Abstract
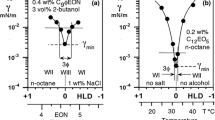
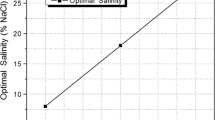
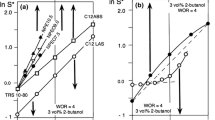
Surfactant–oil–water systems exhibit a low interfacial tension minimum when the interactions between the adsorbed surfactant with water exactly coincide with its interactions with oil. This occurrence takes place at the so-called optimum formulation, which was conceptually derived by Winsor in the 1950s and rendered by numerical correlations for enhanced oil recovery in the 1970s. The actual low value of the interfacial tension minimum has been found to increase or decrease with formulation variables and though some hints are available, no general relationship has been reported up to now, probably because too many variables are involved in complex interactions. It is shown in the present article that a linear relationship between low-tension performance and formulation variables can be found for very simple ternary systems containing a pure ethoxylated alcohol, n-alkane and water at variable temperature, i.e., when there are only four degrees of freedom. In such a case the iso-performance contours studied in bi-dimensional spaces are reported to be almost straight lines and as a consequence the path to lower the tension through formulation adjustments is easy to find as being perpendicular as possible to the contours. On the other hand, it is shown that displacing the limit of restrictions like the surfactant precipitation boundary is a priority issue, thus justifying many trends which have been proposed on empirical grounds in the past years. The reported simple guidelines for a simple surfactant–oil–water ternary is likely to considerably facilitate the formulator's work in a real system with a score of formulation variables.









Similar content being viewed by others
References
Stegemeier GL (1977) Mechanisms of entrapment and mobilization of oil in porous media. In: Shah DO, Schechter RS (eds) Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, pp 55–91
Reed RL, Healy RN (1977) Some physicochemical aspects of microemulsion flooding: a review. In: Shah DO, Schechter RS, (eds) Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, pp 383–347
Morgan JC, Schechter RS, Wade WH. Recent advances in the study of low interfacial tension. In: Shah DO, Schechter RS (eds) Improved oil recovery by surfactant and polymer flooding. Academic Press, New York, pp 101–118
Shinoda K, Hanrin M, Kunieda H, Saito H (1981) Principles of attaining ultra-low interfacial tension: the role of hydrophile-lipophile balance of surfactant at oil/water interface. Colloid Surf 2:301–314
Winsor P (1954) Solvent properties of amphiphilic compounds. Butterworth, London
Bourrel M, Schechter RS (1988) Microemulsions and Related Systems. Marcel Dekker, New York
Salager JL, Bullón J, Pizzino A, Rondón-González M, Tolosa L (2010) Emulsion formulation engineering for the practitioner. In: Somasundaran P (ed) Encyclopedia of surface and colloid science, vol 1. Taylor & Francis, London, pp 1–6
Bourrel M, Salager JL, Schechter RS, Wade WH (1980) A correlation for phase behavior of nonionic surfactants. J Colloid Interf Sci 75:451–461
Salager JL, Vasquez E, Morgan J, Schechter RS, Wade WH (1979) Optimum formulation of surfactant–water–oil systems for minimum interfacial tension and phase behavior. Soc Petrol Eng J 19:107–115
Antón RE, Garcés N, Yajure A (1997) A correlation for three-phase behavior of cationic surfactant–oil–water systems. J Dispers Sci Technol 18:539–555
Salager JL, Márquez N, Graciaa A, Lachaise J (2000) Partitioning of ethoxylated octylphenol surfactants in microemulsion-oil-water systems. Influence of temperature and relation between partitioning coefficient and physicochemical formulation. Langmuir 16:5534–5539
