slugs containing medium molecular weight alcohols. Paper SPE 11783, international symposium on oilfield and geothermal chemistry, Denver CO, 1–3 June 1983.
Fotland P, Skauge A. Ultralow interfacial tension as a function of pressure. J Dispers Sci Technol. 1986;7:563–79.
Skauge A, Fotland P. Effect of pressure and temperature on the phase behavior of microemulsions. Soc Pet Eng Reserv Eval Eng. 1990;5:601–8.
Ghosh S, Johns RT. A modified HLD-NAC equation of state to predict alkali-surfactant-oil-brine phase behavior. Paper SPE 175132, SPE annual technical conference, Houston TX, 28–30 September 2015.
Ghosh S, Johns RT. An equation of state model to predict surfactant/oil/brine phase behavior. Paper SPE 170927, SPE annual technical conference, Amsterdam, 27–29 October 2014.
Ghosh S, Johns RT. Dimensionless equation of state to predict microemulsion phase behavior. Langmuir. 2016;32:8969–79.
Salager JL, Manchego L, Marquez L, Bullon J, Forgiarini AM. Trends to attain a lower interfacial tension in a revisited pure alkyl polyethyleneglycol surfactant-alkane-water ternary system. Basic concepts and straightforward guidelines for improving performance in enhanced oil recovery formulations. J Surfactants Deterg. 2014;17:199–213.
Sottman T, Strey R. Ultralow interfacial tension in water–n-alkane–surfactant systems. J Chem Phys. 1997;106:8606–15.
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL. Solubilization of polar oils with extended surfactants. Colloids Surf A. 1995;100:217–24.
Miñana-Pérez M, Graciaa A, Lachaise J, Salager JL. Solubilization of polar oils in microemulsion systems. Progr Colloid Polymer Sci. 1995;98:177–9.
Solairaj S, Britton C, Lu J, Kim DH, Weerasooriya U, Pope GA. New correlation to predict optimum surfactant structure for EOR. Paper SPE 154262, 18th improved oil recovery symposium, Tulsa, April 14–18, 2012.
Johansson I. Does hydrophobe branching make a surfactant more or less hydrophilic? Spec Chem Mag. 2004;11:38–40.
Salager JL, Bourrel M, Schechter RS, Wade WH. Mixing Rules for optimum phase behavior formulation of surfactant–oil–water systems. Soc Petrol Eng J. 1979;19:271–8.
Antón RE, Anderez JM, Bracho CL, Vejar F, Salager JL. Practical surfactant mixing rules based on the attainment of microemulsion–oil–water three-phase behavior systems. Adv Polym Sci. 2008;218:83–113.
Huh C. Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J Colloid Interface Sci. 1979;71:408–26.
Huh C. Equilibrium of a microemulsion that coexists with oil and water. Soc Pet Eng J. 1983;23:829–47.
Stegemeier GL. Mechanisms of entrapment and mobilization of oil in porous media. In: Shah DO, Schechter RS, editors. Improved oil recovery by surfactant and polymer flooding. New York: Academic Press; 1977. p. 55–91.
Salager JL, Forgiarini AM, Marquez L, Manchego L, Bullon J. How to attain an ultralow interfacial tension and a three-phase behavior with surfactant formulation for enhanced oil recovery—a review. Part 2. Performance improvement trends from Winsor’s premise to currently proposed inter- and intramolecular mixtures. J Surfactants Deterg. 2013;16:631–63.
Salager JL, Bullón J, Pizzino A, Rondon-Gonzalez M, Tolosa L. Emulsion formulation engineering for the practitioner. In: Somasundaran P, editor. Encyclopedia of surface and colloid science, 1:1, 1-6. London: Taylor and Francis; 2010.
Salager JL, Forgiarini AM, Bullon J. How to attain an ultralow interfacial tension and a three-phase behavior with surfactant formulation for enhanced oil recovery—a review: part 3. Practical procedures to optimize the laboratory research according to the current state of the art in surfactant mixing. J Surfactants Deterg. 2013;20:3–19.
Hirasaki G, Miller C, Puerto M. Recent advances in surfactant EOR. Soc Pet Eng J. 2011;51:889–907.
Lin IJ, Marszall L. Partition coefficient, HLB and effective chain length of surface-active agents. Prog Colloid Polymer Sci. 1978;63:99–104.
Harusawa F, Nakajima H, Tanaka M. The hydrophilic-lipophilic balance of mixed nonionic surfactants. J Soc Cosmet Chem. 1982;33:115–29.
Graciaa A, Lachaise J, Sayous JG, Grenier P, Yiv S, Schechter RS, Wade WH. The partitioning of complex surfactant mixtures between oil/water/microemulsion phases at high surfactant concentrations. J Colloid Interface Sci. 1983;93:474–86.
Graciaa A, Lachaise J, Bourrel M, Osborne-Lee I, Schechter RS, Wade WH. Partitioning of nonionic and anionic surfactant mixtures between oil/microemulsion/water phases. SPE Reserv Eng. 1987;2:305–14.
Graciaa A, Anderez JM, Bracho C, Lachaise J, Salager JL, Tolosa L, Ysabertt F. The selective partitioning of the oligomers of polyethoxylated surfactant mixtures between interface and oil and water bulk phases. Adv Colloid Interface Sci. 2006;123–126:63–73.
Koukounis C, Wade WH, Schechter RS. Phase partitioning of anionic and nonionic surfactant mixtures. Soc Petrol Eng J. 1983;23:301–10.
Zhang DL, Liu S, Puerto M, Miller CA, Hirasaki G. Wettability alteration and spontaneous imbibition in oil-wet carbonate formations. J Petrol Sci Eng. 2006;52:213–26.
Shamijazeyi H, Verduzco R, Hirasaki GJ. Reducing adsorption of anionic surfactant for enhanced oil reovery: part I. Competitive adsorption mechanism. Colloids Surf A. 2014;453:162–7.
Shamijazeyi H, Verduzco R, Hirasaki GJ. Reducing adsorption of anionic surfactant for enhanced oil reovery: part II. Applied aspects. Colloids Surf A. 2014;453:168–75.
Salager JL, Forgiarini AM, Bullón J. Progress in designing emulsion properties over a century. Emerging phenomenological guidelines from generalized formulation and prospects to transmute the knowledge into know-how. In: Romsted LS, editor. Surfactant science and technology: retrospects and prospects. Boca Raton, FL: CRC Press; 2014. Chap 18, pp. 459–487.
