Abstract
The genus Anaplasma includes A. marginale, A. centrale, A. bovis, A. ovis, A. platys, and A. phagocytophilum transmitted by ticks, some of which are zoonotic and cause anaplasmosis in humans and animals. In 2012, a new species was discovered in goats in China. In 2015, the same agent was detected in humans in China, and it was provisionally named Anaplasma capra, referring to 2012. The studies conducted to date have revealed the existence of A. capra in humans, domestic animals, wild animals, and ticks from three different continents (Asia, Europe, and Africa). Phylogenetic analyses based on gltA and groEL sequences show that A. capra clearly includes two different genotypes (A. capra genotype-1 and A. capra genotype-2). Although A. capra human isolates are in the genotype-2 group, goat, sheep, and cattle isolates are in both groups, making it difficult to establish a host genotype-relationship. According to current data, it can be thought that human isolates are genotype-2 and while only genotype-1 is found in Europe, both genotypes are found in Asia. Anaplasma capra causes clinical disease in humans, but the situation is not yet sufficient to understand the zoonotic importance and pathogenicity in animals. In the present review, the history, hosts (vertebrates and ticks), molecular prevalence, pathogenic properties, and genetic diversity of A. capra were evaluated from a broad perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction (the great progress in a short time in history of Anaplasma capra)
Anaplasma (family Anaplasmataceae, order Rickettsiales) species are obligate intracellular alphaproteobacteria that multiply within membrane-bound vacuoles and can cause disease in humans and a wide range of domestic animals (Dumler et al. 2001; Rar et al. 2021). The first clinical infections in animals were described in 1910 (Theiler 1910). Today, it still has effects on human and animal health at the global level (Rar et al. 2021). According to the classification based on 16S rRNA and groEL genes, there are six Anaplasma species (A. bovis, A. ovis, A. marginale, A. centrale, A. phagocytophilum, and A. platys) in humans and animals (Dumler et al. 2001).
Developments in molecular genetics and the increased use of these techniques in the identification of pathogens have undoubtedly contributed greatly to the elucidation of the etiologies of diseases. With these techniques, different strains and genotypes of many pathogens have been revealed, progress has been made in their classification, and they have enabled the discovery of previously unidentified species (Dumler et al. 2001). In the study conducted in Central and Southern China in 2012, the isolate obtained from goats, which was different from other Anaplasma species according to its 16S rRNA gene sequence, was recorded in the GenBank as Uncultured Anaplasma sp. (Liu et al. 2012). In 2015, in China, a novel Anaplasma species different from all known Anaplasma species was identified in 28 of 477 (6%) humans with tick bite history. It has been shown that the 16S rRNA gene full sequence (1,499 bp) of this isolate, which is an important marker in genotyping, has 27–73 nucleotide differences with other Anaplasma species (Li et al. 2015). The species revealed in this study was named “Anaplasma capra” because goats were the host from which the agent was first isolated. In the study, A. capra was also detected in a tick species (Ixodes persulcatus) for the first time (Li et al. 2015). There is an important point to point out here; it is thought that A. capra was circulating in domestic and wild animals before 2012 when it was first reported. Phylogenetic analysis based on 16S rRNA has shown that the species (A. centrale Aomori strain, AF283007, Inokuma et al. 2001) identified in cattle in Japan in 2001 is A. capra (Khumalo et al. 2018). Likewise, BLAST analyses of A. centrale (AB211164) detected in deer in Japan in 2005 (Kawahara et al. 2006) and Anaplasma sp. (AB509223) detected in serow in Japan in 2009 (Sato et al. 2009) revealed that they were A. capra. On the other hand, A. capra was detected in 2020 in blood samples taken from goats in 2011 (Zhang et al. 2020).
The detection of the zoonotic potential in a short time (this period reached 60 years for A. phagocytophilum) resulted in the intense interest of the scientific community to A. capra. Anaplasma phagocytophilum was detected in sheep in 1932 (Gordon et al. 1932), but it took 62 years for it to be identified in humans (Chen et al. 1994). A. capra was detected for the first time in sheep and cattle in 2017 and 2018, respectively (Guo et al. 2018; Koh et al. 2018; Yang et al. 2017). In a study conducted in Malaysia in 2018, A. capra was detected for the first time in a country other than China (Koh et al. 2018). In the same year, A. capra was detected in takin, reeves’ muntjac, and forest musk deer, thus showing that it also infects wild animals (Yang et al. 2018). In 2018, A. capra was detected outside the Asian continent, with its discovery in Sweeden (Grandi et al. 2018). In 2019, the agent was detected in dogs in China. Thus, its presence in carnivores was determined for the first time (Shi et al. 2019). With the detection of A. capra in cattle from Angola in 2021, it was detected for the first time on the African continent (Barradas et al. 2021). It has been shown that A. capra invades host erythrocytes in 2021 (Peng et al. 2021a, b). Finally, in 2023, the full genome sequences of A. capra was obtained, and it was determined that the approximately 1.07 Mp genome contained 862 protein-coding genes (Lin et al. 2023a). It took a total of 12 years from the detection of A. capra to the disclosure of its full genome. The cornerstones of the short-fast historical process are summarized in Fig. 1.
The hosts of Anaplasma capra
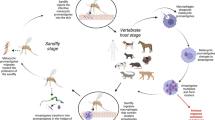
The life cycle of Anaplasma species circulates between vertebrate hosts and ticks (de la Fuente et al. 2016). The fact that A. capra was first detected in goats and then revealed in humans can be considered an indication that the host group will be interesting.
Vertebrates
The domestic and wild animals in which A. capra was detected by molecular methods are given in Fig. 2. Studies on A. capra using molecular methods were, as expected, mostly conducted on goats. This was followed by sheep and cattle. The study in China continued to be the first and only study in which A. capra was detected in humans (Li et al. 2015). While Li et al. (2015) reported that human-derived A. capra was demonstrated to infect HL-60 and THP-1 cells, Peng et al. (2021b) reported that the goat-derived A. capra can infect human erythrocytes, HL-60 and TF-1 cells as in vitro. The results can be considered as strong evidence for the zoonotic potential of A. capra. Except for humans, cattle, sheep, and goats, A. capra was detected in domestic animals such as buffalo (Sahin et al. 2022), dog (Shi et al. 2019), horse (unpublished data, GenBank; ON872236), cat (unpublished data, GenBank; MW520360), and wild animals such as roe deer (Remesar et al. 2022; Wang et al. 2019), sika deer (Kawahara et al. 2006), water deer (Shin et al. 2020), red deer (Jouglin et al. 2019), swamp deer (Jouglin et al. 2019), forest musk deer (Yang et al. 2018), yak (Wang et al. 2021b), onegar (Staji et al. 2021), serow (Sato et al. 2009), takin (Yang et al. 2018), mouflon (Isaq et al. 2022), and reeves’ muntjac (Yang et al. 2018), albeit in one or at most two studies (Fig. 2). Although some studies state that the main host of A. capra may be domestic ruminants, it seems that it is too early to say this. As a matter of fact, studies in this field were mostly conducted on domestic ruminants.