Salager JL, Forgiarini AM, Bullón J (2013) How to attain an ultralow interfacial tension and a three-phase behavior with surfactant formulation for enhanced oil recovery—a review. Part 1. Optimum formulation for simple SOW ternary systems. J Surf Deterg 16:449–472
Salager JL, Antón RE, Andérez JM, Aubry JM (2001) Formulation des micro-émulsions par la méthode HLD. In Techniques de l’Ingénieur. Génie des Procédés J2–157:1–20
Shinoda K, Saito H (1968) The effect of temperature on the phase equilibria and the types of dispersion of the ternary system composed of water, cyclohexane, and nonionic surfactant. J Colloid Interf Sci 26:70–74
Shinoda K, Sagitani H (1978) Emulsifier selection in water/oil type emulsions by the hydrophile-lipophile balance temperature system. J Colloid Interf Sci 64:68–71
Kunieda H, Shinoda K (1982) Phase behavior in systems of nonionic surfactant–water–oil around the hydrophile-lipophile balance temperature (HLB-Temperature). J Dispers Sci Technol 3:233–244
Förster T, Von Rybinski W, Wadle A (1995) Influence of microemulsion phases on the preparation of fine-disperse emulsions. Adv Colloid Interf Sci 58:119–149
Sottmann T, Strey R, Chen SH (1997) A small-angle neutron scattering study of nonionic surfactant molecules at the water–oil interface: area per molecule, microemulsion domain size, and rigidity. J Chem Phys 106:6483–6491
Kahlweit M, Strey R, Firman P (1986) Search for tricritical points in ternary systems: water-oil-nonionic amphiphile. J Phys Chem 90:671–677
Leitao H, Somoza AM, Telo da Gama MM, Sottmann T, Strey R (1996) Scaling of the interfacial tension of microemulsions: a phenomenological description. J Chem Phys 105:2875–2883
Engelskirchen S, Elsner N, Sottmann T, Strey R (2007) Triacylglycerol microemulsions stabilized by alkyl ethoxylate surfactants—a basic study phase behavior, interfacial tension and microstructure. J Colloid Interf Sci 312:114–121
Salager JL, Forgiarini AM, Márquez L, Manchego L, Bullón J (2013) How to attain an ultralow interfacial tension and a three-phase behavior with surfactant formulation for enhanced oil recovery—a review. Part 2. Performance improvement trends from Winsor’s premise to currently proposed inter- and intra-molecular mixtures. J Surf Deterg 16:631–663
Bourrel M, Chambu C (1983) The rules for achieving high solubilization of brine and oil by amphiphilic molecules. Soc Pet Eng J 23:327–338
Salager JL, Forgiarini A, Bullón J (2012) Surfactant formulation guidelines to reach the ultralow interfacial tension for enhanced oil recovery. In: Garcia Sucre M, Lozsan A, Castellanos-Suarez A, Toro-Mendoza J (eds) Topics in colloidal aggregation and interfacial phenomena. Chap. 5. Research Signpost, Trivandrum, pp 125–160
Salager JL (1977) Physico-chemical properties of surfactant–water–oil mixtures: Phase behavior, microemulsion formation and interfacial tension. Ph.D. dissertation. Univ of Texas at Austin, USA
Bourrel M, Salager JL, Lipow AM, Wade WH, Schechter RS (1978) Properties of amphiphile/oil/water systems at an optimum formulation for phase behavior. Paper SPE 7450. In: 53rd SPE Technical Conference, Houston TX October 1–3, 1978
Hayes M, Bourrel M, El-Emary M, Schechter RS, Wade WH (1979) Interfacial tension and phase behavior of nonionic surfactants. Soc Petrol Eng J 19:349–356
Bourrel M, Koukounis Ch, Schechter R, Wade W (1980) Phase and interfacial tension behavior of nonionic surfactant. J Dispers Sci Technol 1:13–35
Salager JL, Antón RE, Sabatini DA, Herwell JH, Acosta EJ, Tolosa LI (2005) Enhancing solubilization in microemulsions—state of the art and current trends. J Surf Deterg 8:3–21