Safran SA, Turkevich LA. Phase diagrams for microemulsions. Phys Rev Lett. 1983;50:1930–3.
De Gennes PG, Taupin C. Microemulsions and the flexibility of oil/water interfaces. J Phys Chem. 1982;86:2294–304.
Taupin C, Dvolaitzky M, Ober R. Structure of microemulsions: role of interfacial flexibility. Il Nuevo Cimento. 1984;3:62–74.
Langevin D, Guest D, Meunier J. Correlation between interfacial tension and microemulsion structure in Winsor equilibria. Role of the surfactant film curvature properties. Colloids Surf. 1986;19:159–70.
Lichterfeld F, Schmeling T, Strey R. Mircostructure of microemulsions of the system H2O-n-tetradecane-C12E5. J Phys Chem. 1986;90:5762–6.
Binks BP, Meunier J, Langevin D. Characteristic sizes, film rigidity and interfacial tensions in microemulsion systems. Progr Colloid Polymer Sci. 1989;79:208–13.
Lee LT, Langevin D, Strey R. Relation between microemulsion structure and surfactant layer bending elasticity. Phys A. 1990;168:210–9.
Langevin D. Microemulsions—interfacial aspects. Adv Colloid Interface Sci. 1991;34:583–95.
Strey R. Microemulsion microstructure and interfacial curvature. Colloid Polym Sci. 1994;272:1005–19.
Sottmann T, Stubenrauch C. Phase behavior, interfacial tension and microstructure of micro-emulsions. In: Stubenrauch C, editors. Microemulsions: background, new concepts, applications, perspectives. Oxford: Blackwell Publishing; 2009. Chap 1, pp. 1–47.
Sottman T, Strey R. Shape similarities of ultra-low interfacial tension curves in ternary microemulsion systems of the water-alkane-CiEj type. Ber Bunsenges Phys Chem. 1996;100:237–41.
Leitao H, Somoza AM, Telo da Gama MM, Sottmann T, Strey R. Scaling of the interfacial tension of microemulsions: a phenomenological description. J Chem Phys. 1996;105:2875–83.
Leitao H, Telo da Gama MM, Strey R. Scaling of the interfacial tension of microemulsions: a Landau theory approach. J Chem Phys. 1998;108:4189–98.
Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Naturforsh. 1973;28C:693–703.
Salager JL, Antón RE, Forgiarini AM, Marquez L. Formulation of microemulsions. In: Stubenrauch C, editor. Micro-emulsions: background, new concepts, applications, perspectives. Oxford: Blackwell Publishing; 2009. Chap 3, pp. 84–121.
Reed RL, Healy RN. Some physicochemical aspects of microemulsion flooding: a review. In: Shah DO, Schechter RS, editors. Improved oil recovery by surfactant and polymer flooding. New York: Academic Press; 1977. p. 383–437.
Graciaa A, Fortney LN, Schechter RS, Wade WH. Yiv S. Criteria for structuring surfactants to maximize solubilization of oil and water: part 1: commercial nonionics. Soc Petrol Eng J. 1982;22:743–9.
Bourrel M, Salager JL, Lipow A, Wade WH, Schechter RS. Properties of amphiphile/oil/water systems at an optimum formulation for phase behavior. Paper SPE 7450, 53rd annual fall technical conference SPE, Houston TX. Oct 1–3, 1978.
Barakat Y, Fortney LN, Schechter RS, Wade WH, Yiv SH. Criteria for structuring surfactants to maximize solubilization of oil and water. Part 2. Alkyl Benzene Sodium Sulfonates. J Colloid Interface Sci. 1983;92:561–74.
Barakat Y, Fortney LN, Schechter RS, Wade WH, Yiv SH. Alpha-olefin sulfonates for enhanced oil recovery. In: Proceedings 2nd european symposium on enhanced oil recovery, Technip, Paris; 1982. pp. 11–20.
Barakat Y, Fortney LN, Lalanne-Cassou C, Schechter RS, Wade WH, Yiv SH. The phase behavior of simple salt tolerant sulfonates. Paper 10679, SPE/DOE 3rd joint symposium on enhanced oil recovery,Tulsa, April 4–7, 1982.
Carmona I, Schechter RS, Wade WH, Weerasooriya U. Ethoxylated oleyl sulfonates as model compounds for enhanced oil recovery. Paper SPE 11771, international symposium oilfield and geothermal chemistry, Denver CO, June 1–3, 1983.
Carmona I, Schechter RS, Wade WH, Weerasooriya U, Weerasooriya V. Synthesis and performance of linear monoisimeric ethylene oxide sulfonate surfactants. J. Dispers Sci Technol. 1983;4:361–70.
Hsieh WC, Shah DO. The effect of chain length of oil and alcohol as well as surfactant to alcohol ratio on the solubilization, phase behavior and interfacial tension of oi-brine-surfactant-alcohol systems. Paper SPE 6594. SPE international symposium on oilfields and geothermal chemistry, La Jolla, CA. June 27–28, 1977.
Bansal VK, Shah DO. The effect of divalent cations (Ca++ and Mg++) on the optimum salinity and salt tolerance of petroleum sulfonate and ethoxylated sulfonate mixture in relation to oil recovery. J Am Oil Chem Soc. 1978;55:367–70.
Bansal VK, Shah DO. The effect of ethoxylated sulfonates on salt tolerance and optimum salinity of surfactant formulation for tertiary oil recovery. Soc Petrol Eng J. 1978;18:167–72.
Liu S, Zhang D, Yan W, Puerto M, Hirasaki G, Miller C. Favorable attributes of alkaline-surfactant-polymer flooding. Soc Petrol Eng J. 2008;13:5–16.
Antón RE, Salager JL. Effect of the electrolyte anion on the salinity contribution to optimum formulation of anionic surfactant microemulsions. J Colloid Interface Sci. 1990;140:75–81.
Bansal VK, Shah DO. The effect of addition of ethoxylated sulfonate on salt tolerance, optimal salinity, and impedance characteristics of petroleum sulfonate solutions. J Colloid Interface Sci. 1978;65:451–9.
Miller DJ, von Halasz SP, Schmidff M, Hoist A, Pusch G. Dual surfactant systems for enhanced oil recovery at high salinities. J Petrol Sci Eng. 1991;6:63–72.