It is known that wild animals, especially wild ruminants such as red deer (Cervus elaphus), roe deer (Capreolus capreolus), white-tailed deer (Odocoileus virginianus), mouflon (Ovis musimon), and chamois (Rupicapra rupicapra), serve as reservoir hosts for Anaplasma species (Rar and Golovljova 2011). Among the animals in which A. capra has been detected, it is seen that wild ruminants are predominant (Fig. 2). Wild ruminates play an important role in the bioecology of Anaplasma species (Woldehiwet 2010). Anaplasma ovis has a high positivity rate in roe deer and red deer, and these species are reservoirs for A. ovis (de la Fuente et al. 2008; Renneker et al. 2013). A similar relationship is observed in A. phagocytophilum and deers (Teodorowski et al. 2020). The prevalence of A. phagocytophilum is up to 98% in roe deer and 87% in red deer (Stuen et al. 2013). It has also been reported that A. marginale is persistent in deer and has a high positivity rate in these animals (Atif 2016). Understanding the reservoir role of wild ruminants for Anaplasma species is important to explain the epidemiology of the species. Although de la Fourniere et al. (2023) recently showed transovarial transmission of A. marginale in Rhipicephalus microplus and Baldridge et al. (2009) demonstrated transovarial transmission of A. phagocytophilum in Dermacentor albipictus, it was generally thought that Anaplasma species were not transovarially transmitted in ticks (Aubry and Geale 2011; Kocan et al. 2010; Woldehiwet 2010). In this case, the reservoir role of wild animals increases their contribution to the epidemiology of A. capra.
Ticks
Ticks are the main vector for numerous haemopathogens such as Anaplasma, Babesia, Theileria, and Hepatozoon (Dumanli et al. 2012; Inci et al. 2016). Anaplasma species are transmitted to hosts biologically through ticks belonging to the Ixodidae family, they are also transmitted mechanically through surgical instruments contaminated with the blood of infected animals, during surgical operations (such as castration or dehorning), through blood-sucking arthropods, or even transplacental (Aubry and Geale 2011; Dumler et al. 2001; Kocan et al. 2010). Many issues need to be clarified regarding the biology of A. capra, but its detection in blood-sucking arthropods is an indication of indirect development. The significantly higher prevalence of the agent in the summer months may be associated with vector activity (Shi et al. 2019; Wang et al. 2021b). The tick species in which A. capra was detected by molecular methods are given in Fig. 2. Haemaphysalis longicornis is the tick species on which the most studies have been conducted and for which A. capra has been detected the most (Guo et al. 2018; Lu et al. 2023; Qin et al. 2018; Seo et al. 2020; Sun et al. 2015; Teng et al. 2023; Yan et al. 2021). In much more limited work, A. capra was detected in H. ginghainensis (Han et al. 2019; Yang et al. 2016), Ixodes persulcatus (Li et al. 2015), I. kashmiricus (Numan et al. 2023), Rhipicephalus sanguineus (unpublished data, GenBank; OK091153), R. microplus (Addo et al. 2023; Guo et al. 2018, 2019) Dermacontor nuttali, D. everestianus, and D. abaensis (Han et al. 2019).
Anaplasma capra has been identified in both parasitic ticks (H. longicornis, R. microplus, I. kashmiricus) collected from domestic animals (Guo et al. 2018; Numan et al. 2023) and host-seeking (H. longicornis, H. qinghaiensis, I. persulcatus, D. abaensis, D. everestianus, D. nuttalli) ticks (Han et al. 2019; Li et al. 2015; Seo et al. 2020). The positivity rate in ticks is quite variable. It was detected in I. persulcatus at 3.0% (Li et al. 2015), in H. longicornis at 0.43–63.27% (Guo et al. 2018; Lu et al. 2023; Seo et al. 2020; Sun et al. 2015; Teng et al. 2023; Yan et al. 2021;), in H. qinghaiensis at 4.5–5.8% (Han et al. 2019; Yang et al. 2016), in R. microplus at 0.81–40.4% positive rates (Addo et al. 2023; Guo et al. 2018, 2019). Almost all of the studies on ticks were conducted in China, where A. capra was first detected and where most studies were conducted on vertebrates. A very high positivity rate (63.27%) was determined in H. longicornis collected from goats in China (Lu et al. 2023). On the other hand, H. longicornis is the most prevalent tick species in China and is especially parasitized in sheep (Teng et al. 2023). It may support the relationship between A. capra infections and both small ruminants and H. longicornis. Although the current studies have revealed the presence of A. capra in ticks, there is a need to be further studies by transmission experiments. Anaplasma marginale is known to be transmitted by more than 20 tick species, including D. andersoni, D. variabilis, D. albipictus, R. microplus, and R. annulatus (Ben Said et al. 2018; Kocan et al. 2010). Anaplasma capra is likely to be identified in many more tick species. It has been detected in R. sanguineus from Portugal (unpublished data, GenBank; OK091153) and I. kashmiricus from Pakistan (Numan et al. 2023). Additionally, in two studies conducted in the same region (Sivas) in Türkiye, A. capra was detected at a rate of 14.28% in buffalos and 0.41% in cattle (Altay et al. 2022a; Sahin et al. 2022). The tick infestation rate is much lower in water buffalos than in cattle. However, the above prevalence contradicts this. It should be taken into consideration that A. capra can be transmitted by other means. It is known that some Anaplasma species are transmitted by other blood-sucking arthropods (Aubry and Geale 2011; Dumler et al. 2001; Kocan et al. 2010). Recently, Ehrlichia and Rickettsia species were detected in all developmental stages of mosquitoes, and it was reported that mosquitoes may transmit these species both transtadially and transovarially (Guo et al. 2016). When all this information is evaluated together, it shows that A. capra circulates among domestic animals, wild animals, and ticks, and that these hosts are important factors determining the epidemiology of A. capra.
The molecular prevalence of Anaplasma capra
Although the studies on A. capra were mostly conducted in China, in a short time its presence has been revealed in 18 different countries including China (Liu et al. 2012), South Korea (Seo et al. 2018), Türkiye (Altay et al. 2022a), Kyrgyztan (Altay et al. 2022b, c), Malaysia (Koh et al. 2018), Japan (Kawahara et al. 2006), Iraq (unpublished data GenBank; ON872236), Iran (Staji et al. 2021), India (Kumar et al. 2023), Pakistan (Isaq et al. 2022), France (Jouglin et al. 2019), Sweeden (Grandi et al. 2018), Portugal (unpublished data, GenBank; OK091153), Spain (Remesar et al. 2022), Greece (Saratsis et al. 2022), Angola (Barradas et al. 2021), Morocco (Elhachimi et al. 2021), and Ghana (Addo et al. 2023) by molecular techniques (Fig. 1). Since, A. capra is a newly discovered species, studies have generally focused on its identification and determination of its phylogenetic position.