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL (1993) Improving solubilization in microemulsions with additives. 1. The lipophilic linker role. Langmuir 9:669–672
Graciaa A, Lachaise J, Cucuphat C, Bourrel M, Salager JL (1993) Improving solubilization in microemulsions with additives. 2. Long chain alcohols as lipophilic linkers. Langmuir 9:3371–3374
Salager JL, Graciaa AA, Lachaise J (1998) Improving solubilization in microemulsions with additives. 3. Lipophilic linker optimization. J Surf Deterg 1:403–406
Acosta E, Uchiyama H, Sabatini DA, Harwell JH (2002) The role of hydrophilic linker. J Surf Deterg 5:151–157
Yuan JS, Ansari M, Samaan M, Acosta EJ (2008) Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm 349:130–143
Frank C, Frielinghaus H, Allgaier J, Richter D (2008) Hydrophilic alcohol ethoxylates as efficiency boosters for microemulsions. Langmuir 24:6036–6043
Allgaier J, Frielinghaus H (2009) Effect if polymers on the properties of microemulsions. In: Stubenrauch C (ed) Microemulsions: background, new concepts, applications, perspectives. Blackwell Pub, Birmingham, pp 122–147
Sottman T (2002) Solubilization efficiency boosting by amphiphilic block copolymers in microemulsions. Curr Opin Colloid Interf Sci 7:57–65
Jakobs B, Sottmann T, Strey R, Allgaier J, Willner L, Richter D (1999) Amphiphilic block copolymers as efficiency boosters for microemulsions. Langmuir 15:6707–6711
Miñana-Perez M, Graciaa A, Lachaise JK, Salager JL (1995) Solubilization of polar oils with extended surfactants. Colloids Surf A 100:217–224
Acosta EJ (2008) The HLD-NAC equation of state for microemulsions formulated with nonionic alcohol ethoxylate and alkylphenol ethoxylate surfactants. Colloids Surf A 320:193–204
Kunz W, Testard F, Zemb T (2009) Correspondence between curvature, packing parameter, and hydrophilic-lipophilic deviation scales around the phase-inversion temperature. Langmuir 25:112–115
Langevin D, Guest D, Meunier J (1986) Correlation between interfacial tension and microemulsion structure in Winsor equilibria. Role of the surfactant film curvature properties. Colloids Surf 19:159–170
Binks BP, Meunier J, Langevin D (1989) Characteristic sizes, film rigidity and interfacial tensions in microemulsion systems. Prog Colloid Polym Sci 79:208–213
Lee LT, Langevin D, Strey R (1990) Relation between microemulsion structure and surfactant layer bending elasticity. Phys A 168:210–219
Huh C (1979) Interfacial tension and solubilizing ability of a microemulsion phase that coexists with oil and brine. J Colloid Interf Sci 71:408–426
Huh C (1983) Equilibrium of a microemulsion that coexists with oil and brine. Soc Petrol Eng J 23:247–829
De Gennes PG, Taupin C (1982) Microemulsions and the flexibility of oil/water interfaces. J Phys Chem 86:2294
Langevin D (1991) Microemulsions—interfacial aspects. Adv Colloid Interf Sci 34:583–595
Choi SM, Chen SH, Sottmann T, Strey R (2002) The existence of three length scales and their relation to the interfacial curvatures in bicontinuous microemulsions. Phys A 304:85–92
Fraaije JGEM, Tandon K, Jain S, Handgraaf J-W, Buijse M (2013) Method of moments for computational microemulsion analysis and prediction in tertiary oil recovery. Langmuir, pp 2136–2151
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Salager, JL., Manchego, L., Márquez, L. et al. Trends to Attain a Lower Interfacial Tension in a Revisited Pure Alkyl Polyethyleneglycol Surfactant–Alkane–Water Ternary System. Basic Concepts and Straightforward Guidelines for Improving Performance in Enhanced Oil Recovery Formulations. J Surfact Deterg 17, 199–213 (2014). https://doi.org/10.1007/s11743-013-1534-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1534-5