Puerto M, Hirasaki G, Miller CA, Barnes J. Surfactant systems for EOR in high-temperature, high-salinity environments. Soc Petrol Eng J. 2012;17:11–9.
Bataweel MA, Yadhali Shivaprasad AK, Nasr-El-Din HA. Low tension polymer flooding using amphoteric surfactant in high salinity/high hardness and high temperature condition in sandstone cores. Paper SPE 155676, SPE EOR conference at oil and gas west Asia, Muscat, Oman. 16–18 April 2012.
Wu Z, Yue X, Cheng T, Yu J, Yang H. Effect of viscosity and interfacial tension of surfactant-polymer flooding on oil recovery in high-temperature and high-salinity reservoirs. J Petrol Explor Prod Technol. 2014;4:9–16.
Lu J, Goudarzi A, Chen P, Kim DH, Delshad M, Mohanty KK, Sepehrnoori K, Weerasooriya U, Pope GA. Enhanced oil recovery from high-temperature, high-salinity naturally fractured carbonate reservoirs by surfactant flood. J Petrol Sci Eng. 2014;124:122–31.
Mardilowich Behr A, Tucker CJ, Daugs ED. Performance of new biodegradable di-sulfonate surfactants as hydrotropes in high-temperature and salinity environments. J Surfactants Deterg. 2015;18:329–38.
Sun Y, Li Y, Li C, Zhang D, Cao X, Song X, Wang Q, Li Y. Molecular array behavior and synergetic effect of sodium alcohol ether sulfate and carboxyl betaine/sulfobetaine in foam under high salt conditions. Colloids Surf A. 2015;480:138–48.
Al-Faraji S, Al-Maamari RS, Aoudia M. Sodium alkyl ether sulfonates (SAES): dual anionic-nonionic behavior in synthetic brine having high salinity and hardness. J Surfactants Deterg. 2015;18:113–21.
Puerto MC, Hirasaki G, Miller CA, Reznik C, Dubey S, Barnes JR, vanKuijk S. Effects of hardness and cosurfactant on phase behavior of alcohol-free alkyl propoxylated sulfate systems. Soc Petrol Eng J. 2015;20:1145–53.
Liu X, Zhai Y, Li Q, Niu J. Surface tension, interfacial tension and emulsification of sodium dodecyl sulfate extended surfactant. Colloid Surf A. 2016;494:201–8.
Budhathoki M, Hsu T-P, Lohateeraparp P, Roberts BL, Shiau B-J, Harwell JH. Design od an optimal middle phase microemulsion for ultra high saline brine using hydrophilic lipophilic deviation (HLD) method. Colloids Surf A. 2016;488:36–45.
Rosen MJ. Surfactants and interfacial phenomena. 3rd ed. Hoboken: Wiley-Interscience; 2004.
Shinoda K, Arai H. The correlation between phase inversion temperature and cloud point in solution of nonionic emulsifier. J Phys Chem. 1964;68:3485–90.
Shinoda K. The comparison between the PIT system and the HLD-value system to emulsifier selection. In: Proceedings of the 5th international congress of surface activity, Barcelona, Spain. 1969. vol. 2, pp 275–283.
Shinoda K, Kunieda H. Conditions to produce so-called microemulsions: factors to increase the mutual solubility of oil and water bu solubilizer. J Colloid Interface Sci. 1973;42:381–7.
Kunieda H, Shinoda K. Solution behavior and hydrophile-lipophile balance temperature in the aerosol OT-isooctane-brine system: correlation between microemulsions and ultra-low interfacial tensions. J Colloid Interface Sci. 1980;75:601–6.
Antón RE, Castillo P, Salager JL. Surfactant–oil–water systems near the affinity inversion. Part IV: emulsion inversion temperature. J Dispers Sci Technol. 1986;7:319–29.
Velasquez J, Scorzza C, Vejar F, Forgiarini A, Antón RE, Salager JL. Effect of the temperature and other variables on the optimum formulation of anionic extended surfactants-alkane-brine systems. J Surfactants Deterg. 2010;13:69–73.
Pes MA, Aramaki K, Nakamura N, Kunieda H. Temperature-insensitive microemulsions in a sucrose monoalkanoate system. J Colloid Interface Sci. 1996;178:666–72.
Garti N, Clement V, Leser M, Aserin A, Fanun M. Sucrose ester microemulsions. J Mol Liq. 1999;80:253–96.
Kabir MH, Aramaki K, Ishitobi M, Kunieda H. Cloud point and formation of microemulsions in sucrose dodecanoate systems. Colloids Surf A. 2003;216:65–74.
Reimer J, Söderman O. Microstructure of alkyl glucoside microemulsions: control of curvature by interfacial composition. Langmuir. 2003;19:10692–702.
Antón RE, Graciaa A, Lachaise J, Salager JL. Surfactant–oil–water systems near the affinity inversion. Part VIII: optimum formulation and phase behavior of mixed anionic-nonionic systems versus temperature. J Dispers Sci Technol. 1992;13:565–79.
Oh K-H, Baran JR, Wade WH. Temperature insensitive microemulsion phase behavior with nonionic surfactants. J Dispers Sci Technol. 1995;16:165–88.
Antón RE, Rivas H, Salager JL. Surfactant–oil–water systems near the affinity inversion. Part X: emulsions made with anionic-nonionic surfactant mixtures. J Dispers Sci Technol. 1996;17:553–66.
Aramaki K, Ozawa K, Kunieda H. Effect of temperature on the phase behavior of ionic-nonionic microemulsions. J Colloid Interface Sci. 1997;196:74–8.
Binks BP, Flether PDI, Taylor DJF. Temperature insensitive microemulsions. Langmuir. 1997;13:7030–8.
Kabir MH, Aramaki K, Ishitobi M, Kinieda H. Cloud and HLB temperatures of mixed-sucrose dodecanoate and poly(oxyethylene) dodecyl ether solutions. Colloids Surf A. 2003;226:87–94.
Stubenrauch C, editor. Microemulsions—background, new concepts, applications, perspectives. Oxford: Blackwell Publishing; 2009.
Antón RE. Contribution à l’étude du comportement de phase des systèmes: Mélanges de surfactifs-eau-huile. PhD Dissertation, University of Pau PA, France 1992.