The prevalence of tick-borne pathogens is affected by many different factors such as the host, age, season, management systems, tick infestation density, climatic characteristics of the region, host immunity, time of sampling, sample size, and detection methods (Belkahia et al. 2017; Ben Said et al. 2018; Kabir et al. 2011; Nguyen et al. 2020; Wang et al. 2021a). According to a meta-analysis study conducted in 2023, the average prevalence of A. capra was found to be 5.9% in humans, 11.3% in animals, and 7.8% in ticks (Lin et al. 2023b). Despite this undoubtedly valuable information, it is still very difficult to determine the limits of the prevalence of A. capra. The positivity rate of A. capra in cattle was 0.28% in Kyrgyzstan (Altay et al. 2022c), 0.30% in South Korea (Miranda et al. 2021), 0.41% in Türkiye (Altay et al. 2022a), and 11.3% in Morocco (Elhachimi et al. 2021). Similarly, while the positivity rate in goats is 0.30% in South Korea (Miranda et al. 2021), this rate reaches 44.6% in China (Wei et al. 2020). Its prevalence in wild animals starts from 0.6% (Wang et al. 2021b) and reaches 17.7% (Amer et al. 2019). The positivity rates obtained from molecular studies conducted on domestic and wild animals can be viewed in Table 1.
Tick activation is generally highest between spring and autumn (Dumanli et al. 2012). The prevalence of A. capra was found to be higher in the summer months, which are more suitable for the activation of ticks, as in other tick-borne pathogens (Seo et al. 2018; Shi et al. 2019). Additionally, it was observed that its prevalence increased with age, and in this case, it was associated with the extension of the tick contact period (Shi et al. 2019; Zhou et al. 2023). It should be taken into consideration that A. capra may be chronic or persistent and its prevalence may increase with age (Rar et al. 2021). Jouglin et al. (2019) reported that A. capra can persist in red deer for four months. The persistently infected hosts may serve as reservoirs for ticks, and these hosts are important in the epidemiology of the Anaplasma species (Brown and Barbet 2016; Kocan et al. 2010). Although the prevalence of A. capra varies, the important point is that its circulation is in a wide geography and a very wide host group. Considering the current prevalence of the agent and the fact that it has been identified in different tick species, studies are needed to determine its situation in other continents.
The genotypes of Anaplasma capra
In recent years, intensive studies have been carried out on the genetic differences of Anaplasma species and the relationship of some genetic groups with geography, vector and host is emphasized (Rar et al. 2021). In the multilocus sequence analysis of 520 samples of A. phagocytophilum, eight clusters that could be separated according to geography, vector, and host were obtained (Langenwalder et al. 2020). Twelve clusters emerged in phylogenetic analyses based on the A. phagocytophilum ankA gene (Langenwalder et al. 2020). In the analysis of groEL sequences of A. phagocytophilum from Europe and Russia, four different ecotypes with host tropism were identified (Jahfari et al. 2014). The 11 5’-UTR microsatellite genotypes and 193 msp1a tandem repeats of A. marginale have been identified worldwide, but it has been reported that they have no geographical relationship (Rar et al. 2021). The 47 msp1aS repeats and 32 genotypes of A. centrale have been identified only in Africa (Khumalo et al. 2018). msp2 gene analyses of A. ovis have revealed between 2 and 17 genotypes in different countries (Belkahia et al. 2014, 2017; Cabezas-Cruz et al. 2019; Torina et al. 2010; Zhou et al. 2017). Anaplasma bovis groEL sequences form four and gltA sequences form three lineages. There are findings that ecotypes formed on this basis show host and vector specificity (Rar et al. 2021).
The 16S rRNA gene is frequently used in molecular survey studies. However, 16S rRNA gene is not very useful in Anaplasma species genotyping studies, more variable genes (groEL, gltA, msp2, and msp4) are preferred in these studies (Caudill and Brayton 2022; Rar et al. 2021). After the naming of A. capra in 2015, especially the groEL and gltA gene sequences were recorded in GenBank (NCBI). Thus, a sequence pool of this isolate was formed, which enabled intraspecific genetic comparisons. Yang et al. (2017) reported that the 16S rRNA gene of A. capra exhibits high sequence similarity (similarity of 99.8–99.9%), but the gltA and groEL genes were relatively less identical (88.6–88.7% for gltA and 90.6–91.0% for groEL). They concluded that; one genotype contains strains isolated from goats, sheep, I. persulcatus, and humans, while the other from deer, serows, and H. qinghaiensis. Wang et al. (2021a) similarly reported that A. capra was divided into two clusters, and cluster I contained isolates with zoonotic potential (from human), and clade II contained isolates obtained from goats. However, it has been reported that A. capra exhibits at least two different genotypes, both are likely zoonotic (Peng et al. 2021a). Jouglin et al. (2022) reported that A. capra divides in two separate clades based on gltA or groEL, clade I includes A. capra sequences from sheep, goats, cattle, dogs, humans, and ticks, and clade II includes from sheep and goats, and also from a variety of wild ruminants. They reported that this grouping had no geographical relationship (Jouglin et al. 2022). In the analysis of 203 gltA gene sequences, water buffalo, sheep, goat, wild animals, and tick isolates were included in the genotype-1 group, and human, sheep, cattle, goat, dog, wild animals, and tick isolates were in the genotype-2 group. In the analysis of 158 groEL gene sequences, water buffalo, sheep, goat, wild animals, and tick isolates were included in genotype-1, and human, dog, cat, sheep, goats, cattle, wild animals, and tick isolates were included in genotype-2 (Sahin et al. 2022). When the gltA DNA sequences of 21 A. capra isolates detected in Kyrgyzstan in 2022 were compared with the existing gene sequences in the Gene Bank, one group with a difference of 0–7 nucleotides within themselves and the second group with a difference of 68–70 nucleotides from those were formed. The first group includes the sequences from red deer, swamp deer, Siberian roe deer, takin, reeves’ muntjac, forest musk deer, D. everestianus, Korean water deer, cattle, and sheep, while the second group includes dog, cattle, sheep, goat, human, H. qinghaiensis, H. longicornis, and R. microplus (Altay et al. 2022b). In another study conducted on the basis of gltA, isolates from France (red deer, swamp deer), South Korea (water deer), China (D. everestianus), Türkiye (cattle and sheep) were classified in the genotype-1 group, China (human, dog, sheep, goat and R. microplus) and South Korea (cattle) were included in the genotype-2 group (Altay et al. 2022a). Anaplasma capra isolates obtained from sheep in Kyrgyzstan are in the genotype-1 group with isolates from France (swamp deer and red deer), and China (roe deer, takin, forest musk deer, revees’ muntjac and D. everestianus) but China (human, goat, sheep, dog, H. longicornis, H. qinghaiensis, and R. microplus) isolates were included in the genotype-2 group (Altay et al. 2022b). According to these data, it can be thought that human isolates are genotype-2 and while only genotype-1 is found in Europe, both genotypes are found in Asia. This situation may change with the addition of more sequences from different isolates. There is a need for comprehensive and detailed research to reveal the relationship between host, tick species, geography, and pathogenicity of these isolates.
In this review, a phylogenetic tree based on gltA and groEL genes, which are more informative to understand genotypic variation of the pathogen than other gene regions, like 16S rRNA was constructed by selecting some of the A. capra isolates detected in different parts of the world. In this phylogenetic tree, A. capra genotype-1 and A. capra genotype-2 are shown (Fig. 3).
Pathogenicity and public health concern of Anaplasma capra
Anaplasma species infect different cells of the host and multiply within these cells. While A. ovis, A. marginale, and A. centrale infect the erythrocytes, A. bovis, A. phagocytophilum, and A. platys infect monocytes, granulocytes, and platelets, respectively (Dumler et al. 2001; Kocan et al. 2010; Woldehiwet 2010). While the morulae of A. marginale are small and localized at the periphery of stained erythrocytes, A. centrale forms smaller and more central morulae (Theiler 1910). Anaplasma ovis settles in the central area of sheep erythrocytes (Bevan 1912). Current information shows that A. capra invades erythrocytes like A. marginale, A. centrale, and A. ovis (Peng et al. 2021b). Li et al. (2015) inoculated A. capra into human cell lines (HL-60 and THP-1 cells) and observed the morulae stages. However, in natural infections, no morulae or other blood agents were detected in any cells of peripheral blood smears by microscopic examination. (Li et al. 2015). Anaplasma capra inclusion bodies were microscopically observed in the erythrocytes of naturally infected onagers, but their morphological features were not defined (Staji et al. 2021). The first detailed study on the morphology of A. capra was carried out by Peng et al. (2021a). The researchers examined A. capra-infected goat erythrocytes using electron microscope. They observed A. capra cells with a diameter of 0.2–0.4 μm on the outer surface of the erythrocyte. They also imaged the round or oval-shaped morulae stages, on the surface (0.2–0.4 μm in diameter) and inside (0.8 × 1 μm) of the erythrocytes. Peng et al. (2021b) showed that goat-derived A. capra can infect human erythrocytes and TF-1 cells.
The clinical course of anaplasmosis varies depending on the species causing the disease. Anaplasma phagocytophilum is the causative agent of tick fever syndrome in ruminants and causes high fever, loss of appetite, cough, decrease in milk yield, and abortions (Woldehiwet 2010). Anaplasma marginale causes severe clinical symptoms in cattle (Kocan et al. 2010) and can cause anemia, weakness, fever, loss of appetite, decrease in milk yield, abortion, and death in untreated cases (Aubry and Geale 2011; Kocan et al. 2010). Anaplasma bovis can cause fever, weight loss, incoordination, lymph node enlargement, and rarely death in ruminants (Chilton et al. 2018). Anaplasma centrale is less pathogenic than A. marginale and usually causes subclinical infections in cattle, it is used as live vaccines against anaplasmosis caused by A. marginale in Israel, South Africa, South America, and Australia (Aubry and Geale 2011; Kolo 2023). While A. ovis generally causes mild clinical symptoms in sheep and goats, it can also cause acute infections with clinical symptoms such as hemolytic anemia, icterus, depression, anorexia, weight loss, and decreased milk yield in case the immune system of infected hosts is suppressed or in mixed infections with different pathogens (Dumler et al. 2001; Renneker et al. 2013). While A. platys causes infectious cyclic thrombocytopenia in dogs, there are usually no symptoms of infection (de la Fuente et al. 2006).
There is not yet sufficient information that A. capra causes clinical disease in animals. The prevalence of the agent was found only to be high in the anemia group in dogs (Shi et al. 2019). However, in the same study, no relationship was found between A. capra and fever, cough, malaise, and depression (Shi et al. 2019). Current information shows that A. capra invades host erythrocytes, such as A. marginale, A. centrale, and A. ovis (Peng et al. 2021a, b). Staji et al. (2021) reported that a slight decrease of RBC, HCT, and HGB, leukopenia, lymphopenia, thrombocytopenia, hypoalbuminemia, and hyperbilirubinemia was detected in two Persian onagers infected with A. capra. The clinical picture of human infections is relatively clearer. It has been reported that A. capra causes influenza-like symptoms, including fever, headache, malaise, dizziness, and chills, and some gastrointestinal symptoms (nausea, vomiting, or diarrhea), rash, eschar, and regional lymphadenopathy in humans. Additionally, high hepatic aminotransferase concentrations, leucopenia, and thrombocytopenia have been detected. The patients recovered when treated with doxycycline (Li et al. 2015).
Conclusions
Tick-borne pathogens are a growing concern for human and animal health. Anaplasma capra has been detected in humans, domestic animals, wild animals, and ticks. Although current studies are not sufficient, the domestic ruminants are considered the main host. Wild animals serve as reservoirs for many tick-borne pathogens. Anaplasma capra has been detected in various wild animal species. The existence of clinical infections in humans has been demonstrated, although in limited studies. The detection of the agent in parasitic and host-seeking ticks indicates that it is a tick-borne disease. All the studies together show that A. capra has a wide host range with an increased risk of infections worldwide. Considering that the clinical symptoms of anaplasmosis infections are significantly nonspecific and are confused with other infections, the importance of A. capra to human health can be better emphasized. Anaplasma capra, as an emerging bacterial infection transmitted by ticks, with global distribution, and a very wide host group, is a species that needs to be investigated in many aspects, especially its epidemiology, biological properties, pathogenicity via experimental infections in different hosts, rapid diagnosis, and, if necessary, treatment protocols and effective control methods.
Data availability
No datasets were generated or analysed during the current study.