Antón RE, Mosquera F, Oduber M, Salager JL. Sistemas micelares óptimos insensibles a la temperatura. II Simposio Internacional sobre Recuperación de Petróleo (II SIREMCRU), Instituto Investigaciones Petroleras, University of Zulia, Maracaibo-Venezuela, February 24–27, 1987.
Antón RE, Graciaa A, Lachaise J. Salager JL (1992), Surfactant–oil–water systems near the affinity inversion. Part VIII optimum formulation and phase behavior of mixed anionic-nonionic systems versus temperature. J Disper Sci Technol. 1992;13:565–79.
Acosta EJ, Bhakta AS. The HLD-NAC model for mixtures of ionic and nonionic surfactants. J Surfactants Deterg. 2009;12:7–19.
Chan KS, Shah DO. The molecular mechanism for achieving ultra low interfacial tension minimum in a petroleun sulfonate/oil/brine system. J Dispers Sci Technol. 1980;1:55–95.
Hirasaki G. Interpretation of the change in optimal salinity with overall surfactant concentration. Soc Pet Eng J. 1982;22:971–82.
Rosen M, Li Z, Zhao F. The relationship between the molar partition coefficient and the ultralow interfacial tension minimum in a petroleum sulfonate/hydrocarbon/brine system. J Dispers Sci Technol. 1983;4:335–45.
Graciaa A, Lachaise J, Morel G, Salager JL, Bourrel M. Optimal phase behavior of water/oil blend/surfactant systems. Progr Colloid Polymer Sci. 1993;93:257–60.
Márquez N, Antón RE, Usubillaga A, Salager JL. Experimental conditions for HPLC analysis of ethoxylated alkyl phenol surfactants in microemulsions systems. Part I. Isocratic mode with mixed solvents. Separation. Sci Technol. 1993;28:1769–82.
Márquez N, Antón RE, Usubillaga A, Salager JL. Experimental conditions for HPLC analysis of ethoxylated alkyl phenol surfactants in microemulsions systems. Part II. Gradient mode for extended EON range as found in the analysis of oligomer fractionation. Separation. Sci Technol. 1993;28:2387–400.
Márquez N, Antón RE, Uusubillaga A, Salager JL. Optimization of HPLC conditions to analyze widely distributed ethoxylated alkylphenol surfactants. J Liq Chromatogr. 1994;17:1147–69.
Márquez N, Antón RE, Graciaa A, Lachaise J, Salager JL. Partitioning of ethoxylated alkylphenol surfactants in microemulsion–oil–water systems. Colloids Surf A. 1995;100:225–31.
Márquez N, Antón RE, Graciaa A, Lachaise J, Salager JL. Partitioning of ethoxylated alkylphenol surfactants in microemulsion–oil–water systems. Part II: influence of hydrophobe branching. Colloids Surf A. 1998;131:45–9.
Tanthakit P, Chavadej S, Scamehorn JF, Sabatini DA, Tongcumpou C. Microemulsion formation and detergency with oily soil: IV. Effect of rinse cycle design. J Surfactants Deterg. 2008;11:117–28.
Tanthakit P, Makrachata-Amorn A, Scamehorn JF, Sabatini DA, Tongcumpou C, Chavadej S. Microemulsion formation and detergency with oily soil: V. Effects of water hardness. J Surfactants Deterg. 2009;12:173–83.
Puig JE, Mares MT, Miller WG, Frances EI. Mechanism of ultralow interfacial tensions in dilute surfactant-oil-brine systems. Colloids Surf. 1985;16:139–52.
Zhao Z, Liu F, Qiao W, Li Z, Cheng L. Novel alkyl methylnaphtalene sulfonate surfactants: a good candidate for enhanced oil recovery. Fuel. 2006;85:1815–20.
Arandia MA. Microemulsiones de formulación robusta a cambios de dilución y/o composición. PhD Dissertation, University of The Andes, Mérida, Venezuela 2011.
Kunieda H, Shinoda K. Evaluation of the hydrophile-lipophile balance (HLB) of nonionic surfactants. I. Multisurfactant systems. J Colloid Interface Sci. 1985;107:107–21.
Yamaguchi Y, Aoki R, Azemar N, Solans C, Kunieda H. Phase behavior of cationic microemulsions near the tricritical point. Langmuir. 1999;15:7438–45.
Sottman T, Lade M, Stolz M, Schomächer R. Phase behavior of nonionic microemulsions prepared from technical-grade surfactants. Tenside Surf Deterg. 2002;39:20–8.
Kunieda H, Yamagata M. Three-phase behavior in a mixed nonionic surfactant system. Colloid Polymer Sci. 1993;271:997–1004.
Kunieda H, Nakano A, Akimaru M. The effect of mixing of surfactants on solubilization in a microemulsion system. J Colloid Interface Sci. 1995;170:78–84.
Miñana-Perez M, Gutron C, Zundel C, Andérez JM, Salager JL. Miniemulsion formation by transitional inversion. J Dispers Sci Technol. 1999;20:893–905.
Wade WH, Vasquez E, Salager JL, El-Emary M, Koukounis C, Schechter RS. Interfacial tension and phase behavior of pure surfactant systems. In: Mittal K, editor. Solution chemistry of surfactants. vol. 2. New York: Plenum Press; 1979. pp. 801–17.
Kahlweit M, Strey R, Busse G. Weakly to strongly structures mixtures. Phys Rev E. 1993;47:4197–209.
Lade O, Beizai K, Sottman T, Strey R. Polymerizable nonionic microemulsions: Phase behavior of H2O-n-alkyl methacrylate-n-alkyl poly(ethylene glycol) ether (CiEj). Langmuir. 2000;16:4122–30.
Kahlweit M, Strey R, Firman P. Search for tricritical points in ternary systems: water–oil-nonionic amphiphile. J Phys Chem. 1986;90:671–7.
Arandia MA, Forgiarini AM, Salager JL. Resolving an enhanced oil recovery challenge: optimum formulation of a surfactant–oil–water system made insensitive to dilution. J Surfactants Deterg. 2010;13:119–26.
Tate JF, Shupe RD, Maddox J. Composition and method for treating scale. US patent 3,975,282. August 17, 1976.
Gale WW, Saunders RK, Ashcraft TL. Oil recovery method using a surfactant, US patent 3,977,471. August 30, 1976.