References
Addo SO, Baako BOA, Bentil RE, Addae CA, Behene E, Asoala V, Sallam M, Mate S, Dunford JC, Larbi JA, Baidoo PK, Wilson MD, Diclaro JW, Dadzie SK (2023) Molecular survey of Anaplasma and Ehrlichia species in livestock ticks from Kassena-Nankana, Ghana; with a first report of Anaplasma Capra and Ehrlichia Minasensis. Arch Microbiol 205(3):92. https://doi.org/10.1007/s00203-023-03430-1
Altay K, Erol U, Sahin OF (2022a) The first molecular detection of Anaplasma Capra in domestic ruminants in the central part of Turkey, with genetic diversity and genotyping of Anaplasma Capra. Trop Anim Health Prod 54:1–8. https://doi.org/10.1007/s11250-022-03125-7
Altay K, Erol U, Sahin OF, Aytmirzakizi A, Temizel EM, Aydin MF, Dumanli N, Aktas M (2022b) The detection and phylogenetic analysis of Anaplasma phagocytophilum-like 1, A. ovis and A. capra in sheep: A. capra divides into two genogroups. Vet Res Commun 46(4):1271–1279. https://doi.org/10.1007/s11259-022-09998-1
Altay K, Erol U, Sahin OF, Aytmirzakızı A (2022c) First molecular detection of Anaplasma species in cattle from Kyrgyzstan; molecular identification of human pathogenic novel genotype Anaplasma Capra and Anaplasma phagocytophilum related strain. Ticks Tick Borne Dis 13(1):101861. https://doi.org/10.1016/j.ttbdis.2021.101861
Amer S, Kim S, Yun Y, Na KJ (2019) Novel variants of the newly emerged Anaplasma Capra from Korean water deer (Hydropotes inermis argyropus) in South Korea. Parasit Vector 12(1):1–9. https://doi.org/10.1186/s13071-019-3622-5
Atif FA (2016) Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 143(6):659–685. https://doi.org/10.1017/S0031182016000238
Aubry P, Geale DW (2011) A review of bovine anaplasmosis. Transbound Emerg Dis 58(1):1–30. https://doi.org/10.1111/j.1865-1682.2010.01173.x
Baldridge GD, Scoles GA, Burkhardt NY, Schloeder B, Kurtti TJ, Munderloh UG (2009) Transovarial transmission of Francisella-like endosymbionts and Anaplasma phagocytophilum variants in Dermacentor albipictus (Acari: Ixodidae). J Med Entomol 46:625–632. https://doi.org/10.1603/033.046.0330
Barradas PF, Mesquita JR, Ferreira P, Gartner F, Carvalho M, Inacio E, Chivinda E, Katimba A, Amorim I (2021) Molecular identification and characterization of Rickettsia spp. and other tick-borne pathogens in cattle and their ticks from Huambo, Angola. Tick Tick Borne Dis 12(1):101583. https://doi.org/10.1016/j.ttbdis.2020.101583
Belkahia H, Ben Said M, El Hamdi S, Yahiaoui M, Gharbi M, Daaloul-Jedidi M, Messadi L (2014) First molecular identification and genetic characterization of Anaplasma ovis in sheep from Tunisia. Small Rum Res 121:404–410. https://doi.org/10.1016/j.smallrumres.2014.07.009
Belkahia H, Ben Said M, El Mabrouk N, Saidani M, Cherni C, Ben Hassen M, Bouattour A, Messadi L (2017) Seasonal dynamics, spatial distribution and genetic analysis of Anaplasma species infecting small ruminants from Northern Tunisia. Infect Genet Evol 54:66–73. https://doi.org/10.1016/j.meegid.2017.06.016
Ben Said M, Belkahia H, Messadi L (2018) Anaplasma spp. in North Africa: a review on molecular epidemiology, associated risk factors and genetic characteristics. Ticks Tick Borne Dis 9:543–555. https://doi.org/10.1016/j.ttbdis.2018.01.003
Bevan LLEW (1912) Anaplasmosis of sheep. Vet J 68:400–401
Brown WC, Barbet AF (2016) Persistent infections and immunity in ruminants to arthropod-borne bacteria in the family Anaplasmataceae. Annu Rev Anim Biosci 4:177–197. https://doi.org/10.1146/annurev-animal-022513-114206
Cabezas-Cruz A, Gallois M, Fontugne M, Allain E, Denoual M, Moutailler S, Devillers E, Zientara S, Memmi M, Chauvin A, Agoulon A, Vayssier Taussat M, Chartier C (2019) Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasit Vector 12: 3. https://doi.org/10.1186/s13071-018-3269-7
Caudill MT, Brayton KA (2022) The use and limitations of the 16S rRNA sequence for species classification of Anaplasma samples. Microorganisms 10:605. https://doi.org/10.3390/microorganisms10030605
Chen SM, Dumler JS, Bakken JS, Walker DH (1994) Identification of a granulocytic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol 32:589–595. https://doi.org/10.1128/jcm.32.3.589-595.1994
Chilton NB, Dergousoff SJ, Lysyk TJ (2018) Prevalence of Anaplasma bovis in Canadian populations of the Rocky Mountain wood tick, Dermacentor andersoni. Ticks Tick Borne Dis 9(6):1528–1531. https://doi.org/10.1016/j.ttbdis.2018.07.003
de la Fourniere S, Guillemi EC, Paoletta MS, Pérez A, Obregón D, Cabezas-Cruz A, Sarmiento NF, Farber MD (2023) Transovarial transmission of Anaplasma marginale in Rhipicephalus (Boophilus) microplus ticks results in a bottleneck for. Strain Divers Pathogens 12(8):1010. https://doi.org/10.3390/pathogens12081010
de la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Kocan KM (2006) Molecular characterization of Anaplasma platys strains from dogs in Sicily, Italy. BMC Vet Res 2(1):1–5. https://doi.org/10.1186/1746-6148-2-24
de la Fuente J, Ruiz Fons F, Naranjo V, Torina A, Rodriguez O, Gortazar C (2008) Evidence of Anaplasma infections in European roe deer (Capreolus capreolus) from southern Spain. Res Vet Sci 84(3):382–386. https://doi.org/10.1016/j.rvsc.2007.05.018
de la Fuente J, Estrada-Pena A, Cabezas Cruz A, Kocan KM (2016) Anaplasma phagocytophilum uses common strategies for infection of ticks and vertebrate hosts. Trends Microbiol 24:173–180. https://doi.org/10.1016/j.tim.2015.12.001
Dumanli N, Altay K, Aydin MF (2012) Tick species of cattle, sheep and goats in Turkey. Turkiye Klinikleri J Vet Sci 3(2):67–72
Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 51:2145–2165. https://doi.org/10.1099/00207713-51-6-2145
Elhachimi L, Rogiers C, Casaert S, Fellahi S, Van Leeuwen T, Dermauw W, Valcarcel F, Olmeda AS, Daminet S, Khatat SEH, Sahibi H, Duchateau L (2021) Ticks and tick-borne pathogens abound in the cattle population of the Rabat-Sale Kenitra Region. Morocco Pathogens 10:1594. https://doi.org/10.3390/pathogens10121594
Gordon WS, Brownlee A, Wilson DR, MacLeod J (1932) Tick-borne fever (a hitherto undescribed disease of sheep). J Comp Pathol 45:301–307. https://doi.org/10.1016/S0368-1742(32)80025-1
Grandi G, Aspan A, Pihl J, Gustafsson K, Engstrom F, Jinnerot T, Soderlund R, Chirico J (2018) Detection of tick-borne pathogens in lambs undergoing prophylactic treatment against ticks on two Swedish farms. Front Vet Sci 5:72. https://doi.org/10.3389/fvets.2018.00072
Guo WP, Tian JH, Lin XD, Ni XB, Chen XP, Liao Y, Yang SY, Dumler JS, Holmes EC, Zhang YZ (2016) Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci Rep 6:38770. https://doi.org/10.1038/srep38770
Guo WP, Huang B, Zhao Q, Xu G, Liu B, Wang YH, Zhou EM (2018) Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an, China. PLoS Negl Trop Dis 12(11): e0006916. https://doi.org/10.1371/journal.pntd.0006916
Guo WP, Zhang B, Wang YH, Xu G, Wang X, Ni X, Zhou EM (2019) Molecular identification and characterization of Anaplasma Capra and Anaplasma platys-like in Rhipicephalus microplus in Ankang, Northwest China. BMC Infect Dis 19:434. https://doi.org/10.1186/s12879-019-4075-3
Han R, Yang JF, Mukhtar MU, Chen Z, Niu QL, Lin YQ, Liu GY, Luo JX, Yin H, Liu ZJ (2019) Molecular detection of Anaplasma infections in ixodid ticks from the Qinghai-Tibet Plateau. Infect Dis Poverty 8(1):83–90. https://doi.org/10.1186/s40249-019-0522-z
He Y, Chen W, Ma P, Wei Y, Li R, Chen Z, Tian S, Qi T, Yang J, Sun Y, Li J, Kang M, Li Y (2021) Molecular detection of Anaplasma spp., Babesia spp. and Theileria spp. in yaks (Bos grunniens) and tibetan sheep (Ovis aries) on the Qinghai-Tibetan Plateau, China. Parasit Vector 14:613. https://doi.org/10.1186/s13071-021-05109-2
Inci A, Yildirim A, Duzlu O, Doganay M, Aksoy S (2016) Tick-borne diseases in Turkey: a review based on one health perspective. PLoS Negl Trop Dis 10:e0005021. https://doi.org/10.1371/journal.pntd.0005021
Inokuma H, Terada Y, Kamio T, Raoult D, Brouqui P (2001) Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other ehrlichiae. Clin Diagn Lab Immunol 8:241–244. https://doi.org/10.1128/CDLI.8.2.241-244.2001
Ishaq M, Ijaz M, Lateef M, Ahmed A, Muzammil I, Javed MU, Raza A, Ghumman NZ (2022) Molecular characterization of Anaplasma Capra infecting captive mouflon (Ovis Gmelini) and domestic sheep (Ovis aries) of Pakistan. Small Rum Res 216:106837. https://doi.org/10.1016/j.smallrumres.2022.106837
Jahfari S, Coipan EC, Fonville M, van Leeuwen AD, Hengeveld P, Heylen D, Heyman P, van Maanen C, Butler CM, Foldvari G, Szekeres S, van Duijvendijk G, Tack W, Rijks JM, van der Giessen J, Takken W, van Wieren SE, Takumi K, Sprong H (2014) Circulation of four Anaplasma phagocytophilum ecotypes in Europe. Parasit Vector 15(7):365. https://doi.org/10.1186/1756-3305-7-365
Jouglin M, Blanc B, de la Cotte N, Bastian S, Ortiz K, Malandrin L (2019) First detection and molecular identification of the zoonotic Anaplasma capra in deer in France. PLoS ONE 14(7):e0219184. https://doi.org/10.1371/journal.pone.0219184
Jouglin M, Rispe C, Grech-Angelini S, Gallois M, Malandrin L (2022) Anaplasma capra in sheep and goats on Corsica Island, France: a European lineage within A. capra clade II? Ticks Tick Borne Dis 13(3):101934. https://doi.org/10.1016/j.ttbdis.2022.101934
Kabir MHB, Mondal MMH, Eliyas M, Mannan MA, Hashem MA, Debnath NC, Miazi OF, Mohiuddin C, Kashem MA, Islam MR, Elahi MF (2011) An epidemiological survey on investigation of tick infestation in cattle at Chittagong District, Bangladesh. Afr J Microbiol Res 5(4):346–352. https://doi.org/10.5897/AJMR10.706
Kawahara M, Rikihisa Y, Lin Q, Isogai E, Tahara K, Itagaki A, Hiramitsu Y, Tajima T (2006) Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl Environ Microbiol 72(2):1102–1109. https://doi.org/10.1128/AEM.72.2.1102-1109.2006
Khumalo ZTH, Brayton KA, Collins NE, Chaisi ME, Oosthuizen MC (2018) Evidence confirming the phylogenetic position of Anaplasma centrale (ex Theiler 1911) Ristic and Kreier 1984. Inter J Syst Evol Microbiol 68:2682–2691. https://doi.org/10.1099/ijsem.0
Kocan KM, de la Fuente J, Blouin EF, Coetzee JF, Ewing SA (2010) The natural history of Anaplasma marginale. Vet Parasitol 167(2–4):95–107. https://doi.org/10.1016/j.vetpar.2009.09.012
Koh FX, Panchadcharam C, Sitam FT, Tay ST (2018) Molecular investigation of Anaplasma spp. in domestic and wildlife animals in Peninsular Malaysia. Vet Parasitol Reg Stud Rep 13:141–147. https://doi.org/10.1016/j.vprsr.2018.05.006
Kolo A (2023) Anaplasma species in Africa - a century of discovery: a review on molecular epidemiology, genetic diversity, and control. Pathogens 12:702. https://doi.org/10.3390/pathogens12050702
Kumar K, Singh K, Verma AK, Maurya PS, Prajapati MR, Kumar A, Sarkar TK (2023) Phylogenetic analysis and molecular characterization of field isolates of Anaplasma spp. from cattle in India. Vet Arhiv 93(5):535–548. https://doi.org/10.24099/vet.arhiv.1659
Langenwalder DB, Schmidt S, Silaghi C, Skuballa J, Pantchev N, Matei IA, Mihalca AD, Gilli U, Zajkowska J, Ganter M, Hoffman T, Salaneck E, Petrovec M, von Loewenich FD (2020) The absence of the drhm gene is not a marker for human-pathogenicity in European Anaplasma phagocytophilum strains. Parasit Vector 13:238. https://doi.org/10.1186/s13071-020-04116-z
Li H, Zheng YC, Ma L, Jia N, Jiang BG, Jiang RR, Huo QB, Wang YW, Liu HB, Chu YL, Song YD, Yao NN, Sun T, Zeng FY, Dumler JS, Jiang JF, Cao WC (2015) Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis 15(6):663–670. https://doi.org/10.1016/S1473-3099(15)70051-4
Lin ZT, Du LF, Zhang MZ, Han XY, Wang BH, Meng J, Yu FX, Zhou XQ, Wang N, Li C, Wang XY, Liu J, Gao WY, Ye RZ, Xia LY, Sun Y, Jia N, Jiang JF, Zhao L, Cui XM, Zhan L, Cao WC (2023a) Genomic characceristics of emerging intraerythrocytic Anaplasma capra and high prevalence in goats, China. Emerg Infect Dis 29(9):1780–1788. https://doi.org/10.3201/eid2909.230131