Tyler TN, Mills ME, Wells JA, Carlin JT. Salinity tolerant surfactant oil reovery process. Patent 4,110,228. August 29, 1978.
Shupe RD. Surfactif oil recovery process usable in high temperature formations containing high salinity water. US patent 4,088,189. May 9, 1978.
Gale WW, Puerto MC, Ashcraft TL, Saunders RK, Reed RL. Propoxylated ehoxylated surfactants and methods of recovering oil therewith. US patent 4,293,428. October 6, 1981.
Hirasaki G, Miller CA, Puerto MA. Recent advances in surfactant EOR. Paper SPE 115386. International Petroleum Technology Conference, Kuala Lumpur Malaysia, 3–5 December 2008.
Abe M, Schechter D, Schechter RS, Wade WH, Weerasooriya U, Yiv S. Microemulsion formation with branched tail polyoxyethylene sulfonate surfactants. J Colloid Interface Sci. 1986;114:342–56.
Hornof V, Hombek R. Surface-active agents based on propoxylated lignosulfonate. J Appl Polymer Sci. 1990;41:2391–8.
Aoudia M, Wade Wh, Weerasooriya V. Optimum microemulsions formulated with propoxylated Guerbet alcohol and propoxylated tridecyl alcohol sodium sulfate. J Dispers Sci Technol. 1995;16:115–35.
Baran JR, Pope GA, Wade Wh, Weerasooriya V. Phase behavior of water/perchloroethylene/anionic surfactant systems. Langmuir. 1994;10:1146–50.
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL. Improving solubilization in microemulsions with additives—1: the lipophilic linker role. Langmuir. 1993;9:669–72.
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL. Improving solubilization in microemulsions with additives—2: long chain alcohol as lipophilic linkers. Langmuir. 1993;9:3371–4.
Salager JL, Graciaa A, Lachaise J. Improving solubilization in microemulsions with additives—3: optimization of the lipophilic linker. J Surfactants Deterg. 1998;1:403–6.
Miñana M, Graciaa A, Lachaise J, Salager JL. Systems containing mixtures of extended surfactants and conventional nonionics. Phase behavior and solubilization in microemulsion. In: Roger de Llúria, editors. (Edited for A.E.P.S.A.T) Proceedings of 4th World surfactants congress; 1996, vol. 2, pp. 226–34, Barcelona, Spain, June 3–7.
Scorzza C, Godé P, Goethals G, Martin P, Miñana M, Salager JL, Usubillaga A, Villa P. Another new family of “extended” glucidoamphiphiles. Synthesis and surfactant properties for different sugar head groups and spacer arm lengths. J Surfactants Deterg. 2002;5:337–43.
Shusharina NP, Alexandridis P, Linse P, Balijepalli S, Gruenbuer HJM. Phase behavior and structure of an ABC triblock copolymer dissolved in selective solvent. Eur Phys J E. 2003;10:45–54.
Shusharina NP, Balijepalli S, Gruenbauer HJM, Alexandridis P. Mean field theory prediction of the phase behavior and phase structure of alkyl-propoxy-ethoxylate “graded” surfactants in water: temperature and electrolyte effects. Langmuir. 2003;19:4483–92.
Smith G, Nguyen D. Formulating cleaning products with microemulsions. Paper # 164, CD Proceedings 6th world surfactant congress CESIO, Berlin-Germany, June 21–23, 2004.
Huang L, Lips A, Co CC. Microemulsification of triglyceride sebum and the role of interfacial structure on bicontinuous phase behavior. Langmuir. 2004;20:3559–63.
Fernandez A, Scorzza C, Usubillaga A, Salager JL. Synthesis of new extended surfactants containing carboxylate or sulfate group. J Surfactants Deterg. 2005;8:187–91.
Fernandez A, Scorzza C, Usubillaga A, Salager JL. Synthesis of new extended surfactants derived from a xylitol polar group. J Surfactants Deterg. 2005;8:193–8.
Salager JL, Antón RE, Sabatini DA, Harwell JH, Acosta EJ, Tolosa L. Enhancing solubilization in microemulsion—state of the art and current trends. J Surfactants Deterg. 2005;8:3–21.
Childs JD, Acosta E, Scamehorn JF, Sabatini DA. Surfactant-enhanced treatment of oil-based drill cuttings. J Energy Resour Technol. 2005;127:153–62.
Aoudia M, Al-Shibli MN, Al-Kasimi L, Al-Maamari R, Al-Bemani A. Novel surfactants for ultralow interfacial tension in a wide range of surfactant concentration and temperature. J Surfactants Deterg. 2006;9:287–93.
Witthayapanyanon A, Acosta EJ, Harwell JH, Sabatini DA. Formulation of ultralow interfacial tension systems using extended surfactants. J Surfactants Deterg. 2006;9:331–9.
Lamalle P, Fungerlings T, Scholtissek M, Tropsch J, Ruland A, Bohn R, Oetter G, Hackmann C, Pabst G. Leather degreasing agent. US Patent 2006/0143833 A1. July 6, 2006.
Smith GA, Hand KR. Enhanced solubilization using extended chain surfactants. US patent 2006/0211593 A1. September 21, 2006.
Klaus A, Tiddy GJT, Touraud D, Schramm A, Stühler G, Drechsler M, Kunz W. Phase behavior of an extended surfactant in water and a detailed characterization of the dilute and semidilute phases. Langmuir. 2010;26:5435–43.
Watcharasing S, Kongkowit W, Chavadej S. Motor oil removal from water by continuous froth flotation using extended surfactant. Effects of air bubble parameters and surfactant concentration. Sep Purif Technol. 2009;70:179–89.
Do LD, Sabatini DA. Aqueous extended-surfactant based method for vegetable oil extraction: proof of concept. J Am Oil Chem Soc. 2010;87:1211–20.
Sarkar B, Alexandridis P. Alkyl propoxy ethoxylate “graded” surfactants. Micelle formation and structure in aqueous solutions. J Phys Chem B. 2010;114:4485–94.
Forgiarini AM, Scorzza C, Velasquez J, Zambrano E, Salager JL. Influence of the mixed propoxy/ethoxy spacer arrangement order and of the ionic head group nature on the adsorption and aggregation of extended surfactants. J Surfactants Deterg. 2010;13:451–8.
Phan TT, DA Harwell Sabatini. Effects of triglyceride molecular structure on optimum formulation of surfactant–oil–water systems. J Surfactants Deterg. 2010;13:189–94.