Lin ZT, Ye RZ, Liu JY, Wang XY, Zhu WJ, Li YY, Cui XM, Cao WC (2023b) Epidemiological and phylogenetic characteristics of emerging Anaplasma capra: a systematic review with modeling analysis. Infec Genet Evol 115:105510. https://doi.org/10.1016/j.meegid.2023.105510
Liu Z, Ma M, Wang Z, Wang J, Peng Y, Li Y, Guan G, Luo J, Yin H (2012) Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl Environ Microbiol 78(2):464–470. https://doi.org/10.1128/AEM.06848-11
Lu M, Meng C, Li Y, Zhou G, Wang L, Xu X, Li N, Ji Y, Tian J, Wang W, Li K (2023) Rickettsia sp. and Anaplasma spp. in Haemaphysalis longicornis from Shandong Province of China, with evidence of a novel species Candidatus Anaplasma shandongensis. Ticks Tick Borne Dis 14:102082. https://doi.org/10.1016/j.ttbdis.2022.102082
Miranda EA, Han SW, Cho YK, Choi KS, Chae JS (2021) Co-infection with Anaplasma species and novel genetic variants detected in cattle and goats in the Republic of Korea. Pathogens 10(1):28. https://doi.org/10.3390/pathogens10010028
Nguyen AH, Tiawsirisup S, Kaewthamasorn M (2020) Molecular detection and genetic characterization of Anaplasma marginale and Anaplasma platys-like (Rickettsiales: Anaplasmataceae) in water buffalo from eight provinces of Thailand. BMC Vet Res 16:1–12. https://doi.org/10.1186/s12917-020-02585-z
Numan M, Alouffi A, Almutairi MM, Tanaka T, Ahmed H, Akbar H, Rashid MI, Tsai KH, Ali A (2023) First detection of Theileria sinensis-like and Anaplasma Capra in Ixodes kashmiricus: with notes on cox1-based phylogenetic position and new locality records. Animals 13:3232. https://doi.org/10.3390/ani13203232
Oguz B, Deger MS, Al Olayan E, El Ashram S (2023) Molecular survey of Anaplasma Capra in goats in Van province, eastern Türkiye. Acta Parasitol. https://doi.org/10.1007/s11686-023-00758-y
Peng Y, Wang K, Zhao S, Yan Y, Wang H, Jing J, Jian F, Wang R, Zhang L, Ning C (2018) Detection and phylogenetic characterization of Anaplasma Capra: an emerging pathogen in sheep and goats in China. Front Cell Infect Microbiol 30(8):283. https://doi.org/10.3389/fcimb.2018.00283
Peng Y, Lu C, Yan Y, Shi K, Chen Q, Zhao C, Wang R, Zhang L, Jian F, Ning C (2021a) The first detection of Anaplasma Capra, an emerging zoonotic Anaplasma sp., in erythrocytes. Emerg Microbes Infect 10(1):226–234. https://doi.org/10.1080/22221751.2021.1876532
Peng Y, Lu C, Yan Y, Song J, Pei Z, Gong P, Wang R, Zhang L, Jian F, Ning C (2021b) The novel zoonotic pathogen, Anaplasma Capra, infects human erythrocytes, HL-60, and TF-1 cells in vitro. Pathogens 10(5):600. https://doi.org/10.3390/pathogens10050600
Qin XR, Han FJ, Luo LM, Zhao FM, Han HJ, Zhang ZT, Liu JW, Xue ZF, Liu MM, Ma DQ, Huang YT, Yue S, Sun XF, Li WQ, Zhao L, Hao Y, Yu XJ (2018) Anaplasma species detected in Haemaphysalis longicornis tick from China. Ticks Tick Borne Dis 9(4):840–843. https://doi.org/10.1016/j.ttbdis.2018.03.014
Rar V, Golovljova I (2011) Anaplasma, Ehrlichia, and Candidatus Neoehrlichia bacteria: pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol 11:1842–1861. https://doi.org/10.1016/j.meegid.2011.09.019
Rar V, Tkachev S, Tikunova N (2021) Genetic diversity of Anaplasma bacteria: twenty years later. Infect Genet Evol 91:104833. https://doi.org/10.1016/j.meegid.2021.104833
Remesar S, Prieto A, Garcia-Dios D, Lopez-Lorenzo G, Martinez-Calabuig N, Diaz-Cao JM, Panadero R, Lopez CM, Fernandez G, Díez-Banos P, Morrondo P, Diaz P (2022) Diversity of Anaplasma species and importance of mixed infections in roe deer from Spain. Transbound Emerg Dis 69(4):e374–e385. https://doi.org/10.1111/tbed.14319
Renneker S, Abdo J, Salih DE, Karagenç T, Bilgiç H, Torina A, Oliva AG, Campos J, Kullmann B, Ahmed J, Seitzer U (2013) Can Anaplasma ovis in small ruminants be neglected any longer? Transbound Emerg Dis 60(2):105–112. https://doi.org/10.1111/tbed.12149
Sahin OF, Erol U, Altay K (2022) Buffaloes as new hosts for Anaplasma capra: molecular prevalence and phylogeny based on gtlA, groEL, and 16S rRNA genes. Res Vet Sci 152: 458–464. https://doi.org/10.1016/j.rvsc.2022.09.008
Saratsis A, Ligda P, Aal F, Jelicic M, Polgar J, de Vries M, Mastranestasis I, Musella V, Rinaldi L, Jongejan F, Sotiraki S (2022) The scenario of ticks and tick-borne pathogens of sheep on a Mediterranean Island. Microorganisms 10(8):1551. https://doi.org/10.3390/microorganisms10081551
Sato M, Nishizawa I, Fujihara M, Nishimura T, Matsubara K, Harasawa R (2009) Phylogenetic analysis of the 16S rRNA gene of Anaplasma species detected from Japanese serows (Capricornis crispus). J Vet Med Sci 71(12):1677–1679. https://doi.org/10.1292/jvms.001677
Seo MG, Ouh IO, Lee H, Geraldino PJL, Rhee MH, Kwon OD, Kwak D (2018) Differential identification of Anaplasma in cattle and potential of cattle to serve as reservoirs of Anaplasma Capra, an emerging tick-borne zoonotic pathogen. Vet Microbiol 226:15–22. https://doi.org/10.1016/j.vetmic.2018.10.008
Seo MG, Kwon OD, Kwak D (2020) Genotypic analysis of piroplasms and associated pathogens from ticks infesting cattle in Korea. Microorganisms 8(5):728. https://doi.org/10.3390/microorganisms8050728
Shi K, Li J, Yan Y, Chen Q, Wang K, Zhou Y, Li D, Chen Y, Yu F, Peng Y, Zhang L, Ning C (2019) Dogs as new hosts for the emerging zoonotic pathogen Anaplasma Capra in China. Front Cell Infect Microbiol 9:394. https://doi.org/10.3389/fcimb.2019.00394
Shi Y, Yang J, Guan G, Liu Z, Luo J, Song M (2020) Molecular investigation of Anaplasma species in sheep from Heilongjiang Province, northeast China identified four Anaplasma species and a novel genotype of Anaplasma Capra. Parasitol Int 76:102072. https://doi.org/10.1016/j.parint.2020.102072
Shin SU, Park YJ, Ryu JH, Jang DH, Hwang S, Cho HC, Park J, Han JI, Choi KS (2020) Identification of zoonotic tick-borne pathogens from Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis 20(10):745–754. https://doi.org/10.1089/vbz.2019.2609
Staji H, Yousefi M, Hamedani MA, Tamai IA, Khaligh SG (2021) Genetic characterization and phylogenetic of Anaplasma Capra in Persian onagers (Equus hemionus onager). Vet Microbiol 261:109199. https://doi.org/10.1016/j.vetmic.2021.109199