Sabatini DA, Harwell J, Do L, Witthayapanyanon A, Nguyen T, Acosta E, Roberts B. Aqueous-based surfactant solution and method of making and using the same. WO patent 2010/114559A1. October 10, 2010.
Yang H, Liyanage PJ, Solairaj S, Kim DH, Nguyen Q, Weerasooriya U, Pope GA. Low-cost, high-performance chemicals for enhanced oil recovery. Paper SPE 129978, SPE improved oil recovery symposium, Tulsa OK, 24–28 April 2010.
Hammond C, Acosta EJ. On the characteristic curvature of alkyl-polypropylene oxide sulfate extended surfactants. J Surfactants Deterg. 2012;15:157–65.
Man VF-P, Denoma MC, Mloney Viall S, Lentsch SE, Killeen YM. Cleaning composition employing extended chain anionic surfactants. US patent 2013/0281352 A1. October 24, 2013.
Lewitt DB, Jackson AC, Heinson C, Britton LN, Malik T, Dwarakanath V, Pope GA. Identification and evaluation of high-performance EOR surfactants. Paper SPE 100089 SPE-DOE symposium on improved oil recovery, Tulsa OK 22–26 April, 2006.
Barnes JR, Smit JP, Smit JR, Shpakoff PG, Raney KH, Puerto MC. Development of surfactants for chemical flooding at difficult reservoir conditions. Paper SPE 113313, SPE/DOE improved oil recovery symposium, Tulsa OK, 19–23 April 2008.
Adkins A, Liyanage PJ, Pinnawala GWP, Mudiyanselage T, Weerasooriya U, Pope GA. A new process for manufacturing and stabilizing high-performance EOR surfactants at low cost for high temperature, high-salinity oil reservoirs. Paper SPE 129923, SPE improved oil recovery symposium, Tulsa OK, 24–28 April, 2010.
Sahni V, Dean RM, Britton C, Kim DH, Weerasooriya U, Pope GA. The role of co-solvents and co-surfactants in making chemical floods robust. Paper 130007, SPE improved oil recovery symposium, Tulsa OK, 24–28 April, 2010.
Hirasaki G, Miller CA, Puerto MA. Recent advances in surfactant EOR. Soc Pet Eng J. 2011;16:889–907.
Shinoda K, Arai HH. The effect of phase volume on the phase inversion temperature of emulsions stabilized with nonionic surfactants. J Colloid Interface Sci. 1967;25:429–31.
Shinoda K, Saito H. The effect of temperature on the phase equilibria and the types of dispersions of the ternary system composed of water, cyclohexane, and nonionic surfactant. J Colloid Interface Sci. 1968;26:70–4.
Kahlweit M, Faulhaber B, Busse G. Microemulsions with mixtures of nonionic and ionic amphiphiles. Langmuir. 1994;10:2528–32.
Salager JL, Miñana M, Pérez M, Ramirez M, Rojas CI. Surfactant–oil–water systems near the affinity inversion part III: the two kinds of emulsion inversion. J Dispers Sci Tehnol. 1983;4:313–29.
Shinoda K. Solution behavior of surfactants: the importance of surfactant phase and the continuous change in HLB of surfactant. Progr Colloid Polymer Sci. 1963;68:1–7.
Miñana M, Jarry P, Pérez M, Ramirez M, Salager JL. Surfactant–oil–water systems near the affinty inversion. Part V: properties of emulsions. J Dispers Sci Tehnol. 1986;7:331–43.
Kunieda H, Ishikawa N. Evaluation of the hydrophile-lipophile balance (HLB) of nonionic surfactants. II. Commercial surfactant systems. J Colloid Interface Sci. 1985;107:122–8.
Kunieda H, Hanno K, Yamaguchi S, Shinoda K. The three-phase behavior of a brine/ionic surfactant/nonionic surfactant/oil system: evaluation of the hydrophile-lipophile balance (HLB) of ionic surfactants. J Colloid Interface Sci. 1985;107:129–37.
Miñana M, Jarry P, Salager JL. Sistemas surfactante-agua-aceite: comportamiento de fase al equilibrio y propiedades de las emulsiones. Ciencia e Ingenieria. 1985;17:121–36.
Salager JL, Lopez G, Miñana M. Surfactant–oil–water systems near the affinity inversion. Part VI: emulsions with viscous hydrocarbons. J Dispers Sci Technol. 1990;11:397–407.
Fukuda K, Söderman O, Lindman B, Shinoda K. Microemulsions formed by alkyl polyglucosides and an alkyl glycerol ether. Langmuir. 1993;9:2921–5.
Mendez Z, Antón RE, Salager JL. Surfactant–oil–water systems near the affinity inversion. Part XI. pH sensitive emulsions containing carboxylic acids. J Dispers Sci Technol. 1999;20:883–92.
Wormuth KR, Kaler EW. Microemulsifying polar oils. J Phys Chem. 1989;93:4855–61.
Pizzino A, Molinier V, Catté M, Ontiveros JF, Salager JL, Aubry JM. Relationship between phase behavior and emulsion inversion for a well-defined surfactant (C10E4)/n-octane-water ternary system at different temperatures and water/oil ratios. Ind Eng Chem Res. 2013;52:4527–38.
Burauer S, Sachert T, Sottmann T, Strey R. On microemulsion phase behavior and the monomeric solubility of surfactant. Phys Chem Chem Phys. 1999;1:4299–306.
Pizzino A, Molinier V, Catté M, Salager JL, Aubry JM. Bidimensional analysis of the phase behavior of a well-defined surfactant (C10E4)/oil (n-octane)/water—temperature system. J Phys Chem B. 2009;113:16142–50.
Márquez N, Subero N, Antón RE, Lachaise J, Graciaa A, Salager JL. Effect of the alkylate isomerism upon the surfactant separation in HPLC column and partitioning between water and oil. Sep Sci Technol. 1997;32:1087–98.
Márquez N, Graciaa A, Lachaise J, Salager JL. Partitioning of ethoxylated alkylphenol surfactants in microemulsion–oil–water systems: influence of physicochemical formulation variables. Langmuir. 2002;18:6021–4.
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL. Interfacial segregation of an ethyl oleate/hexadecane oil mixture in microemulsion systems. Langmuir. 1993;9:1473–8.
Salager JL, Márquez N, Anteon RE, Graciaa A, Lachaise J. Retrograde transition in the phase behavior of surfactant–oil–water systems produced by an alcohol scan. Langmuir. 1995;11:37–41.
Ysambertt F, Antón RE, Salager JL. Retrograde transition in the phase behavior of surfactant–oil–water systems produced by an oil equivalent alkane carbon number scan. Colloids Surf A. 1997;125:131–6.
Johnson CE. Status of caustic and emulsion methods. J Petrol Technol. 1970;22:85–92.
Cooke CE, Williams RE, Kolodzie PA. Oil recovery by alkaline waterflooding. J Petrol Technol. 1974;26:1365–74.
Jenninghs HY, Johnson CE, McAuliffe CD. A caustic waterflooding process for heavy oils. J Petrol Technol. 1974;26:1344–52.
Mayer EH, Weinbrandt RM, Irani MR, Krumrine PH. Alkaline waterflooding. Its theory, applications and status. In: Proceedings 2nd European symposium on enhanced oil recovery. Technip. Paris 8–10 November 1982.
Shaw JE. Enhanced oil recovery using carboxylate surfactant systems. J Am Oil Chem Soc. 1984;61:1389–94.
Shaw JE. Carboxylate surfactant systems exhibiting phase behavior suitable for enhanced oil recovery. J Am Oil Chem Soc. 1984;61:1395–9.
Rudin J, Wasan DT. Mechanisms for lowering of interfacial tension in alkali/acidic oil systems. 1. Experimental studies. Colloids Surf. 1992;68:67–79.
Rudin J, Wasan DT. Mechanisms for lowering of interfacial tension in alkali/acidic oil systems. 2. Theoretical studies. Colloids Surf. 1992;68:81–94.
Rivas H, Gutierrez X, Zirrit JL, Antón RE, Salager JL. Microemulsion and optimal formulation occurrence in pH-dependent systems as found in alkaline-enhanced oil recovery. In: Solans C, Kunieda H, editors. Industrial applications of microemulsions. New York: Marcel Dekker; 1997. pp. 305–29.
Sheng JJ. Alkaline flooding. In: Sheng JJ, editor. Enhanced oil recovery field case studies. Waltman, MA: Gulf Professional Publishing; 2013. Chap 6, pp. 143–167.
Cratin PD. Mathematical modeling of some pH-dependent surface and interfacial properties of stearic acid. J Dispers Sci Technol. 1993;14:559–602.
Yuan F-Q, Cheng Y-Q, Wang H-Y, Xu Z-C, Lei Zhang, Zhang Lu, Zhao S. Effect of organic alkali on interfacial tension of surfactant solutions against crude oils. Colloids Surf A. 2015;470:171–8.
Sharma H, Dufour S, Pinnawala GWP, Weerasooriya U, Pope GA, Mohanty K. Alternative alkali for ASP flooding in anhydrite containing oil reservoir. Fuel. 2015;140:407–30.
Chen F, Jiang H, Bai X, Zheng W. Evaluation of the performance of sodium metaborate as a novel alkali in alkali/surfactant/polymer flooding. J Ind Eng Chem. 2013;19:450–7.
Chen Z, Zhao X, Wang Z, Fu M. A comparative study of inorganic alkaline/polymer flooding and organic alkaline/polymer flooding for enhanced oil recovery. Colloids Surf A. 2015;469:150–7.
Salager JL, Antón RE. Ionic microemulsions. In: Kumar P, Mittal KL, editors. Handbook of microemulsion science and technology. New York: Marcel Dekker;1999. Chap 8, pp. 247–80.
Qutubuddin S, Miller CA, Fort T. Phase behavior of pH-dependent microemulsions. J Colloid Interface Sci. 1984;101:46–58.
Antón RE, Graciaa A, Lachaise J, Salager JL. Phase behavior of pH-dependent systems containing oil–water and fatty acid, fatty amine or both. In: Roger de Llúria, editor. (Edited for AEPSAT) Proceedings of 4th world surfactants congress; 1996, vol. 2, pp. 244–56, Barcelona, Spain, June 3–7.
Li X, Kunieda H. Catanionic surfactants: microemulsions formation and solubilization. Curr Opin Colloids Interface Sci. 2003;8:327–36.
Mehreteab A, Loprest F. Formation of pseudo-nonionic complexes of anionic and cationic surfactants. J Colloid Interface Sci. 1988;125:602–9.
Bourrel M, Bernard D, Graciaa A. Properties of binary mixtures of anionic and cationic surfactants: micellization and microemulsions. Tenside Deterg. 1984;21:311–8.
Stellner KL, Amante JC, Scamehorn JF, Harwell JH. Precipitation phenomena in mixtures of anionic and cationic surfactants in aqueous solutions. J Colloid Interface Sci. 1988;123:186–200.
Amante JC, Scamehorn JF, Harwell JH. Precipitation of mixtures of anionic and cationic surfactants. II. Effect of surfactant structure, temperature and pH. J Colloid Interface Sci. 1991;144:243–53.
Shiau B-J, Harwell JH, Scamehorn JF. Precipitation of mixtures of anionic and cationic surfactants. J Colloid Interface Sci. 1994;167:332–45.
Mehreteab A. Anionic-cationic surfactant mixtures. In: Broze G, editor. Hanbook of detergents. Part A: properties. New York: Marcel Dekker; 1999. Chap 5, pp 133–55.
Antón RE, Gómez D, Graciaa A, Lachaise J, Salager JL. Surfactant–oil–water systems near the affinity inversion. Part IX: optimum formulation and phase behavior of mixed anionic-cationic systems. J Dispers Sci Technol. 1993;14:401–16.
Doan T, Acosta E, Scamehorn JF, Sabatini DA. Formulating middle-phase microemulsions using mixed anionic and cationic surfactant systems. J Surfactants Deterg. 2003;6:215–24.
Upadhyaya A, Acosta E, Scamehorn JF, Sabatini DA. Microemulsion phase behavior of anionic-cationic surfactant mixtures: effect of tail branching. J Surfactants Deterg. 2006;9:169–79.
Ruan K, Zhang L, Tang J, Xiao J. Interfacial tension of the aqueous two-phase systems of cationic-anionic surfactant mixtures. Acta Phys Chim Sin. 2006;22:1451–5.