Stuen S, Granquist EG, Silaghi C (2013) Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 3:31. https://doi.org/10.3389/fcimb.2013.00031
Sun XF, Zhao L, Wen HL, Luo LM, Yu XJ (2015) Anaplasma species in China. Lancet Infect Dis 15(11):1263–1264. https://doi.org/10.1016/S1473-3099(15)00377-1
Teng Z, Shi Y, Zhao N, Zhang X, Jin X, He J, Xu B, Qin T (2023) Molecular detection of tick-borne bacterial and protozoan pathogens in Haemaphysalis longicornis (Acari: Ixodidae) ticks from free-ranging domestic sheep in Hebei Province, China. Pathogens 12(6): 763. https://doi.org/10.3390/pathogens12060763
Teodorowski O, Radzki R, Kalinowski M, WIniarczyk S, Garcia Bocanegra I, Winiarczyk D, Adaszek L (2020) Molecular detection of Anaplasma phagocytophilum in roe deer (Capreolus capreolus) in eastern Poland. Ann Agric Environ Med 27(4):702–705. https://doi.org/10.26444/aaem/124902
Theiler A (1910) Anaplasma marginale (Gen. and Spec. Nov.): the marginal points in the blood of cattle suffering from a specific disease. Government Printing and Stationary Office, Pretoria
Torina A, Galindo RC, Vicente J, Di Marco V, Russo M, Aronica V, Fiasconaro M, Scimeca S, Alongi A, Caracappa S, Kocan KM, Gortazar C, de la Fuente J (2010) Characterization of Anaplasma phagocytophilum and A. ovis infection in a naturally infected sheep flock with poor health condition. Trop Anim Health Prod 42:1327–1331. https://doi.org/10.1007/s11250-010-9580-8
Wang H, Yang J, Mukhtar MU, Liu Z, Zhang M, Wang X (2019) Molecular detection and identification of tick-borne bacteria and protozoans in goats and wild siberian roe deer (Capreolus pygargus) from Heilongjiang Province, northeastern China. Parasit Vector 12(1):296. https://doi.org/10.1186/s13071-019-3553-1
Wang K, Yan Y, Zhou Y, Zhao S, Jian F, Wang R, Zhang L, Ning C (2021a) Seasonal dynamics of Anaplasma spp. in goats in warm-temperate zone of China. Ticks Tick Borne Dis 12(3):101673. https://doi.org/10.1016/j.ttbdis.2021.101673
Wang Y, Zhang Q, Han S, Li Y, Wang B, Yuan G, Zhang P, Yang Z, Zhang H, Sun Y, Chen J, Han X, He H (2021b) Ehrlichia chaffeensis and four Anaplasma species with veterinary and public health significance identified in tibetan sheep (Ovis aries) and yaks (Bos grunniens) in Qinghai, China. Front Vet Sci 8:727166. https://doi.org/10.3389/fvets.2021.727166
Wei W, Li J, Wang YW, Jiang BG, Liu HB, Wei R, Jiang RR, Cui XM, Li LF, Yuan TT, Wang Q, Zhao L, Xia LY, Jiang JF, Qiu YF, Jia N, Cao WC, Hu YL (2020) Anaplasma platys-like infection in goats, Beijing, China. Vector Borne Zoonotic Dis 20(10):755–762. https://doi.org/10.1089/vbz.2019.2597
Woldehiwet Z (2010) The natural history of Anaplasma phagocytophilum. Vet Parasitol 167(2–4). https://doi.org/10.1016/j.vetpar.2009.09.013. 108 – 22
Yan Y, Wang K, Cui Y, Zhou Y, Zhao S, Zhang Y, Jian F, Wang R, Zhang L, Ning C (2021) Molecular detection and phylogenetic analyses of Anaplasma spp. in Haemaphysalis longicornis from goats in four provinces of China. Sci Rep 11(1): 14155. https://doi.org/10.1038/s41598-021-93629-3
Yang J, Liu Z, Niu Q, Liu J, Han R, Liu G, Shi Y, Luo J, Yin H (2016) Molecular survey and characterization of a novel Anaplasma species closely related to Anaplasma Capra in ticks, northwestern China. Parasit Vector 9(1):603. https://doi.org/10.1186/s13071-016-1886-6
Yang J, Liu Z, Niu Q, Liu J, Han R, Guan G, Hassan MA, Liu G, Luo J, Yin H (2017) A novel zoonotic Anaplasma species is prevalent in small ruminants: potential public health implications. Parasit Vector 10(1):264. https://doi.org/10.1186/s13071-017-2182-9
Yang J, Liu Z, Niu Q, Mukhtar MU, Guan G, Liu G, Luo J, Yin H (2018) A novel genotype of Anaplasma Capra in wildlife and its phylogenetic relationship with the human genotypes. Emerg Microbes Infect 7(1):210. https://doi.org/10.1038/s41426-018-0212-0
Zhang Y, Cui Y, Sun Y, Jing H, Ning C (2020) Novel Anaplasma variants in small ruminants from Central China. Front Vet Sci 7:580007. https://doi.org/10.3389/fvets.2020.580007
Zhou M, Cao S, Sevinc F, Sevinc M, Ceylan O, Ekici S, Jirapattharasate C, Moumouni PF, Liu M, Wang G, Iguchi A, Vudriko P, Suzuki H, Xuan X (2017) Molecular detection and genetic characterization of Babesia, Theileria and Anaplasma amongst apparently healthy sheep and goats in the central region of Turkey. Ticks Tick Borne Dis 8(2):246–252. https://doi.org/10.1016/j.ttbdis.2016.11.006
Zhou S, Huang L, Lin Y, Bhowmick B, Zhao J, Liao C, Guan Q, Wang J, Han Q (2023) Molecular surveillance and genetic diversity of Anaplasma spp. in cattle (Bos taurus) and goat (Capra aegagrus hircus) from Hainan island/province, China. BMC Vet Res 19(1):213. https://doi.org/10.1186/s12917-023-03766-2
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by the Scientific and Technical Research Council of Turkey (TUBITAK) under project number 123O919 and by the Scientific Research Project Fund of Sivas Cumhuriyet University under project number V-2023-132. The APC of this article was covered by The Scientific and Technological Research Council of Türkiye (TUBITAK).
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
K.A. wrote the main manuscript text U.E. literature search and selection, data analys, preparation figures and table O.F.S. literature research, preparation figures and table All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. Because no application was performed on animals in this study.
Consent to participate
Not applicable.
Consent for publication
All authors have seen and approved the final version of the manuscript being submitted. They warrant that the article is the authors’ original work, has not received prior publication, and is not under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altay, K., Erol, U. & Sahin, O.F. Anaplasma capra: a new emerging tick-borne zoonotic pathogen. Vet Res Commun 48, 1329–1340 (2024). https://doi.org/10.1007/s11259-024-10337-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-024-10337-9