Kume G, Gallotti M, Nunes G. Review on anionic/cationic surfactant mixtures. J Surfactants Deterg. 2008;11:1–11.
Kayali IH, Liu S, Miller CA. Microemulsions containing mixtures of propoxylated sulfates with slightly branched hydrocarbon chains and catanionic surfactants with short hydrophobes or PO chains. Colloids Surf A. 2010;354:246–51.
Kayali IH, Qamhieh K, Olsson U. Microemulsion phase behavior of aerosol OT combined with a cationic hydrotrope in the dilute region. J Dispers Sci Technol. 2010;31:183–7.
Li Y, Zhang W, Kong B, Puerto M, Bao X, Sha O, Shenz Yang Y, Liu Y, Gu S, Miller CA, Hirasaki G. Mixtures of anionic/cationic surfactants: a new approach for enhanced oil recovery in low-salinity, high-temperature sandstone reservoir. Soc Petrol Eng J. 2016;21:1164–77.
Li Y, Puerto M, Bao X, Zhang W, Jin J, Su Z, Shen S, Hirasaki G, Miller CA. Synergism and performance for systems containing binary mixtures of anionic/cationic surfactants for enhanced oil recovery. J Surfactants Deterg. 2017;20:21–34.
Ysambertt F. Contribution à l’étude des mélanges de surfactifs dans des systèmes microémulsion-eau-huile présentant un partage préférentiel des différentes espèces. PhD Dissertation, University of Pau PA France 1997.
Hansen CM. Hansen solubility parameters—a user’s handbook. Boca Raton: CRC Press; 2000.
Hansen CM. 50 years with solubility parameters—past and future. Progr Org Coat. 2004;51:77–84.
Winsor PA. Solvent properties of amphiphilic compounds. London: Butterworth; 1954.
Winsor PA. Binary and multicomponent solutions of amphiphilic compounds. Solubilization and the formation, structure, and theoretical significance of liquid crystalline solutions. Chem Rev. 1968;68:1–40.
Knikerbocker BM, Pesheck CV, Davis HT, Scriven LE. Patterns of three-liquid-phase behavior illustrated by alcohol-hydrocarbon-water-salt mixtures. J Phys Chem. 1982;86:393–400.
Kahlweit M. Microemulsions. Annual Rep Prog Chem Sec C. 1999;95:89–115.
Bellocq AM, Bourbon D, Lemanceau B. Thermodynamic, interfacial and structural properties of alcohol-brine-hydrocabon systems. J Dispers Sci Technol. 1981;2:27–52.
Kahlweit M, Lessner E, Strey R. Influence of the properties of the oil and the surfactant on the phase behavior of systems of the type H2O-oil-nonionic surfactant. J Phys Chem. 1983;87:5032–40.
Bellocq AM, Biais J, Bothorel P, Clin B, Fourche G, Lalanne P, Lemaire B, Lemanceau B, Roux D. Microemulsions. Adv Colloid Interface Sci. 1984;20:167–272.
Kahlweit M, Strey R, Haase D, Kunieda H, Schmeling T, Faulhaber B, Borkovec M, Eicke HF, Busse G, Eggers F, Funck Richmann H, Magid L, Soderman O, Stilbs P, Winkler J, Dittrich A, Jahn W. How to study microemulsions. J Colloid Interface Sci. 1987;118:436–53.
Lang JC, Widom B. Equilibrium of the three liquid phases and approach to the tricritical point in benzene-ethanol–water-ammonium sulfate mixtures. Physica. 1975;81A:190–213.
Antón RE. Comportamiento de fase de un sistema surfactante-alcohol-salmuera-hidrocarburo. Representaciones pseudoternaria y cuaternaria. Ciencia e Ingeniería. 1985;17:79–94.
Kilpatrick PK, Scriven LE, Davis HT. Thermodynamic modeling of quaternary systems: oil/brine/surfactant/alcohol. Soc Petrol Eng J. 1985;25:330–42.
Bellocq AM, Gazeau D. Phase behavior of the quinary mixture: H2O-NaCl-dodecane-pentanol-SDS Origin of the Winsor III equilibria. Progr Colloid Polymer Sci. 1988;76:203–10.
Yamaguchi S. Three-phase behavior in a water/C12EO8/propanol/cyclohexane/heptane system. J Colloid Interface Sci. 1999;218:282–8.
Acosta E, Szekeres E, Sabatini DA, Harwell JH. Net-average curvature model for solubilization and supersolubilization in surfactant microemulsions. Langmuir. 2003;19:186–95.
Ghosh S, Johns RT. An equation-of-state model to predict surfactant/oil/brine-phase behavior. Soc Petrol Eng J. 2016;21:106–25.
Khorsandi S, Johns RT. Robust flash calculation algorithm for microemulsion phase behavior. J Surfactants Deterg. 2016;19:1273–87.
Torrealba VA, Johns RT. Microemulsion phase behavior using empirical trends in chemical potentials. In: SPE-184555-MS SPE international conference oilfield chemistry, Montgomery TX 3–5 April, 2017.
Kalhweit M, Strey R. Phase behavior of quinary systems: tracing the three-phase body. J Phys Chem. 1987;91:1553–7.
Kalhweit M, Strey R. Phase behavior of quinary mixtures of the type H2O-oil-nonionic amphiphile-ionic amphiphile-salt. J Phys Chem. 1988;92:1557–63.
Hofsäss T, Kleinert H. Towards custom-made microemulsions. Chem Phys Lett. 1988;145:407–10.
Acknowledgements
The authors thank CEPSA for providing various linear alkyl benzene sulfonate mixture samples indicated as xϕCNLAS and SASOL for providing a specially prepared extended surfactant of the branched-dodecyl polypropoxylated (20PO) sulfate type indicated as Guerbert C12 PO20 S. The authors thanks researchers Aram Quijada and Mairis Guevara for their measurements of interfacial tension in special surfactant mixture systems.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Salager, JL., Antón, R.E., Arandia, M.A. et al. How to Attain Ultralow Interfacial Tension and Three-Phase Behavior with Surfactant Formulation for Enhanced Oil Recovery: A Review. Part 4: Robustness of the Optimum Formulation Zone Through the Insensibility to Some Variables and the Occurrence of Complex Artifacts. J Surfact Deterg 20, 987–1018 (2017). https://doi.org/10.1007/s11743-017-2000-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-2000-6